Abstract
Objective
The risk of urinary tract infection (UTI) among women undergoing elective gynecologic surgery where a catheter is placed is high: 10 to 64% following catheter removal. We conducted the first randomized, double-blind, placebo-controlled trial of the therapeutic efficacy of cranberry juice capsules in preventing UTI post surgery.
Study Design
We recruited patients from a single hospital between August 2011 and January 2013. Eligible participants were undergoing elective gynecologic surgery that did not involve a fistula repair or vaginal mesh removal. 160 patients were randomized and received two cranberry juice capsules two times a day, equivalent to two 8-ounce servings of cranberry juice, for 6 weeks after surgery, or matching placebo. The primary endpoint was the proportion of participants who experienced clinically-diagnosed and treated UTI with or without positive urine culture. Kaplan-Meier plots and logrank tests compared the two treatment groups.
Results
The occurrence of UTI was significantly lower in the cranberry treatment group compared to the placebo group (15/80 (19%) versus 30/80 (38%); OR=0.38; 95% CI: 0.19, 0.79; p=0.008). After adjustment for known confounders, including frequency of intermittent self-catheterization in the post- operative period, the protective effects of cranberry remained (OR=0.42; 95% CI: 0.18, 0.94). There were no treatment differences in the incidence of adverse events; including gastrointestinal upset (56% vs. 61% for cranberry vs. placebo).
Conclusions
Among women undergoing elective benign gynecologic surgery involving urinary catheterization, use of cranberry extract tablets during the postoperative period reduced the rate of UTI by half.
Keywords: catheter associated urinary tract infection, cranberry extract, clinical trial
INTRODUCTION
Urinary tract infections (UTI) remain one of the most common hospital-acquired infections (1). The vast majority of hospital-associated UTI are attributed to use of a urinary catheter (2). Even following removal of the catheter, risk of UTI remains high, with postoperative patients being particularly vulnerable. Among women undergoing elective urogenital surgery the 6-week cumulative incidence of a symptomatic urinary tract infection (UTI) following catheter removal is 10 to 64% (reviewed in (3)), compared to 3–4% per year for women in the general population (4). Within hours following insertion, bacteria colonize the urinary catheter surface; the incidence of significant bacteriuria is 5% per day of catheterization regardless of gender (5). A meta-analysis of 7 studies of short-term catheterized patients (including men and women and surgeries of various types) found that antibiotics given at time of catheter removal reduced UTI incidence by ~50%, but the risk remained significant: 4.7% within 4 weeks (6). However, antibiotic prophylaxis is not an ideal solution as the prevalence of antibiotic resistance among urinary bacterial isolates is very high and continues to increase. Escherichia coli is the most common urinary pathogen; among hospitalized patients in the US and Europe, ~10% E. coli from urinary-associated bacteremia had the extended spectrum beta-lactamase phenotype, and ~26% were resistant to levofloxacin (7).
The American cranberry (Vaccinium macrocarpon) has been widely used for the prevention of urinary tract infections. Cranberry juice may prevent UTI by selecting against more adhesive strains in the stool, by directly preventing E. coli from adhering to uroepithelial cells, or by both of these mechanisms (8,9). Other effects might include influence on fimbrial subunit synthesis, assembly, or attachment; these effects would be similar to those seen with sub-inhibitory concentrations of antibiotics (10). A 2012 systematic review and meta-analysis found an overall protective effect of cranberry on UTIs, but there was considerable heterogeneity in results across trials that varied in dose administered, population study, and sample size (11).
No previous studies of cranberry have addressed effectiveness in reducing risk of UTI post catheterization. We begin to fill this gap by conducting a randomized clinical trial of the effectiveness of cranberry juice capsules in preventing UTI among women undergoing elective gynecologic surgery where a catheter is in place. This is an especially high risk group as these operations involve surgery adjacent to the bladder and delayed bladder emptying is common. Because catheter insertion and removal introduces bacteria and causes trauma that may increase UTI risk, we also take into account frequency of intermittent catheterization following removal of the Foley catheter.
MATERIALS AND METHODS
Study Design
We conducted a randomized, double-blind, placebo-controlled study of the therapeutic efficacy of two cranberry juice capsules two times a day, equivalent to two 8-ounce servings of cranberry juice, for 6 weeks after elective gynecological surgery, in preventing UTI post catheterization.
The Institutional Review Board at the University of Michigan approved the study protocol; all participants gave written informed consent. The use of cranberry capsules for this trial was approved by the Food and Drug Administration (IND 111959); ClinicalTrials.gov identifier NCT01346774.
