Abstract
Background and Purpose
The relationship between carotid artery intima media thickness (IMT) and cognitive function in midlife remains relatively unexplored. We examined the association between IMT and cognitive function in a middle-aged epidemiologic cohort of 2,747 stroke-free participants.
Methods
At the Year 20 visit (our study baseline), participants from the Coronary Artery Risk Development in Young Adults study had IMT measured by ultrasound at the common carotid artery. Five years later, participants completed a cognitive battery consisting of the Rey Auditory-Verbal Learning Test of verbal memory, the Digit Symbol Substitution Test of processing speed, and the Stroop test of executive function. We transformed cognitive scores into standardized z-scores, with negative values indicating worse performance.
Results
Mean age at baseline was 45.3 years (SD=3.6). Greater IMT (per 1SD difference of 0.12mm) was significantly associated with worse performance on all cognitive tests (z-scores) in unadjusted linear regression models (Verbal Memory=−0.16, 95%CI=−0.20 to −0.13; Processing Speed=−0.23, 95%CI=−0.27 to −0.19; and Executive Function=−0.17, 95%CI= −0.20 to −0.13). In models adjusted for socio-demographics and vascular risk factors that lie earlier in the causal pathway, greater IMT remained negatively associated with processing speed (−0.06,95%CI=−0.09 to −0.02; p=0.003) and borderline associated with executive function (−0.03, 95%CI=−0.07 to 0.00; p=0.07) but not with verbal memory.
Conclusions
We observed an association between greater IMT and worse processing speed – a key component of cognitive functioning- at middle-age above and beyond traditional vascular risk factors. Efforts targeted at preventing early stages of atherosclerosis may modify the course of cognitive aging.
Keywords: Cognition, Epidemiology, Intima Media Thickness, Risk Factors, Stroke-Free
INTRODUCTION
Carotid artery intima media thickness (IMT), a measure of subclinical vascular disease, has been associated with structural brain changes1-3 and reduced cognitive function.3-11 However, the majority of prior work on IMT and cognitive function has focused on samples with older adults and results have been inconsistent.3-11 While it is becoming increasingly clear that cognitive impairment and dementia have a long preclinical period and that vascular risk factors are key determinants of cognitive function,12-21 evidence of whether there is an association between IMT and cognitive function earlier in the life course is yet to be determined.
Possible mechanisms of an association between atherosclerosis of the carotid artery and cognitive function include subclinical cerebral infarctions and associated structural brain changes,1,2,22 stroke and other cerebrovascular diseases23,24 which are in turn associated with reduced cognitive function.3,12,25 Therefore, studying the association between carotid artery IMT and cognitive function in subjects without clinically evident cerebrovascular disease (stroke) has important implications for early intervention.
We studied participants enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Our goal was to investigate the association between carotid artery IMT and cognitive function in a cohort of middle-aged adults who were stroke-free. We hypothesized that greater IMT is associated with worse cognitive performance, above and beyond traditional vascular risk factors.
METHODS
Study population
The CARDIA study is an ongoing prospective cohort study in which participants were aged 18 to 30 years old at baseline in 1985-86. A detailed description of the CARDIA study design and recruitment has been described elsewhere.26 In brief, participants were recruited from 4 field centers: : the University of Alabama at Birmingham (Birmingham, AL), the University of Minnesota (Minneapolis, MN), Northwestern University (Chicago, IL), and Kaiser Permanente (Oakland, CA). Recruitment was conducted by telephone or door-to-door in areas where telephone recruitment was not possible. Those recruited were then screened for eligibility, and then invited to participate in the study. All 4 filed centers followed the same eligibility criteria regarding age, race, residence in the target areas, and health and medical status (participants had to be free of long-term disease or disability). A total of 5,115 black and white adult participants were recruited from the 4 field centers and recruitment was balanced within center by sex, age and education and included 52% blacks and 48% whites. Participants were asked to participate at baseline and at follow-up examinations spanning 25 years. In the present analysis we focus on carotid artery IMT which we measured at year 20 and on cognitive function which we assessed five years later. We obtained appropriate informed consent from all study participants, and the study was approved by the institutional review boards from each field center and the coordinating center. The present analysis was also approved by the Publications & Presentations committee of the CARDIA study.
