Abstract
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) have a well-established analgesic efficacy for inflammatory pain. These drugs exert their effect by inhibiting the enzyme cyclooxygenase (COX) and are commonly used for the management of pain following endodontic treatment. There are two distinct isoforms of COX: COX-1, which is constitutively expressed; and COX-2, which is primarily induced by inflammation. Previous studies have shown that functional human genetic variants of the COX-2 gene may explain individual variations in acute pain. The present study extends this work by examining the potential contribution of the two COX isoforms to pain after endodontic treatment.
Methods
Ninety-four patients treated by endodontic residents at the University of North Carolina School of Dentistry were enrolled into a prospective cohort study. Data on potential predictors of post-treatment pain was collected and all patients submitted saliva samples for genetic analysis. Non-surgical root canal therapy was performed and participants recorded pain levels for five days following.
Results
In this study, 63% of patients experienced at least mild pain after root canal therapy and 24% experienced moderate to severe pain. Presence of pretreatment pain was correlated with higher post-treatment pain (p=0.01). Elevated heart rate (p=0.02) and higher diastolic blood pressure (p=0.024) were also correlated with decreased post-treatment pain. Finally, we identified genetic variants in COX-2 (haplotype composed of rs2383515 G, rs5277 G, rs5275 T, and rs2206593 A) associated with post-treatment pain following endodontic treatment (p= 0.025).
Conclusion
Understanding the genetic basis of pain following endodontic treatment will advance its prevention and management.
Keywords: Cyclooxygenase, COX-1, COX-2, single nucleotide polymorphism, postoperative pain, odontogenic
INTRODUCTION
The general population commonly associates root canal treatment with pain, and post-treatment pain remains frequent sequelae of endodontic treatment. A systematic review of sixteen studies on pain following endodontic treatment found that the prevalence of at least mild pain ranged from 3% to 58% (1). Up to 20% of patients report moderate to severe post-treatment pain with the greatest intensity of pain reported during the first 24 hours following treatment (2, 3). It is well established that high levels of preoperative pain are predictive of higher levels of discomfort both intra- and postoperatively after endodontic treatment (2, 4). While many studies have evaluated other factors that could potentially affect post-treatment pain, none have examined genetic variants associated with pain in this patient population. While inter-individual differences in the experience of pain have been noted by clinicians, the genetic basis for acute pain conditions such as post-treatment endodontic pain remains unknown. Animal studies indicate that genetic variations account for 28 to 76% of pain experience (5). Potential candidate genes for pain modulation include the two isoforms of cyclooxygenase. Cyclooxygenase-1 (COX-1; prostaglandin H synthase1; PTGS1) is the constitutively expressed main enzyme involved in the synthesis of prostaglandin H2 from arachidonic acid, which is the committed step in prostaglandin (PG) synthesis. Cyclooxygenase-2 (COX-2; prostaglandin H synthase2; PTGS2) is inducible and has a role primarily in the production of PGs that promote inflammation (6–8). In vivo studies on orofacial pain clearly show that COX-1 is expressed in normal (uninflamed) tissues while COX-2 is induced by inflammation in a time-dependent manner (9,10). Inhibition of cyclooxygenase isoforms by drugs such as aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) results in decreased synthesis of PGs and decreased pain. Ibuprofen, the prototypical NSAID, is the drug of choice among endodontists for the management of post-treatment pain (4).
Genetic variations of COX-1 and COX-2 have been explored in several studies on pain (10–12). These studies suggest that functional polymorphisms in COX-2 may account, in part, for inter-individual variations in pain (10, 13). In addition, polymorphisms in COX-2 may also account for inter-individual differences in analgesic responses to NSAIDs and selective COX-2 inhibitors (10). However, the associations between COX-1 and COX-2 polymorphisms and post-treatment pain in endodontic patients are yet to be explored.
Thus, we conducted a prospective study to examine the contribution of COX-1 and COX-2 genetic variants as well as phenotypic and physiological factors to the development of post-treatment pain in endodontic patients.
MATERIALS AND METHODS
Study Participants
Patients were recruited from the University of North Carolina-Chapel Hill School of Dentistry Graduate endodontic clinic. The inclusion criteria were patients of age ≥18 years old and American Society of Anesthesiologists class I or II. Patients who were taking corticosteroid medications and those unable to take ibuprofen were excluded from the study. This study was approved by our Institutional Review Board and written informed consent was obtained from all study participants.