Participants
We recruited study participants between August 2, 2011 and January 23, 2013 from patients referred by physicians from the Urogynecology and Minimally Invasive Surgery (MIS) clinics of the University of Michigan Division of Gynecology at the time of their pre-operative visit for elective gynecologic surgery. The Urogynecology clinic specializes in the surgical management of pelvic organ prolapse, urinary incontinence and anal incontinence; the MIS clinic specializes in the surgical management of fibroids, endometriosis and pelvic pain. All surgeries were performed at within the University of Michigan Hospital System and followed the hospital’s standard operating procedures.
Eligibility criteria
Eligible participants were non-pregnant women at least 18 years of age without a history of nephrolithiasis, congenital urogenital anomaly or neurogenic bladder, any known allergy to cranberry products, who did not require therapeutic anticoagulant medicine during the six weeks after surgery, or whose surgery did not involve a fistula repair or vaginal mesh removal.
Endpoints
The primary endpoint was the proportion of participants who experienced clinically-diagnosed and treated UTI whether or not results from a urine culture were available. Diagnosis and treatment were up to the treating physician. Secondary endpoints included the incidence of UTI caused by E. coli and time from randomization to UTI. Safety endpoints included adverse events and serious adverse events.
Therapeutic Regimen
TheraCran® cranberry and placebo capsules were provided by Theralogix, LLC (Rockville, MD), gratis, asking only that results be made available to them at time of publication. Based upon proanthocyanidin content, the four cranberry capsules are equivalent to two 8-ounce servings of cranberry juice.
Administration
Participants were directed to take two capsules by mouth twice each day (once in the morning and once in the evening) starting at time of discharge for 4–6 weeks, or until their return for their post-operative doctor’s visit. Participants were instructed to drink an 8 oz glass of water while taking the capsule with or without food.
Concomitant Medications, Foods and Beverages
Participants were instructed not to consume any cranberry products (including whole fruit, jellies, juices or dietary supplement capsules containing cranberry), or vitamin C supplements beyond the assigned regimen. Compliance was assessed at each follow-up contact.
Randomization
Participants were randomized at time of surgery, a median of 18.5 days following enrollment (range 0 to 146 days). As bacteriuria increases with age, we balanced treatment groups by age (< 60 v. > 60 years), based on the expected median age of our study population. Stratified randomization (1:1 Theracran® cranberry capsules:placebo) was performed using computer-generated permuted blocks, with a block size known only to the Data Coordinating Center. The Data Coordinating Center provided a randomization schedule for the supplier who printed labels that were placed on each bottle of capsules.
After participants were deemed eligible, study coordinators performed the randomization using the next available randomization number on a stratum-specific list provided by the DCC.
Masking
All study personnel (with the exception of designated individuals at the Data Coordinating Center), treating physicians, and patients were masked to treatment assignment. The Investigational Drug Service at the University of Michigan stored and managed the capsules, and conducted drug accountability.
Study Procedures and Data Collection
After giving informed consent, each participant completed a self-administered questionnaire regarding her medical and sexual history, health behaviors and symptoms. Following hospital admission, a urine specimen was collected upon catheter insertion in the operating room, and catheter removal (from the catheter port). At the time of hospital discharge, research staff provided the participant with enough capsules for 8 weeks of the assigned regimen, and administered the first dose. Participants were reminded to take the cranberry capsules daily, continuing 2×/day until their post-operative visit, usually at six weeks. Research staff also instructed participants to collect a urine sample if they experienced urinary symptoms consistent with UTI (painful urination, an urgent need to urinate, pain/pressure in lower abdomen or pubic area, fever of 100 0F or more, or flank pain), provided a urine collection kit (containing a preservative), orders for a urine culture (if needed) that included instructions to the laboratory to forward results to the treating physician at the University of Michigan Health System if the culture was performed elsewhere, and written instructions for at-home urine collection. Participants were given a symptom diary to record any urinary symptoms.
Participants were advised to contact research staff immediately and to collect a urine specimen should they experience urinary symptoms consistent with UTI. When participants contacted research staff, they were administered a brief structured interview regarding the type and duration of symptoms and referred to their physicians for diagnosis and treatment. Urine was cultured using standard microbiologic techniques for the presence of uropathogens at the clinical laboratory selected by their treating physician. Study staff contacted participants within three days, and at two and four weeks after hospital discharge to assess compliance to study protocol, to identify if the participant had urinary symptoms consistent with UTI, and to elicit any adverse events. These items were also assessed at the 6 week post-operative doctor’s visit using a self-administered questionnaire. At study exit, capsule bottles were collected so capsules could be counted to assess compliance.
Medical records of all randomized study participants were reviewed to identify any missed post-operative symptomatic UTI episodes, adverse events, and medications prescribed. All study participants received an intravenous antibiotic administration prior to the start of the surgical procedure, including urinary catheter insertion (as per hospital protocol).