Of the 3,498 subjects who participated in the study examination at year 25 (72% of the surviving cohort), we excluded 113 subjects with missing cognitive test battery, 582 participants with missing carotid ultrasound, 8 subjects with prevalent adjudicated stroke, and 177 subjects with incomplete risk factor data. Our analytical sample included a total of 2,618 participants.
Carotid Artery IMT
At year 20, we performed ultrasound studies for all CARDIA participants at end diastole according to a standard protocol followed across all four field centers. Sonographers who performed the studies were certified and trained centrally at an ultrasound reading center. We used GE-Logiq-700 (Issaquoah) at all filed centers and used a high-resolution transducer operating at a frequency of 13 MHz to image the common carotid artery (CCA) in a real-time imaging sequence. The images were then manually read and analyzed at the central ultrasound reading center and the readers were blinded to the participant’s characteristics. Further details can be found in the carotid ultrasound manual.27 Our predictor of interest is IMT of the CCA. We defined maximum IMT as the mean, in mm, of the maximal IMT of the near and far wall on both the left and right sides. Based on 58 replicate studies, the Pearson correlation coefficients were 0.86 for the CCA IMT.
At year 20, we also assessed stenosis according to visual examination separately for the right and left carotid artery. We defined the presence of stenosis as the development of sufficient atherosclerotic plaque of any type resulting in any narrowing of the lumen at any arterial segment (almost all are 1% to 25% narrowing). We used the measure of stenosis in a sensitivity analysis which we describe later.
Cognitive Function
At year 25, we administered to all CARDIA participants a cognitive battery that included 3 cognitive tests in an attempt to characterize cognitive performance at midlife. The delayed verbal memory test on the Rey Auditory-Verbal Learning Test (RAVLT, range 0 to 15) assesses the ability to memorize and retrieve words, with higher score (in words) indicating better performance.28 The Digit Symbol Substitution Test (DSST, range 0 to 133) is a subtest of the Wechsler Adult Intelligence Scale and measures performance on speed domains, with higher score (in symbols) indicating better performance.29 The interference score on the Stroop (executive skills) measures the additional amount of processing needed to respond to one stimulus while suppressing another, with higher score (in seconds+errors) indicating worse executive performance.30 For ease of interpretation, we transformed all cognitive test scores into standardized z scores, with positive values indicating better performance and negative values indicating worse performance.
Other Covariates
CARDIA participants reported their years of education completed which we categorized into completed high school (HS) or less vs. more than HS. We classified cigarette smoking as never smoked, former smoker, and current smoker, and we calculated daily alcohol use in mL based on an interviewer-administered questionnaire. Participants reported the amount of time per week spent in 13 categories of physical activity over the past year, and then we calculated the total amount in exercise units which we then categorized into tertiles of low, moderate and high intensity. We measured body weight using a calibrated scale and measured height with a vertical ruler. We calculated body mass index (BMI) as weight in kilograms divided by height in meters squared (kg/meters2). We measured blood pressure while seated using a standard automated blood pressure measurement monitor (OmROn model HEM907XL; Omron). We ascertained hypertension based on systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications. We ascertained type 2 diabetes based on fasting glucose levels ≥126 mg/dl, self-report of oral hypoglycemic medications or insulin, a 2-hour postload glucose ≥200 mg/dl, or a glycated hemoglobin A1c ≥6.5%. We measured plasma total cholesterol concentrations enzymatically at Northwest Lipids Research Laboratory (Seattle, WA). We assessed kidney function using eGFR (estimated Glomerular Filtration Rate) cystatin C using the equation eGFRcys = 76.7x cystatin C−1.19.31 We assessed symptoms of depression using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D; range 0 to 60)32 and then classified participants as having elevated depressive symptoms when CES-D score was greater or equal to 16.