Patients were asked to complete questionnaires prior to treatment to establish baseline pain levels as well as tooth pain experienced in the last 24 hours using a Likert scale (with the anchors “No pain” and “Worst pain imaginable”). After completion of the pretreatment pain questionnaires the subjects resting arterial blood pressure and heart rate were measured once using a wrist cuff blood pressure monitor on the right arm. The monitor used was the OMRON Model HEM 605 (OMRON Vernon Hills, Illinois). These measurements were taken approximately 15 minutes after seating the patient in an upright position. Salivary DNA was collected prior to the initiation of root canal treatment using the Oragene Self-Collection System (DNA Genotek, Ontario, Canada). This system yields high quality DNA, similar to that purified from blood. It is convenient, proven, and specimens remain intact for years in their collected media at room temperature.
While the protocol for root canal treatment was not standardized, the basic protocol used by UNC endodontic graduate students includes chemomechanical debridement by shaping the canals to a minimal apical size of 40.04 and irrigation with sodium hypochlorite and ethylenediaminetetraacetic acid. The root canal system was then either obturated with Resilon/Epiphany™ (SybroEndo Co., Orange, CA) or calcium-hydroxide powder mixed with 2% chlorhexidine was placed as an interappointment medicament, and the access was restored.
Study participants completed a pain diary for five consecutive days after treatment. The instructions were to complete the diary at bedtime and record the time of day and the worst pain they felt in the teeth or mouth during the past 24 hours on a Likert scale. While no medications were prescribed, patients were asked to report any analgesics, including dosage and frequency, which may have been self-administered during the postoperative period.
Genetic data was processed at Cogenics™ Genomics Services Company (Cary, NC). DNA was extracted from the saliva using the 4 ml Oragene DNA Purifier System and quantified using Pico Green Assay kit (Life Technologies, Grand Island, NY) to ensure DNA concentration of at least 10 ng/µl. SNP genotyping was performed using iPLEX Gold chemistry (Sequenom, San Diego, CA). SNP Assays were designed by MassARRAY Assay Design 3.1 (Sequenom, San Diego, CA). iPLEX Gold chemistry utilizes multiplex PCR. After the PCR, remaining nucleotides are deactivated by Shrimp Alkaline Phosphatase (SAP) treatment. Then single base primer extension is performed, and the primer extension products are analyzed using MALDI TOF Mass Spectrometer. From the MALDI TOF Mass Spectrometer peak information we are able to accurately genotype the SNP of interest using Typer 4 software (Sequenom, San Diego, CA.) Samples that failed to amplify sufficiently for allelic discrimination were not included in the final analysis.
Statistical Analysis
Data were analyzed with R. software (version 2.8.1; 2008, Vienna, Austria) Linear regression was used to evaluate the association between postoperative pain and preoperative pain, heart rate, and blood pressure. The association between each genetic variable and postoperative pain was evaluated using ANOVA. Haplotypes (and associated posterior probabilities) were constructed for each participant using the EM algorithm (implemented in the “haplo.stats” R package) (14). All values are reported as mean ± standard error.
RESULTS
Patient Characteristics
This study enrolled 69 subjects (39 male and 30 female) aged 18–85 years for whom we obtained baseline data, post-operative data, and salivary DNA. Subject demographics and baseline characteristics are displayed in Table 1. Of the 69 subjects, 48 were self-identified as Caucasian, 7 as African-American, 7 as Hispanic, 6 as Asian, and 1 as other. Only 12 subjects (17%) reported preoperative pain within the past 24 hours on the day of enrollment. Pulpal and periapical diagnoses varied across patients. Thirty-five (51%) subjects presented with a periapical radiolucency. There were no statistically significant differences between male and female subjects in any of the demographic characteristics measured. The majority of treated teeth (66%) were molars. An equal number of teeth were treated in the maxillary and mandibular arches.
Table 1.