There were no changes to the methods or trial outcomes after the trial began. There were no interim analyses or stopping guidelines.
The study protocol, including definitions of adverse events, is available upon request.
Statistical Methods
Sample size was based on logistical and statistical principles. Estimates of the incidence of symptomatic UTI in our patient population were unavailable and thus we assumed the UTI rate to be similar to that in the literature for patients receiving prophylactic antibiotics (15–18%). (6) We believed we could enroll approximately 200 participants (100/treatment group) and calculated that this sample size would provide at least 80% power with a two-sided type I error of 5% to detect a large treatment difference (65% to 75% relative risk reduction) if the UTI rate was 15–20% in the placebo group (based on a two-sample binomial test, performed using EAST version 5.1, Cytel, 2007). Recruitment was stopped at 160 randomized participants because of budgetary and timing constraints.
Baseline socio-demographic and medical history characteristics were summarized using descriptive statistics. Primary analyses used an intent-to-treat approach. We used logistic regression to model the incidence of the primary endpoint and taking into account all pre-specified risk factors including age, UTI history, presence of a Foley catheter, and intermittent self-catheterization. We also summarized treatment differences in time to UTI using Kaplan-Meier methods and tested these differences with logrank tests. A sensitivity analysis was performed for the primary endpoint, using a modified ITT analysis population that included only randomized participants who took at least one dose of study medication. In addition, we assessed treatment differences in culture-confirmed UTI, and culture-confirmed when E. coli was the pathogen. We tested for differences in compliance and safety using the chi square test.
All analyses were performed using SAS® software, version 9.3.
RESULTS
Study Population
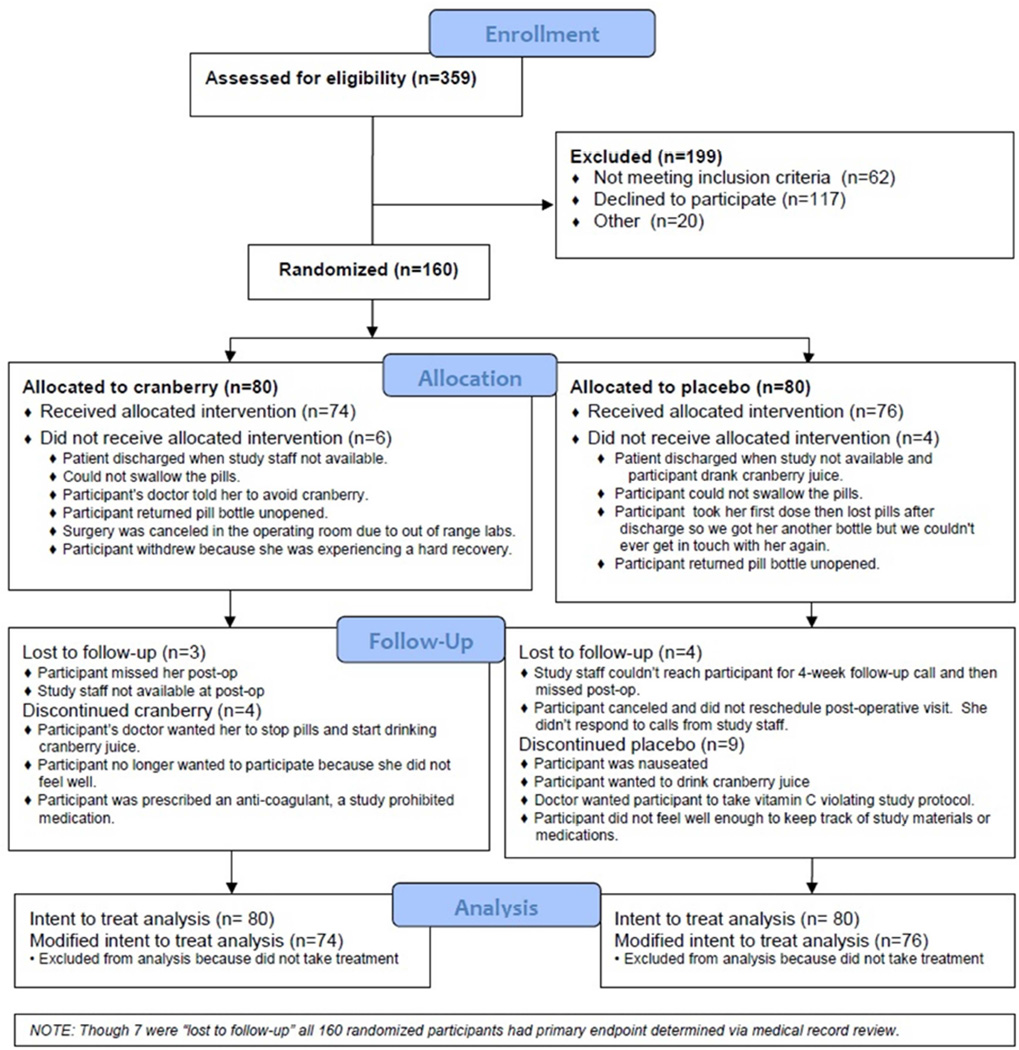
359 women presenting for elective gynecologic surgery were screened for eligibility between June 2011 and March 2013, of which 160 were randomized. Almost all (94%) received the allocated intervention (cranberry: 74/80 vs. placebo: 76/80). Reasons for not receiving the intervention included patient was discharged when staff were not available (n=2), the participant could not swallow the capsules or returned the bottle unopened (n=4), capsules were lost (n=1), participant’s physician told her to avoid cranberry (n=1), the surgery was cancelled (n=1) or the participant withdrew (n=1) (Figure 1). Follow-up was not statistically different by group: 73/80 (91%) of those allocated to cranberry and 72/80 (90%) assigned to placebo completed at least one follow-up (p=0.79); and 41/80 (51%) of those allocated to cranberry and 32/80 (40%) allocated to placebo completed all 3 follow-ups. The primary endpoint was obtained for all randomized participants via review of medical records.