Statistical analysis
We compared mean IMT across categories of baseline characteristics using analysis of variance and estimated the increase of average IMT associated with 1 standard deviation (SD) difference for continuous variables. We used multivariable linear regression models to examine the associations between IMT and performance on each of the three cognitive tests. We modeled IMT as continuous, and we presented the results for 1 standard deviation increase in IMT. In model assessment, we checked for modification of the effects of IMT by sex, and race/ethnicity, using interaction terms. No sex-based or racial/ethnic-based differences were present. We presented the unadjusted model then adjusted for age, sex, race and education as potential confounders, then additionally adjusted for factors that lie earlier in the causal pathway including smoking, physical activity, depressive symptoms, body mass index, type-2 diabetes, hypertension, antihypertension medication use, and kidney function. We included covariates in the adjusted models based on their associations with year 25 cognitive function and year 20 common carotid artery IMT. We assessed potential confounders at year 20. We illustrated the association between IMT and performance on the three cognitive tests by presenting the average predicted cognitive scores (z scores) at two specific values of IMT, from fitted linear regression models.
We performed two sensitivity analyses. First, we examined the associations between IMT at other regions of the carotid artery (the internal carotid artery (ICA) and bulb/ICA) and cognitive function. Second, we examined the associations between IMT and cognitive function after excluding subjects with subclinical disease, i.e. visible stenosis (n=458). All analyses were performed using Stata version 12.
RESULTS
Compared with included participants, excluded CARDIA participants at year 25 had larger percentages of black individuals (57% vs. 44%), larger percentages of individuals with less than high school education (35% vs. 20%), and larger percentages of individuals with baseline elevated depressive symptoms (22% vs. 16%). Excluded individuals had slightly higher IMT than included individuals (mean [SD], 0.81 [0.01] vs. 0.80 [0.002]). Other baseline demographic, behavioral and cardiometabolic risk factors did not significantly differ between included and excluded CARDIA participants.
In Table 1, we present the baseline characteristics of our study sample. The mean age of our participants at baseline was 45.3 years old (SD=3.6). A total of 37.5% of the participants were obese (BMI ≥30 kg/m2), 36.4% had hypertension, and 13.6% had type-2 diabetes. Participants had a mean IMT of 0.80 mm (SD=0.12, range 0.5mm to 1.8mm, Figure 1). Year 25 mean cognitive scores were 8.5 (SD=3.2) for verbal memory, 70.9 (SD=15.8) for processing speed, and 22.1 (SD=10.6) for executive function (Table 2). Results in Table 3 show that being male, black, less educated, a smoker and having co-morbidities such as a BMI ≥ 30kg/m2, hypertension and type-2 diabetes were associated with having greater IMT.
Table 1.
Distribution of sample characteristics available to this analysis (n=2,618)
| Characteristic | |
|---|---|
| Socio-demographics | |
| Age (years), mean (SD) | 45.3 (3.6) |
| Male, n (%) | 1,125 (42.9) |
| Blacks, n (%) | 1,138 (43.5) |
| ≤ High School, n (%) | 533 (20.4) |
| Traditional risk factors for cardiovascular disease | |
| Cigarette smoking, n (%) | |
| Non-smoker | 1,639 (62.6) |
| Former smoker | 525 (20.1) |
| Current smoker | 454 (17.3) |
| Physical activity (exercise units), mean (SD) | |
| Low PA (Tertile 1) | 82.7 (52.1) |
| Moderate PA (Tertile 2) | 281.8 (63.9) |
| High PA (Tertile 3) | 650.6 (220.2) |
| Body Mass Index ≥30 Kg/m2, n (%) | 971 (37.1) |
| Hypertension, n (%) | 950 (36.3) |
| Type-2 diabetes, n (%) | 355 (13.6) |
| Elevated depressive symptoms, n (%) | 423 (16.2) |
| eGFRcys (per 18 ml/min/1.73 m2), mean (SD) | 100.8 (18.4) |
| Anti-hypertensive medication use, n (%) | 425 (16.2) |
| Ultrasound measures | |
| CCA-IMT (mm), mean (SD) | 0.80 (0.12) |
eGFRcys, cystatin C–based estimated glomerular filtration rate
Figure 1.
Distribution of Common Carotid IMT in the analytical sample (Range: 0.5-1.8 mm).