Demographics and Baseline Characteristics of Study Subjects
| BASELINE CHARACTERISTIC |
SUBJECTS- MALES |
SUBJECTS- FEMALES |
OVERALL | |
|---|---|---|---|---|
| Total Enrolled | 39 (57) | 30 (43) | 69 | |
| Average Age (years) | 54 ± 3.02 | 42 ± 2.80 | 48 ± 2.19 | |
| Racial Distribution* | ||||
| White | 27 (69) | 21 (70) | 48 (70) | |
| Black | 4 (10) | 3 (10) | 7 (10) | |
| Hispanic | 5 (13) | 2 (7) | 7 (10) | |
| Asian | 2 (5) | 4 (13) | 6 (9) | |
| Other | 1 (3) | 0 (0) | 1 (1) | |
| Subjects with Pretreatment Pain | 6 (15) | 6 (20) | 12 (17) | |
| Subjects without Pretreatment Pain | 33 (85) | 24 (80) | 57 (83) | |
| Presence of Periapical Disease | 25 (64) | 17 (57) | 42 (61) | |
| Presence of Periapical Radiolucency | 22 (56) | 13 (43) | 35 (51) | |
Values are expressed as number (percentage) unless otherwise indicated
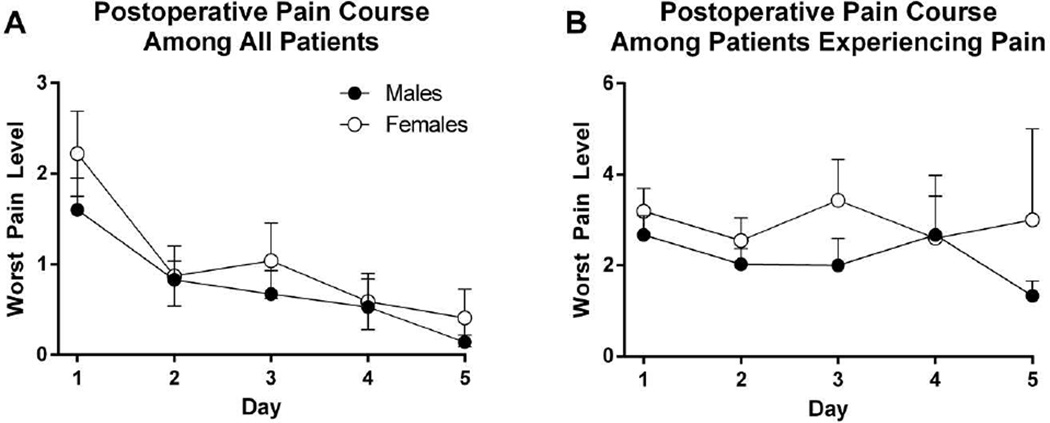
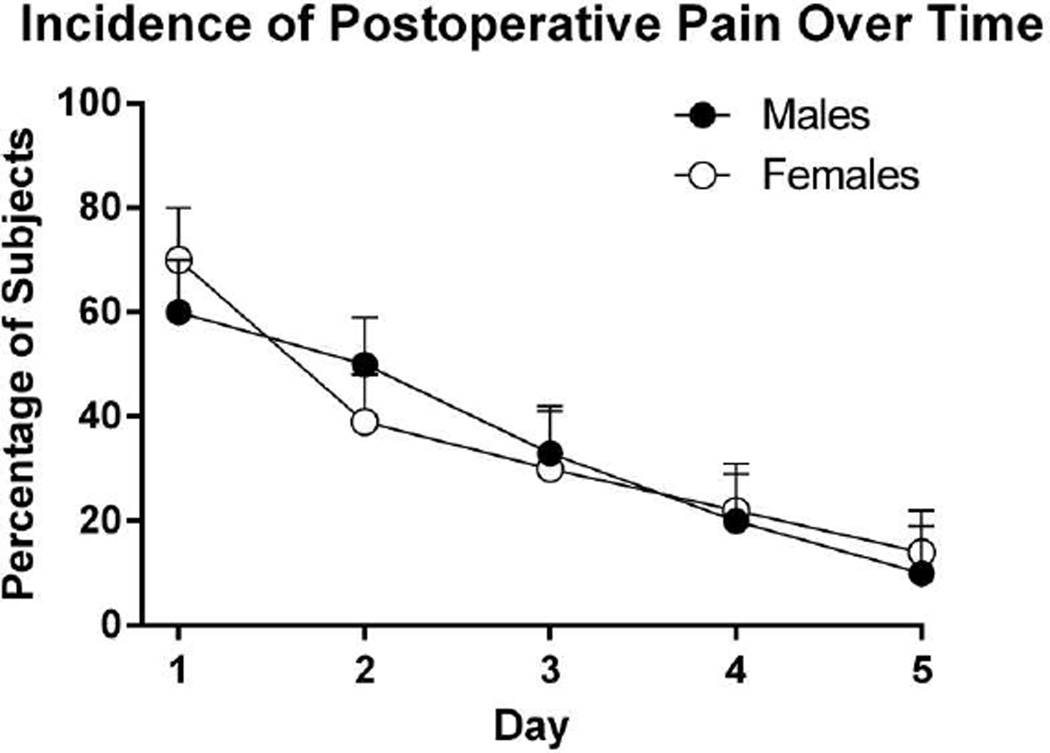
The course of mean postoperative endodontic pain by gender is shown in Figure 2. No differences between males and females were observed in the worst pain intensity reported. As shown in Figure 3, 63% of subjects reported at least mild pain postoperatively. Reported mean worst pain intensity decreased gradually over the five-day period. Most subjects (88%) were pain-free by Day 5.