Figure 1. CONSORT flow diagram.
Enrollment and exclusions, allocation to treatment, follow-up and reasons for loss.
Participants ranged in age from 23 to 88 years (mean ± SD for cranberry: 56 ±− 12.5; placebo: 56 ± 14.3). Socio-demographic characteristics and most medical history features were not statistically significantly different by treatment group (Table 1), including number of UTI in the 12 months prior to surgery (cranberry: 0.39 ± 0.88; placebo: 0.38 ± 0.80) and treating physician (data not shown). However, those assigned to cranberry were significantly less likely to have required intermittent self-catheterization during the recovery period (31% versus 50%; p=0.02), and reported a lower number of self-catheterization episodes per day (cranberry 3.7 ± 3.1 vs. placebo 5.5 + 4.9; p=0.08).. Participants who self-catheterized were more likely to have a history of UTI (72% vs. 54%; p=0.01; data not shown). Seven women used an indwelling Foley catheter at home for part of the follow-up period (cranberry: n=3, placebo: n=4). The decision to discharge a patient with intermittent self-catheterization or a Foley was left to the individual physicians. Patients discharged with a Foley usually are unable to perform catheterize or decline to do so.
Table 1.
Socio-demographic, baseline and post-operative characteristics of randomized participants by treatment group
| Cranberry N=80 |
Placebo N=80 |
|||
|---|---|---|---|---|
| Characteristic | n | (%) | n | (%) |
| Age <60 years | 47 | (59) | 48 | (60) |
| Married | 54 | (68) | 54 | (68) |
| Education (highest level completed) | ||||
| < High School | 17 | (21) | 12 | (15) |
| College (at least some) | 40 | (50) | 48 | (60) |
| Post College Education (at least some) | 23 | (29) | 20 | (25) |
| Average Annual Household Income | ||||
| ≤ $21,000 | 7 | (9) | 8 | (11) |
| $21,001 to $62,000 | 28 | (36) | 30 | (41) |
| $62,001 or more | 43 | (55) | 36 | (49) |
| Race/Ethnicity | ||||
| Non Hispanic White | 66 | (84) | 70 | (88) |
| Non Hispanic Black | 4 | (5) | 4 | (5) |
| Other or Mixed Race | 9 | (11) | 6 | (7) |
| Use of NSAIDS in 4 weeks prior to enrollment | 57 | (72) | 62 | (78) |
| Use of NSAIDS in post-operatory period | 45 | (68) | 41 | (68) |
| Ever experienced UTI in life | 49 | (64) | 50 | (63) |
| Experienced a UTI in the past 12 months | 18 | (23) | 19 | (24) |
| Post-menopausal | 54 | (69) | 54 | (68) |
| Reasons for surgerya | ||||
| Fibroids | 11 | (14) | 9 | (11) |
| Pelvic organ prolapse | 51 | (64) | 56 | (70) |
| Stress urinary incontinence | 21 | (26) | 23 | (29) |
| Chronic pelvic pain | 2 | (3) | 3 | (4) |
| Endometriosis | 0 | (0) | 2 | (3) |
| Abnormal uterine bleeding | 11 | (14) | 6 | (8) |
| Adnexal mass | 4 | (5) | 4 | (5) |
| Other | 9 | (11) | 18 | (23) |