Table 2.
Cognitive Test Scores at Year 25
| N | Mean (SD) | Range | |
|---|---|---|---|
| Verbal Memory (Rey Auditory Verbal Learning Test) |
2601 | 8.5 (3.2) | 0 – 15 |
| Processing Speed (Digit Symbol SubstitutionTest) |
2606 | 70.9 (15.8) | 8 – 119 |
| Executive Function (Stroop Interference) |
2598 | 22.1 (10.6) | −46 – 127 |
Table 3.
Bivariate associations between variables and common carotid artery (CCA) IMT
| CCA IMT Range (0.5mm; 1.8mm) |
||
|---|---|---|
|
Bivariate associations for
dichotomous variables |
Respective mean values of
CCA-IMT (mm) |
P-value |
| Sex (male/female) | 0.82/0.77 | <0.01 |
| Race (black/white) | 0.83/0.77 | <0.01 |
| Education (≤HS/ >HS) | 0.82/0.79 | <0.01 |
| Current smoker (Y/N) | 0.82/0.79 | <0.01 |
| Low physical activity (Y/N) | 0.80/0.79 | 0.05 |
| Body Mass Index ≥30 Kg/m2 | 0.83/0.77 | <0.01 |
| Hypertension (Y/N) | 0.83/0.77 | <0.01 |
| Diabetes (Y/N) | 0.84/0.79 | <0.01 |
| Elevated depressive symptoms (Y/N) | 0.80/0.79 | 0.20 |
| Anti-hypertensive medication use (Y/N) | 0.85/0.78 | <0.01 |
|
Bivariate associations for continuous
variables |
Increase in average CCA-
IMT (SE)* |
P-value |
| Age (per 4 years) | 0.03 (0.002) | <0.01 |
| eGFRcys (per 18 ml/min/1.73 m2) | −0.02 (0.002) | <0.01 |
eGFRcys, cystatin C–based estimated glomerular filtration rate
In Table 4, we present the results of linear regression models of the associations between IMT and performance on the three cognitive tests. In unadjusted models, greater IMT (per 1 SD difference of 0.12 mm) was significantly associated with worse performance on all 3 cognitive tests (z scores) (Verbal Memory=−0.17, 95%CI=−0.20 to −0.13; Processing Speed=−0.23, 95%CI=−0.27 to −0.19; and Executive Function=−0.17, 95%CI= −0.20 to −0.13). In models adjusted for potential confounders, including age, sex, race, and education, greater IMT remained negatively associated with processing speed and executive function (Processing Speed=−0.08, 95%CI=−0.11 to −0.04; and Executive Function=−0.05, 95%CI= −0.10 to −0.02) but not with verbal memory. In other words, with every one SD increase in IMT, subjects significantly scored 1.2 points (symbols) lower on the test of processing speed and about 0.5 seconds+errors more to respond to the executive function task. Additional adjustment for behavioral risk factors, including smoking, physical activity, and elevated depressive symptoms, attenuated the associations but greater IMT remained significantly associated with processing speed and executive function (Processing Speed=−0.07, 95%CI=−0.10 to −0.03; Executive function=−0.05, 95%CI=−0.09 to −0.01). Finally, additional adjustment for cardiometabolic risk factors that lie earlier in the causal pathway further attenuated the associations, but greater IMT remained negatively associated with processing speed and borderline associated with executive function (Processing Speed=−0.06, 95%CI=−0.09 to −0.02; and Executive Function=−0.03, 95%CI= −0.07 to 0.00) and not with verbal memory.
Table 4.