Figure 2.
Postoperative pain among (A) all patients and (B) patients experiencing pain after endodontic treatment. Patients recorded their worst pain intensity in a daily pain diary using a Likert scale with the anchors “No pain” and “Worst pain imaginable.
Figure 3.
Incidence of post-operative pain. Endodontic patients recorded their odontogenic pain in a pain diary for five consecutive days after treatment.
On day 1, 20 subjects (39%) reported taking oral analgesics for postoperative pain. On postoperative day 2, only 9 subjects (18%) reported taking pain medication and by postoperative day 5, only 2 subjects (4%) reported taking pain medication. All but 1 subject who used postoperative analgesics took NSAIDs (ibuprofen and naproxen).
Phenotypic Factors Associated with Post-treatment Pain Level
None of the demographic characteristics were found to be associated with mean Day 1 worst pain or average pain. The relationships between preoperative and postoperative pain are shown in Tables 2 and 3. Twenty-five subjects (59%) who did not experience pretreatment pain did experience at least mild postoperative pain on Day 1, while 7 (78%) subjects who experienced preoperative pain also experienced postoperative pain. Specifically, preoperative pain was associated with higher intensity of reported mean Day 1 worst pain level (p = 0.017; R2 = 0.10).
Table 2.
Postoperative Pain Course in Patients Experiencing Pain
| Males | Females | Overall | ||
|---|---|---|---|---|
| Day 1 | ||||
| Worst pain level** | 2.67 (0.42) | 3.19 (0.50) | 2.91 (0.32) | |
| Average pain level | 1.81 (0.29) | 2.43 (0.36) | 2.10 (0.23) | |
| Day 2 | ||||
| Worst pain level | 2.03 (0.34) | 2.55 (0.50) | 2.23 (0.28) | |
| Average pain level | 1.36 (0.17) | 2.22 (0.49) | 1.70 (0.23) | |
| Day 3 | ||||
| Worst pain level | 2.00 (0.60) | 3.43 (0.90) | 2.59 (0.52) | |
| Average pain level | 1.86 (0.59) | 2.29 (0.57) | 2.07 (0.40) | |
| Day 4 | ||||
| Worst pain level | 2.67 (1.31) | 2.60 (0.93) | 2.64 (0.79) | |
| Average pain level | 3.33 (1.86) | 2.20 (0.73) | 2.63 (0.78) | |
| Day 5 | ||||
| Worst pain level | 1.33 (0.33) | 3.00 (2.00) | 2.17 (0.98) | |
| Average pain level | 1.00 (NA) | 2.67 (1.67) | 2.25 (1.25) | |
Value expressed as N Subjects/Total N (percentage)
Values are expressed as mean score % (standard error of the mean) unless otherwise indicated; Pain was expressed on a 9-point Likert scale with anchors 0 (No Pain) to 9 (Worst pain imaginable)
Table 3.