| Surgery typea | ||||
| Urinary incontinence operation | 24 | (30) | 24 | (30) |
| Kelly plication | 2 | (3) | 1 | (1) |
| Mid-urethral–retropubic | 16 | (20) | 20 | (25) |
| Pubovaginal sling | 5 | (6) | 2 | (3) |
| Other | 1 | (1) | 1 | (1) |
| Prolapse/Reconstructive | 52 | (65) | 58 | (73) |
| Abdominal or laparoscopic sacrocolpopexy | 10 | (13) | 11 | (14) |
| Anterior (cystocele) repair | 24 | (30) | 34 | (43) |
| Colpocleisis | 2 | (3) | 2 | (3) |
| Enterocele Repair (closure of peritoneum) | 1 | (1) | 5 | (6) |
| McCalls culdoplasty | 0 | (0) | 2 | (3) |
| Posterior (rectocele) repair (colpoperineorrhaphy) | 30 | (38) | 34 | (43) |
| Sacrospinous Ligament Suspension | 19 | (24) | 18 | (23) |
| Uterosacral ligament suspension | 5 | (6) | 9 | (11) |
| Other | 1 | (1) | 4 | (5) |
| Hysterectomy/Other extirpative | 35 | (44) | 42 | (53) |
| Abdominal total hysterectomy | 0 | (0) | 1 | (1) |
| Laparoscopic supracervical hysterectomy | 9 | (11) | 6 | (8) |
| Laparoscopic total hysterectomy | 3 | (4) | 10 | (13) |
| Supracervical hysterectomy | 1 | (1) | 0 | (0) |
| Vaginal hysterectomy | 9 | (11) | 20 | (25) |
| Ureterolysis | 0 | (0) | 2 | (3) |
| Removal of tube(s) | 8 | (10) | 20 | (25) |
| Removal of one ovary | 1 | (1) | 3 | (4) |
| Removal of both ovaries | 5 | (6) | 10 | (13) |
| Exploratory laparotomy | 1 | (1) | 2 | (3) |
| Laparoscopy | 3 | (4) | 2 | (3) |
| Myomectomy (laparotomy) | 1 | (1) | 1 | (1) |
| Myomectomy (laparoscopy) | 5 | (6) | 2 | (3) |
| Lysis of adhesions | 3 | (4) | 4 | (5) |
| Resection of endometriosis | 0 | (0) | 3 | (4) |
| Other | 5 | (6) | 7 | (9) |
| Other | 4 | (5) | 4 | (5) |
| Intermittent catheterization during post-operative periodb | 25 | (31) | 40 | (50) |
| Sent home with an indwelling catheter (Foley) | 3 | (4) | 4 | (5) |
Categories not mutually exclusive
p<0.05
Percentages based on total responses. Incomplete responses were received for marital status (cranberry: n=1); income (cranberry: n=2, placebo: n=6); race (cranberry: n=1); NSAIDS use in the post-operative period (cranberry: n=14, placebo: n=20); UTI history (cranberry: n=3, placebo: n=2); and post-menopausal status (cranberry: n=2).
Association of cranberry with UTI
Overall, 45 (28%) of the 160 participants had a UTI. The incidence of UTI was significantly lower in the cranberry than the placebo group (15/80 (19%) versus 30/80 (38%); OR=0.38; 95% CI: 0.19, 0.79; p=0.008). Among participants with a UTI diagnosis, the percentage of those confirmed by culture did not differ between the women assigned placebo or cranberry (77% (23/35) vs 80% (12/15), p=0.31). The association remained significant when the endpoint was limited to those with culture confirmed diagnosis (>103 cfu/mL urine (12)) (OR=0.44, P=0.037).