Adjusted association of 1 standard deviation increase in common carotid artery IMT and cognitive function Among CARDIA Participants
| Standardized Difference in Mean Score (95%CI) |
|||
|---|---|---|---|
| Verbal Memory | Processing Speed | Executive Function | |
| Unadjusted | −0.16 (−0.20; −0.13)† | −0.23 (−0.27; −0.19)† | −0.17 (−0.20; −0.13)† |
| + Sociodemographic factors | −0.02 (−0.06; 0.02) | −0.08 (−0.11; −0.04)† | −0.05 (−0.10; −0.02)‡ |
| + Behavioral risk factors | −0.02 (−0.06; 0.02) | −0.07 (−0.10; −0.03)† | −0.05 (−0.09; −0.01)‡ |
| + Cardiometabolic risk factors | −0.02 (−0.05; 0.02) | −0.06 (−0.09; −0.02)‡ | −0.03 (−0.07; 0.00)§ |
Sociodemographic factors include age, sex, race, and education; Behavioral risk factors include smoking, physical activity, and elevated depressive symptoms; and Cardiometabolic risk factors include Body Mass Index, type-2 diabetes, hypertension, cystatin C–based estimated glomerular filtration rate (eGFRcys), and antihypertensive medication use.
1SD common carotid IMT= 0.124mm.
P<0.001
P<0.01
P<0.10
We present the average predicted cognitive z-scores [raw scores] on all three cognitive tests at select values of IMT (IMT of 0.68 corresponding to mean IMT − 1SD; and IMT of 0.92 corresponding to mean IMT + 1SD) from fitted linear regression models adjusted for age, sex, race and education (Figure 2). For a black male who completed high school education or less and with mean value of age, the average predicted score on verbal memory was −0.82 (95%CI= −0.93 to −0.72) [or 5.6 points; 95%CI=5.3 to 5.9] at an IMT of 1SD below the mean and was −0.87 (95%CI=−0.96 to −0.78) [or 5.5 points; 95%CI=5.2 to 5.8] at an IMT of 1SD above the mean (P=0.2). The average predicted score on processing speed was −0.90 (95%CI=−1.01 to −0.80) [or 55.3 points; 95%CI=53.6 to 57.0] at an IMT of 1SD below the mean and was −1.05 (95%CI= −1.14 to −0.97) [or 52.9 points; 95%CI=51.5 to 54.3] at an IMT of 1SD above the mean (P<0.001). And the average predicted score on executive function was −0.59 (95%CI=−0.70 to −0.49) [or 29.3 points; 95%CI=28.1 to 30.4] at an IMT of 1SD below the mean and was −0.70 (95%CI= −0.79 to −0.61) [or 30.4 points; 95%CI=29.4 to 31.4] at an IMT of 1SD above the mean (P<0.01).
Figure 2.
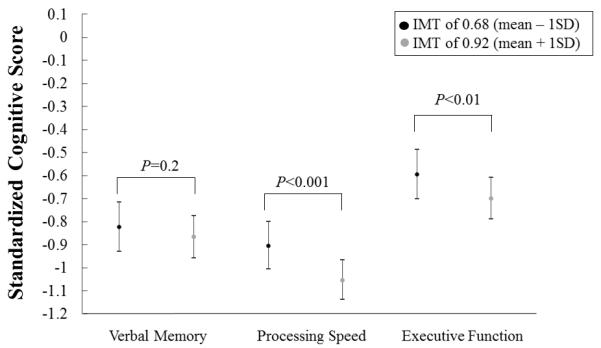
Average predicted* standardized cognitive scores at each of the following IMT levels (mean +/− 1SD), from age, sex, race and education-adjusted linear regression models. *Predicted for a black male who completed high school education or less, and with mean value of age (45 years old).
In sensitivity analysis, we did not find significant associations between IMT at other regions of the carotid artery (bulb/ICA and ICA) and cognitive function. In another sensitivity analysis where we excluded subjects with subclinical disease (i.e. visible stenosis, n=458), the association between IMT and processing speed remained significant (P<0.01).
DISCUSSION
This is the first study to examine the association between IMT and cognitive function in a cohort of middle-aged adults who were free of stroke. In models adjusted for potential confounders, including age, race and sex, we found that greater IMT was significantly associated with reduced cognitive performance on domains of processing speed and executive function which are key components of cognitive functioning. These associations, especially IMT with processing speed, were independent of sociodemographic and traditional vascular risk factors, therefore suggesting that IMT may provide additional information in predicting cognitive function independent of these factors. Finally, given our relatively young cohort, our findings underscore the importance of early identification and intervention to delay or prevent atherosclerosis before its clinical manifestation as a way to possibly improve cognitive function.