Post-Operative Pain in Study Subjects Presenting with or without Preoperative Pain
| Subjects Presenting without Preoperative Pain |
Subjects Presenting with Preoperative Pain |
|
|---|---|---|
| N (%) | N (%) | |
| Males | 14 (58) | 3 (60) |
| Females | 11(61) | 4(100) |
| Overall | 25(59) | 7 (78) |
Physiologic Factors Associated with Post-treatment Pain Level
Alterations in heart rate and blood pressure were linked to postoperative pain, such that higher resting heart rate (p = 0.020) and diastolic arterial blood pressure (p = 0.024) were associated with lower mean Day 1 average pain. Systolic arterial blood pressure was not significantly associated with postoperative pain on Day 1 (p = 0.21 and p = 0.15).
Genetic Variation and Post-treatment Pain Levels
The genotype frequencies of each tested SNP in COX-1 and COX-2 are shown in Table 4. The 4 COX-2 SNPs tested were in high linkage disequilibrium (D’ =0.99–1) and formed a haploblock composed of 1 SNP in the promoter region (rs2383515 G/T), 1 SNP in the coding region (rs5277 C/G), and 2 SNPs in the 3’ UTR (rs5275 C/T and rs2206593 A/G) (Table 5). The GGTA haplotype was associated with Day 1 worst pain postoperative. (p = 0.025).
Table 4.
Variations in Pain Levels (mean worst pain score) Among Tested SNPs of the COX-1, and COX-2 Genes
| SNPs | Genotype | Number of subjects |
Genotypes Frequencies |
Mean (SE) Worst Pain |
ANOVA R2 |
P-value | Gene |
|---|---|---|---|---|---|---|---|
| rs2206593 | A/G | 6 | 0.118 | 3.7 (0.8) | 0.099 | 0.025* | COX-2 |
| G/G | 45 | 0.882 | 1.6 (0.3) | ||||
| rs2383515 | G/G | 29 | 0.569 | 2.3 (0.4) | 0.056 | 0.094 | COX-2 |
| G/T | 22 | 0.431 | 1.3 (0.4) | ||||
| rs5275 | C/C | 7 | 0.200 | 1.3 (0.7) | 0.12 | 0.12 | COX-2 |
| C/T | 15 | 0.429 | 1.5 (0.5) | ||||
| T/T | 13 | 0.371 | 2.8 (0.5) | ||||
| rs5277 | C/C | 1 | 0.020 | 2.0 (2.1) | 0.010 | 0.77 | COX-2 |
| C/G | 12 | 0.235 | 1.5 (0.6) | ||||
| G/G | 38 | 0.745 | 2.0 (0.3) | ||||
| rs1236913 | C/C | 47 | 0.922 | 1.7(0.3) | 0.069 | 0.062 | COX-1 |
| C/T | 4 | 0.078 | 3.8(1.0) | ||||
| rs3842803 | C/T | 2 | 0.039 | 0.0(1.5) | 0.033 | 0.20 | COX-1 |
| T/T | 49 | 0.961 | 2.0(0.3) | ||||
| rs10306202 | A/A | 1 | 0.020 | 0.0(2.1) | 0.034 | 0.43 | COX-1 |
| A/G | 11 | 0.216 | 2.5(0.6) | ||||
| G/G | 39 | 0.765 | 1.8(0.3) | ||||
| rs5789 | A/C | 1 | 0.020 | 0.0(2.0) | 0.016 | 0.37 | COX-1 |
| C/C | 50 | 0.980 | 1.9(0.3) | ||||
| rs10306114 | A/A | 44 | 0.863 | 2.0(0.3) | 0.032 | 0.46 | COX-1 |
| A/G | 5 | 0.098 | 1.2(0.9) | ||||
| G/G | 2 | 0.039 | 0.5(1.5) | ||||
| rs1213266 | A/A | 1 | 0.020 | 0.0(2.1) | 0.017 | 0.67 | COX-1 |
| A/G | 10 | 0.196 | 1.9(0.7) | ||||
| G/G | 40 | 0.784 | 1.9(0.3) | ||||
| rs3842788 | A/A | 1 | 0.020 | 0.0(2.1) | 0.017 | 0.67 | COX-1 |
| A/G | 10 | 0.196 | 1.9(0.7) | ||||
| G/G | 40 | 0.784 | 1.9(0.3) |
Table 5.