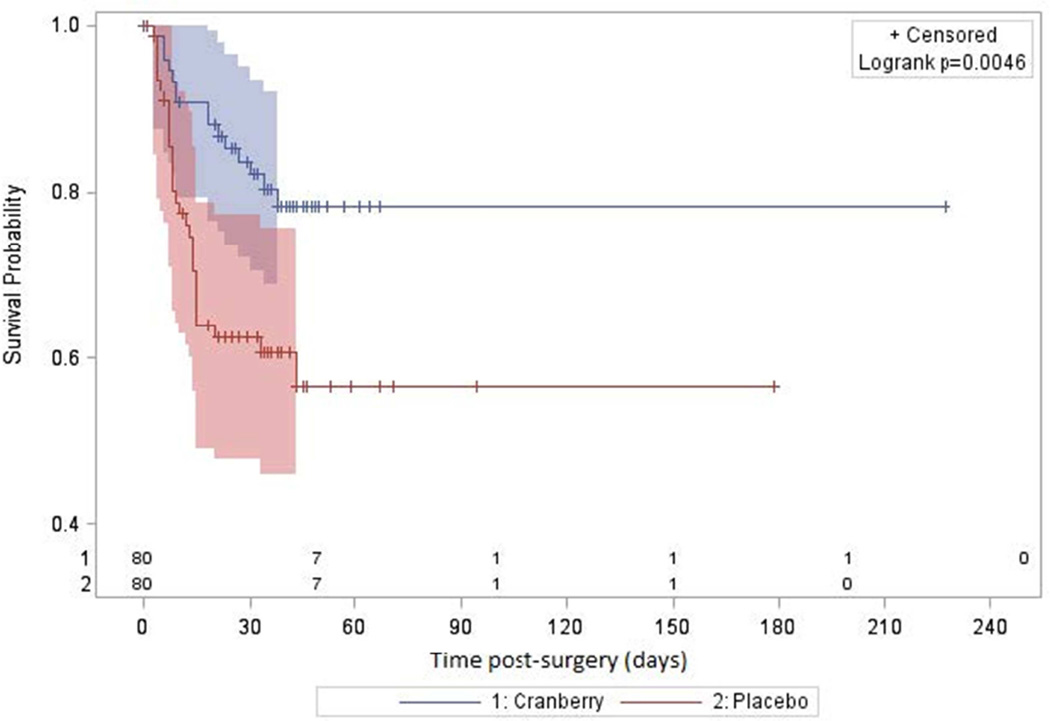
The median time to UTI was significantly longer among those in the cranberry than the placebo group (18 days versus 8.5 days, Figure 2, log rank test p=0.0005). When considering only those patients with culture-confirmed UTI, the incidence of UTI was also significantly lower in the cranberry than the placebo group (12/80 (15%) versus 23/80 (29%), OR=0.44; 0.20, 0.96; p=0.04). Results did not change in the sensitivity analysis using the modified intent to treat analysis population, which excluded the ten participants who did not take a single dose of study medication.
Figure 2. Time to urinary tract infection by treatment group.
Kaplan-Meier estimates and 95% Hall-Wellner confidence bands are provided by treatment group. The number at risk for UTI is shown on the horizontal axis.
The beneficial effect of cranberry treatment remained after adjusting for frequency of post-operative self-catheterization and having an indwelling Foley catheter at home in a logistic regression model (OR=0.42; 95% CI: 0.18, 0.94; p=0.037) (Table 2). Women who self-catheterized during the post-operative period had a significantly higher incidence of UTI (33/65 (51%) versus 12/95 (13%) (OR=7.13; 95% CI: 3.28, 15.5; p<<0.001), but there was no interaction between frequency of self-catheterization and treatment (p=0.63). Further, of the 7 participants with an indwelling catheter for part or all of the post-operative recovery period, 1/3 assigned to cranberry and 3/4 assigned to placebo had a UTI. (The one woman who had a Foley catheter throughout the entire study period developed a UTI based on endpoint criteria). The increased risk of UTI associated with discharge with an indwelling Foley catheter remained after adjusting for frequency of intermittent catheterization following Foley removal during the post-operative period and the effects of cranberry (OR=7.3; 95% CI: 1.42, 42.07; p=0.018 (Table 2)). There was no association between UTI history (both in the previous 12 months and any time during life) and UTI risk post-surgery (data not shown).
Table 2.
Effect of cranberry juice pills on risk of urinary tract infection, adjusting for having an indwelling catheter at home, and frequency of self-catheterization
| Standard | ||||
|---|---|---|---|---|
| Parameter | Estimate | Error | OR | (95% CI) |
| Cranberry juice pills | −0.8661 | 0.4152 | 0.42 | (0.18, 0.94) |
| Indwelling catheter (Foley) | 1.9876 | 0.8368 | 7.30 | (1.42, 42.07 |
| Log frequency of self-catheterizationa | 0.6299 | 0.1244 | 1.88 | (1.48, 2.42) |
| Intercept | −1.5553 | 0.3344 | -- | -- |
Variable was log transformed because highly skewed. Odds ratio is interpreted as an 81% increase in UTI risk for each 10 times a woman self-catheterized.
Escherichia coli was the most common infecting organism (cranberry: 38%; placebo: 46% (Table 3)). Considering only those with a UTI caused by E. coli, the risk of UTI among those taking cranberry was reduced by an estimated 62% (OR=0.38; 95% CI: 0.13, 1.13; p=0.07). The second most common infecting bacterial species was Klebsiella pneumoniae among the cranberry group (23%) and Enterococcus among the placebo group (23%). The vast majority of cultures had ≥105 cfu/mL urine of the infecting bacteria (92% for cranberry and 81% for placebo) and all urine cultures had at least one species occurring at >104 cfu/mL urine; four positive cultures reported two bacterial species).
Table 3.