Our results are supported by several studies including primarily older adults. For example, our results were consistent with the findings from the Beaver Dam study7 which found a significant association between carotid IMT and executive function as measured by the Trail Making B test, from regression models adjusted for socio-demographic and behavioral risk factors. Our results were also consistent with the findings from several studies showing a null association between IMT and verbal memory.3-7 For example, in the Framingham Offspring Study of stroke-free subjects,3 higher CCA-IMT was not associated with performance on verbal memory tests such as the immediate and delayed verbal recall tests. Similarly, in the Tromso Study of stroke-free subjects from Norway,6 CCA-IMT was not associated with verbal memory. Finally, our results showed an association between IMT and processing speed – this finding was however inconsistent with the findings from previous studies including primarily older adults such as the ARIC study (Atherosclerosis Risk in Communities study),4 the Tromso Study of stroke-free subjects from Norway,6 and the Beaver Dam study.7 The latter studies used a measure of IMT averaged across all regions of the carotid artery which may have obscured potential significant associations at individual regions.
Our results show a significant association between IMT and processing speed but not verbal memory. This domain-specific association between IMT and cognitive function is not surprising as vascular risk factors influence to a greater extent performance on processing speed, and are not necessarily related to memory.9 Our findings were independent of sociodemographic and vascular risk factors of cognitive function, such as smoking, physical activity, BMI, depressive symptoms, type-2 diabetes, hypertension, and kidney function thus suggesting that IMT may predict cognitive function, processing speed in particular, in middle-aged adults independent of these risk factors that lie earlier in the causal pathway. While the association between IMT and verbal memory was not statistically significant even in socio-demographic adjusted models, the association between IMT and executive function was strongly significant and became borderline significant only after adjustment for cardiometabolic risk factors, kidney function in particular. In our study, kidney function was strongly associated with both IMT and cognitive function. Future studies need to address the role of kidney function in the associations between subclinical cardiovascular disease and cognitive function.
Our results show significant associations between IMT of the common carotid artery and cognitive function, but not with other regions of the carotid artery. This is not surprising as compared to the IMT of other regions, IMT of the common carotid artery is suggested to be more strongly associated with blood pressure and stroke,33 which are in turn important risk factors for reduced cognitive function and dementia,12,25,34 and less strongly associated with lipids. In other words, our findings suggest that the relationship between IMT and cognitive function is driven by hypertensive-like changes. While hypertension is a manifestation of loss of vascular integrity and a precursor for atherosclerosis, the latter has been shown to be present even in the early development of hypertension when blood pressure is slightly elevated.35 In our study, a total of 6.6% of the participants had non-optimal SBP (≥140 mmHg) and nearly 8% of the participants had non-optimal DBP (≥90 mmHg). When we adjusted for blood pressure instead of hypertension, the associations were only slightly attenuated.
Our findings are based on a cohort of middle-aged adults who were stroke-free at baseline, and remained relatively similar after excluding subjects with subclinical disease (i.e. obvious plaque deposition). As such, our findings provide support for an early cardiovascular prevention and suggest that IMT developing in an earlier phase of the atherosclerotic process may provide a window of time during which appropriate interventions may delay or prevent cognitive deterioration. This is important as there exist potential therapies, such as statins, that have been shown to slow down the process of atherosclerosis in its subclinical stages.36,37
Our work has major strengths that are worth noting. While previous studies included mostly older adults and thus had an average mean age of 60 years and above, our study was the first to examine the association between IMT and cognitive function strictly at midlife (mean age of 45 years) and thus sheds light on potential mechanisms occurring earlier in the life course. Furthermore, while the effect sizes are relatively small, they are clinically meaningful in light of the relatively younger cohort. The strengths of the associations, especially for processing speed, and statistical significance which persists beyond traditional cognitive risk factors that lie earlier in the causal pathway, is particularly notable considering mean age in our cohort was around 45 years old. Furthermore, while prior work4,6,7 focused primarily on an overall measure of IMT averaged across all regions of the carotid artery, we examined separately IMT at the different regions of the carotid artery. Previous studies3-8 were either based on international cohorts or on US samples of relatively small size, however our findings were derived from a large US population-based cohort (N=2,618) which gave us enough power to detect clinically significant associations. Finally, our study included validated measures of cognitive performance across several domains thus providing rich findings. However, we did not have measures of cognitive function at baseline when IMT was assessed; as such, we could not assess change in function. Furthermore, given that cognitive function was not assessed until the last study visit, our sample size was smaller than the original cohort. Finally, our findings are based on an observational study and there may be residual confounding.