COX-2 Haploblock Comprised of Four SNPs.
| rs2206593 | rs5275 | rs5277 | rs2383515 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D' | R^2 | p-value | D' | R^2 | p-value | D' | R^2 | p-value | D' | R^2 | p-value | |
| rs2206593 | 1.00 | 1.00 | 0.0000 | |||||||||
| rs5275 | 0.99 | 0.02 | 0.0985 | 1.00 | 1.00 | 0.0000 | ||||||
| rs5277 | 0.99 | 0.01 | 0.2739 | 1.00 | 0.09 | 0.0007 | 1.00 | 1.00 | 0.0000 | |||
| rs2383515 | 0.99 | 0.01 | 0.1665 | 0.89 | 0.39 | 0.0000 | 1.00 | 0.04 | 0.0044 | 1.00 | 1.00 | 0.0000 |
The Four Cox-2 SNPs genotyped in this study were tested to determine the likelihood they were inherited together by calculating the normalized linkage disequilibrium statistic (D’) and the square of the correlation coefficient between each SNP (R2). D’ and R2 lie in the range from 0 to 1, with 1 indicating the strongest possible linkage disequilibrium/correlation.
DISCUSSION
We employed a prospective cohort study to identify phenotypic, physiologic, and genetic factors that contribute to the incidence and intensity of postoperative pain following endodontic treatment. Among patients presenting to the UNC School of Dentistry for root canal treatment, the incidence and intensity of pain after nonsurgical root canal treatment reached its maximum within the first 24 hours of treatment, when 63% of patients reported pain (mean = 1.88 ± 0.28). Nearly 25% of patients reported Day 1 worst pain levels of more than four on a nine point Likert scale and 38% felt the need to control their postoperative pain symptoms with non-steroidal anti-inflammatory drugs (NSAIDs), with ibuprofen being the most frequently used. These findings are in line with those of other studies demonstrating that 68–70% of root canal treatments are associated with postoperative pain, with 17% reporting symptoms of severe nature (15,16). By day 3 after root canal treatment, the incidence of pain decreased to 30%. This estimate is higher than that of Genet and colleagues, showing very little pain 3 days posttreatment (17.
No differences were observed in pain levels in teeth with or without periapical radiolucency. This finding is in agreement with those of Harrison, Georgopoulou, and O’Keefe (2, 3,16), but not with those of Marshall and Liesinger (18), who found that patients with no radiographic periapical lesions had significantly more pain than patients with lesions. The age and sex of the patient and dental arch treated appeared not to influence the incidence of pain; this is in accord with the results of Harrison, Georgopoulou, O’Keefe, and Albashaireh and Alnegrish (2, 3, 16,19).
Consistent with previous studies (3, 4,17), we found a strong association between preoperative pain and postoperative pain. Specifically, the intensity of preoperative pain was correlated with more severe pain on postoperative Day 1 ((p = 0.017). Furthermore, we found that 10% of worst pain on postoperative Day 1 could be explained by the presence or absence of pretreatment pain (R2 = 0.10).
We also found associations between physiologic measures and post-operative pain, such that lower resting heart rate and lower diastolic arterial blood pressure were associated with higher mean Day 1 average. That higher resting blood pressure is associated with decreased pain sensitivity is well-established (20,21). Specifically, normal pain-free subjects with higher blood pressures at rest have a higher pain threshold and tolerance compared with subjects with lower blood pressures (20). King et al. observed significant correlations between preoperative systolic blood pressure and postoperative pain after non-surgical root canal treatment (2). The exact mechanism for the contribution of arterial blood pressure to experimental pain sensitivity remains unknown. One proposed mechanism involves activation of the carotid sinus baroreceptors (23).
Finally, we explored the association between COX-1 and COX-2 genetic variants and postoperative pain. While our results are tentative due to a small sample size, they show that in COX-2, a haplotype composed of rs2383515 G, rs5277 G, rs5275 T, and rs2206593 A was associated with worst pain scores on postoperative Day 1. Little is known about a genetic association between the COX-2 SNPs we identified in this study and acute pain. The rs5277 polymorphism of COX-2 has been identified as a potential modifier of breast cancer risk (4) and pancreatic cancer susceptibility (25), though it is not currently considered a risk factor. The rs5275 SNP in the COX-2 3’UTR has potential miRNA-binding sites in lymphoblastoic cell lines (26) and has been associated with a reduced risk of premalignant lesions in the oral cavity (27). Currently, no literature exists discussing the role of rs2383515 and rs2206593 in pain or inflammatory processes.