Distribution of micro-organisms causing urinary tract infection by treatment groupa
| Cranberry (n=12) | Placebo (n=23) | |||
|---|---|---|---|---|
| Organism | n | (%) | n | (%) |
| Escherichia coli | 5 | (38) | 12 | (46) |
| Klebsiella pneumoniae | 3 | (23) | 2 | (8) |
| Enterococcus species | 1 | (8) | 6 | (23) |
| Enterobacter species | 2 | (15) | 2 | (8) |
| Streptococcus aglactiae | 1 | (8) | 1 | (4) |
| Proteus mirabilis | 1 | (8) | 0 | |
| Lactobacillus | 0 | 1 | (4) | |
| Yeast | 0 | 1 | (4) | |
| Mixed | 0 | 1 | (4) | |
| Total species detected | 13 | (100) | 26 | (101) |
Of the 45 participants who had a primary outcome (UTI) culture results were not available for 10 participants (cranberry: n=3, placebo: n=7) with clinically diagnosed and treated urinary tract infection. Numbers may not sum to totals, as more than one species was present in 4 cultures (cranberry: n=1, placebo: n=3). Greater than 104 cfu/mL urine of a potential uropathogen were detected in all positive cultures. In the culture positive for yeast, there were 105 cfu/mL urine; the culture positive for Lactobacillus (103 cfu/mL urine) also had 105 cfu/mL urine of E. coli. There were 105 cfu/mL urine reported for the ‘mixed’ culture.
Compliance
The average amount of time from a participant’s surgery, when they started study drug, to their termination from the study was 38 days (SD: 24, Range: 0–227). At study exit, of the 130 participants with information available, 110 (85%) reported taking two capsules twice a day, most or all of the time. Most participants reported following the prescribed regimen at the 2-week (89%) and 4-week (82%) follow-up contacts. Pill counts were available for a similar number of participants by treatment assignment (cranberry: n= 57, placebo: n=60); participants averaged three capsules per day in both treatment groups. Compliance was not statistically significantly different by treatment group using any measure of compliance.
Adverse effects
The proportion of participants with AEs and SAEs did not differ by treatment groups (p=0.28, p=1.00). Seventy-five participants experienced 328 adverse events in the cranberry group, and 78 participants experienced 423 in the placebo group. Four participants experienced 5 severe adverse events in the cranberry group and 4 participants experience 7 adverse events in the placebo group. Gastrointestinal upset was the most commonly reported event in both groups (cranberry: n=45 (56%); placebo: 49 (61%)).
COMMENT
This is the first report of a double-blind, placebo-controlled randomized clinical trial demonstrating a statistically and clinically significant benefit of taking cranberry in preventing UTI after elective gynecologic surgery where a urinary catheter is placed. This reduction in UTI risk is similar in magnitude to that reportedly obtained by administering antibiotics at time of catheter removal (6), and avoids the collateral damage associated with antibiotic use, including pressure for antibiotic resistance.
Our study addresses the major limitations identified in previous studies of the effects of cranberry products (13). We compared cranberry capsules to placebo; sub-analyses of previous trials suggest capsules are somewhat more effective than juice, perhaps because of increased compliance (11). In our previous cranberry trial conducted among college students where we used juice, the placebo juice contained similar levels of vitamin C to that found in cranberry juice. We observed no effect of cranberry in that trial, perhaps because vitamin C has been observed to have a beneficial effect on UTI risk (14).
Several strengths in the study design and analysis of this trial make it unlikely that the difference in outcome was due to (alpha) error. The design, conduct and analysis of the trials, including randomization scheme, blinding of study personnel and participants, and active surveillance for outcomes were held to the highest standards. Furthermore, after analyses taking into account known risk factors - need for intermittent self-catheterization, UTI history and age - UTI history age - the findings remained consistent with cranberry mitigating the risk for UTI. This suggests that our results might be generalized to women undergoing laparoscopic or vaginal surgery for benign gynecologic procedures where a catheter is placed.
Developing practices to reduce UTI risk is a promising area for quality improvement. Considering the use of intermittent catheterization is a potential example. Each time a catheter is inserted, the bladder is inoculated with bacteria, and there is some subtle trauma, increasing risk of infection. Amongst the 65 women that reported intermittent catheterization, the median number of times was 15 (mean of 32; range 1 to 216 times) during the postoperative period. Developing patient education to reduce the daily number of times that intermittent catheterization is undertaken might reduce UTI risk. Moreover, our results suggest that discharging a patient from the hospital with an indwelling catheter in place should be avoided if possible. These women experienced a seven-fold increase in UTI risk after adjusting for cranberry use and frequency of intermittent catheterization.