Our data on COX-1 show a trend towards an association between rs1236913 SNP and worst pain scores on postoperative Day 1. This SNP is located in exon 2, produces a nonsynonymous change in amino acid from tryptophan to arginine at codon 102 (28,29), and is associated with the severity of ankylosing spondylitis, a chronic inflammatory disease of the axial skeleton (30).
While these results suggest that specific COX-1 and COX-2 genetic variants may be useful to predict patient risk of postoperative pain and benefit of drugs, further studies are required to replicate associations between COX genetic variants and postoperative pain in a larger population of endodontic patients as well as to evaluate potential functional changes in the expression of corresponding COX and downstream PG proteins. Understanding acute pain conditions on a genetic level has important implications for their future management. NSAIDs that target COX-1 and COX-2 are the frontline treatment for post-treatment pain in endodontics. The discovery of functional polymorphisms in the COX genes may ultimately improve the safe and effective use of NSAIDS by better tailoring drug dosage in accordance with an individual’s genetic variation.
Figure 1.

Tested SNP locations on COX-1 (rs1236913; rs3842803; rs10306202; rs5789; rs10306114; rs1213266; rs3842788) on chromosome 9 and COX-2 (rs2206593; rs2383515; rs5275; rs5277) on chromosome 1.
Acknowledgements
The authors thank Dr Jason Lambert for his help.
This work was supported by
The American Association of Endodontists Foundation to Elizabeth Applebaum
NIH/NINDS R01 NS072205 to Andrea G. Nackley
NIH/NINDS P01 NS045685 to William Maixner and Andrea G. Nackley
NIH/NIDCR U01 DE017018 to William Maixner
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors deny any conflicts of interest.
The individual contributions of the authors were
Elizabeth Applebaum: Lead author, patient recruitment, data collection, manuscript preparation
Andrea Nackley: Lead author, study design, data interpretation, manuscript preparation
Eric Bair: Statistical analysis
William Maixner: Study design
Asma Khan- Study design, data interpretation, manuscript preparation
REFERENCES
- 1.Sathorn C, Parashos P, Messer H. The prevalence of postoperative pain and flare-up in single- and multiple-visit endodontic treatment: a systematic review. Int Endod J. 2008;41:91–99. doi: 10.1111/j.1365-2591.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 2.Harrison JW, Baumgartner JC, Svec TA. Incidence of pain associated with clinical factors during and after root canal therapy. Part 2. Postobturation pain. J Endod. 1983;9:434–438. doi: 10.1016/S0099-2399(83)80259-3. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe EM. Pain in endodontic therapy: preliminary study. J Endod. 1976;2:315–319. doi: 10.1016/S0099-2399(76)80047-7. [DOI] [PubMed] [Google Scholar]
- 4.Torabinejad M, Dorn SO, Eleazer PD, Frankson M, Jouhari B, Mullin RK, et al. Effectiveness of various medications on postoperative pain following root canal obturation. J Endod. 1994;20:427–431. doi: 10.1016/S0099-2399(06)80031-2. [DOI] [PubMed] [Google Scholar]
- 5.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 6.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 7.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994;9:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature: New biology. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 9.Khan AA, Iadarola M, Yang HY, Dionne RA. Expression of COX-1 and COX-2 in a clinical model of acute inflammation. J Pain. 2007;8:349–354. doi: 10.1016/j.jpain.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YS, Kim H, Wu TX, Wang XM, Dionne RA. Genetically mediated interindividual variation in analgesic responses to cyclooxygenase inhibitory drugs. Clin Pharm Ther. 2006;79:407–418. doi: 10.1016/j.clpt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Halushka MK, Walker LP, Halushka PV. Genetic variation in cyclooxygenase 1: effects on response to aspirin. Clin Pharm Therap. 2003;73:122–130. doi: 10.1067/mcp.2003.1. [DOI] [PubMed] [Google Scholar]
- 12.Dasdemir S, Cetinkaya Y, Gencer M, Ozkok E, Aydin M, Cakmakoglu B. Cox-2 gene variants in migraine. Gene. 2013;518:292–295. doi: 10.1016/j.gene.2012.12.110. [DOI] [PubMed] [Google Scholar]
- 13.Rausch SM, Gonzalez BD, Clark MM, Patten C, Felten S, Liu H, et al. SNPs in PTGS2 and LTA predict pain and quality of life in long term lung cancer survivors. Lung cancer. 2012;77:217–223. doi: 10.1016/j.lungcan.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walton RE, Chiappinelli J. Prophylactic penicillin: effect on posttreatment symptoms following root canal treatment of asymptomatic periapical pathosis. J Endod. 1993;19:466–470. doi: 10.1016/S0099-2399(06)80535-2. [DOI] [PubMed] [Google Scholar]
- 16.Georgopoulou M, Anastassiadis P, Sykaras S. Pain after chemomechanical preparation. Int Endod J. 1986;19:309–314. doi: 10.1111/j.1365-2591.1986.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 17.Genet JM, Wesselink PR, Thoden van Velzen SK. The incidence of preoperative and postoperative pain in endodontic therapy. Int Endod J. 1986;19:221–229. doi: 10.1111/j.1365-2591.1986.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 18.Marshall JG, Liesinger AW. Factors associated with endodontic posttreatment pain. J Endod. 1993;19:573–575. doi: 10.1016/S0099-2399(06)81290-2. [DOI] [PubMed] [Google Scholar]
- 19.Albashaireh ZS, Alnegrish AS. Postobturation pain after single- and multiple-visit endodontic therapy. A prospective study. J Dent. 1998;26:227–232. doi: 10.1016/s0300-5712(97)00006-7. [DOI] [PubMed] [Google Scholar]
- 20.Maixner W, Fillingim R, Kincaid S, Sigurdsson A, Harris MB. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med. 1997;59:503–511. doi: 10.1097/00006842-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, Maixner W. The influence of resting blood pressure and gender on pain responses. Psychosom Med. 1996;58:326–332. doi: 10.1097/00006842-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 22.King JW, Bair E, Duggan D, Maixner W, Khan AA. The relationship between resting arterial blood pressure and acute postoperative pain in endodontic patients. J Orofac Pain. 2012;26:321–327. [PMC free article] [PubMed] [Google Scholar]
- 23.Mini A, Rau H, Montoya P, Palomba D, Birbaumer N. Baroreceptor cortical effects, emotions and pain. Int J Psych. 1995;19:67–77. doi: 10.1016/0167-8760(94)00084-r. [DOI] [PubMed] [Google Scholar]
- 24.Yu KD, Chen AX, Yang C, Qiu LX, Fan L, Xu WH, et al. Current evidence on the relationship between polymorphisms in the COX-2 gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat t. 2010;122:251–257. doi: 10.1007/s10549-009-0688-3. [DOI] [PubMed] [Google Scholar]
- 25.Ozhan G, Lochan R, Leathart JB, Charnley R, Daly AK. Cyclooxygenase-2 polymorphisms and pancreatic cancer susceptibility. Pancreas. 2011;40:1289–1294. doi: 10.1097/MPA.0b013e31821fcc3b. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Zhao Y, Wang Y, Wang Z, Guan X. Association between a functional variant at PTGS2 gene 3'UTR and its mRNA expression in lymphoblastoid cell lines. Cell Biol Int. 2013;37:516–519. doi: 10.1002/cbin.10066. [DOI] [PubMed] [Google Scholar]
- 27.Pu X, Lippman SM, Yang H, Lee JJ, Wu X. Cyclooxygenase-2 gene polymorphisms reduce the risk of oral premalignant lesions. Cancer. 2009;115:1498–1506. doi: 10.1002/cncr.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi J, Misso NL, Duffy DL, Bradley B, Beard R, Thompson PJ, et al. Cyclooxygenase-1 gene polymorphisms in patients with different asthma phenotypes and atopy. Euro Rest J l. 2005;26:249–256. doi: 10.1183/09031936.05.00140104. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S, Ushida M, Komine R, Shimodaira A, Uchida T, Ishihara H, et al. Platelet responsiveness to in vitro aspirin is independent of COX-1 and COX-2 protein levels and polymorphisms. Thrombosis Res. 2008;121:509–517. doi: 10.1016/j.thromres.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Cortes A, Maksymowych WP, Wordsworth BP, Inman RD, Danoy P, Rahman P, et al. Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204835. [DOI] [PMC free article] [PubMed] [Google Scholar]