Future studies might aim to optimize the time of catheter insertion and removal, and to develop strategies to reduce the frequency of intermittent catheterization. At least one previous trial compared different timings of urinary catheter removal following abdominal hysterectomy. The risks of postoperative urinary retention and over-distension need to be weighed against UTI risk. Removal immediately following surgery was associated with increased urinary retention, and removal after 24 hours with increased risk of UTI; removal at 6 hours resulted in the fewest adverse events (15). Strategies to decrease the length of time associated with catheterization may need to be carefully considered in each case but are promising areas to decrease this morbidity.
Acknowledgments
The authors thank Dr. Jack Sobel for serving as data and safety monitor; staff at Statistical Analysis of Biomedical and Educational Research unit (SABER) for technical assistance; and the participating patients, physicians, physician assistants and nurses at the Urogynecology and Minimally Invasive Gynecologic Surgery clinics and University of Michigan Hospitals; and Alexandra Beach, Erin Case and Marian Turner for outstanding work as recruiters. We are very grateful to Bill Reisdorph of the Michigan Institute for Clinical & Health Research (MICHR) (2UL1TR000433-06) for his assistance with obtaining and the reporting requirements for an investigational new drug designation, and MICHR for clinical monitoring. We also thank Theralogix, LLC (Rockville, MD) for providing cranberry juice capsules and placebo for use in this study.
This work was supported by the National Institutes of Health (R21-DK-085290). MBB received investigator support through the University of Michigan BIRCWH Career Development Program (K12-HD-001438).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
REFERENCES
- 1.Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, Boland B, et al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol. 2012 Mar;33(3):283–291. doi: 10.1086/664048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010 Mar 1;50(5):625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Athanasiou S, Iavazzo C, Tokas T, Antsaklis A. Urinary tract infections after pelvic floor gynecological surgery: prevalence and effect of antimicrobial prophylaxis. A systematic review. Int Urogynecol J Pelvic Floor Dysfunct. 2008 Aug;19(8):1165–1172. doi: 10.1007/s00192-008-0584-0. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B. Urinary Tract Infection Syndromes: Occurrence, Recurrence, Bacteriology, Risk Factors, and Disease Burden. Infect Dis Clin North Am. 2014 Mar;28(1):1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7(2):342–347. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marschall J, Carpenter CR, Fowler S, Trautner BW. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013 Jan;346:f3147. doi: 10.1136/bmj.f3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sader HS, Flamm RK, Jones RN. Frequency of occurrence and antimicrobial susceptibility of Gram-negative bacteremia isolates in patients with urinary tract infection: results from United States and European hospitals (2009–2011) J Chemother. 2014 Jun;26(3):133–138. doi: 10.1179/1973947813Y.0000000121. [DOI] [PubMed] [Google Scholar]
- 8.Sobota AE. Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. J Urol. 1984 May;131(5):1013–1016. doi: 10.1016/s0022-5347(17)50751-x. [DOI] [PubMed] [Google Scholar]
- 9.Ofek I, Goldhar J, Zafriri D, Lis H, Adar R, Sharon N. Anti-Escherichia coli adhesin activity of cranberry and blueberry juices. N Engl J Med. 1991 May 30;324(22):1599. doi: 10.1056/NEJM199105303242214. [DOI] [PubMed] [Google Scholar]
- 10.Lee YL, Owens J, Thrupp L, Cesario TC. Does cranberry juice have antibacterial activity? JAMA. 2000 Apr 5;283(13):1691. doi: 10.1001/jama.283.13.1691. [DOI] [PubMed] [Google Scholar]
- 11.Wang C-H, Fang C-C, Chen N-C, Liu SS-H, Yu P-H, Wu T-Y, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012 Jul 9;172(13):988–996. doi: 10.1001/archinternmed.2012.3004. [DOI] [PubMed] [Google Scholar]
- 12.Rubin RH, Shapiro ED, Andriole VT, Davis RJ, Stamm WE. Evaluation of new anti-infective drugs for the treatment of urinary tract infection. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992 Nov;15(Suppl 1):S216–S227. doi: 10.1093/clind/15.supplement_1.s216. [DOI] [PubMed] [Google Scholar]
- 13.Jepson R, Craig J, Williams G. Cranberry products and prevention of urinary tract infections. JAMA. 2013 Oct 2;310(13):1395–1396. doi: 10.1001/jama.2013.277509. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011 Jan 1;52(1):23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed MR, Sayed Ahmed WA, Atwa KA, Metwally L. Timing of urinary catheter removal after uncomplicated total abdominal hysterectomy: a prospective randomized trial. Eur J Obstet Gynecol Reprod Biol. 2014 May;176:60–63. doi: 10.1016/j.ejogrb.2014.02.038. [DOI] [PubMed] [Google Scholar]