SUMMARY/CONCLUSIONS
In conclusion, our study supports a role for early-life IMT development in influencing key components of cognitive function at midlife, including processing speed, independent of traditional vascular risk factors. Future studies are needed to examine whether early atherosclerotic development is associated with longitudinal cognitive change and whether intervening on the atherosclerotic process may indeed prevent or delay cognitive deterioration.
Acknowledgments
Sources of Funding
Dr. Zeki Al Hazzouri was supported by a grant from the National Institutes of Health, National Institute on Aging (K01AG047273). Dr. Yaffe was supported by a grant from the NIH/NIA (k24AG031155). The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content.
Footnotes
Disclosures:
Dr. Yaffe has received funding for this work from the National Heart, Lung, and Blood Institute (NHLBI) and has served on data safety monitoring boards for a trial funded by the National Institute on Aging and a trial funded by Takeda Inc, and has served on an Advisory Board for the Alzheimer’s Association and the NIH Beeson Award. Dr. Sidney is employed at Kaiser (The Permanente Medical Group- TPMG) and has received funding for this work from NHLBI. The other authors report no conflicts.
References
- 1.Bots ML, van Swieten JC, Breteler MM, de Jong PT, van Gijn J, Hofman A, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet. 1993;341:1232–1237. doi: 10.1016/0140-6736(93)91144-b. [DOI] [PubMed] [Google Scholar]
- 2.Manolio TA, Burke GL, O’Leary DH, Evans G, Beauchamp N, Knepper L, et al. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults : the Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol. 1999;19:356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 3.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke; a journal of cerebral circulation. 2009;40:1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 5.Singh-Manoux A, Britton A, Kivimaki M, Gueguen A, Halcox J, Marmot M. Socioeconomic status moderates the association between carotid intima-media thickness and cognition in midlife: evidence from the Whitehall II study. Atherosclerosis. 2008;197:541–548. doi: 10.1016/j.atherosclerosis.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects - the Tromso study. Cerebrovasc Dis. 2012;33:159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 7.Zhong W, Cruickshanks KJ, Schubert CR, Acher CW, Carlsson CM, Klein BE, et al. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012;224:506–510. doi: 10.1016/j.atherosclerosis.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke; a journal of cerebral circulation. 2009;40:3180–3185. doi: 10.1161/STROKEAHA.109.557280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller M, Grobbee DE, Aleman A, Bots M, van der Schouw YT. Cardiovascular disease and cognitive performance in middle-aged and elderly men. Atherosclerosis. 2007;190:143–149. doi: 10.1016/j.atherosclerosis.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Zhong W, Cruickshanks KJ, Huang GH, Klein BE, Klein R, Nieto FJ, et al. Carotid atherosclerosis and cognitive function in midlife: the Beaver Dam Offspring Study. Atherosclerosis. 2011;219:330–333. doi: 10.1016/j.atherosclerosis.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komulainen P, Kivipelto M, Lakka TA, Hassinen M, Helkala EL, Patja K, et al. Carotid intima-media thickness and cognitive function in elderly women: a population-based study. Neuroepidemiology. 2007;28:207–213. doi: 10.1159/000108112. [DOI] [PubMed] [Google Scholar]
- 12.Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22:646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laitala VS, Kaprio J, Koskenvuo M, Raiha I, Rinne JO, Silventoinen K. Association and Causal Relationship of Midlife Obesity and Related Metabolic Disorders with Old Age Cognition. Current Alzheimer Research. 2011:699–706. doi: 10.2174/156720511796717186. [DOI] [PubMed] [Google Scholar]
- 14.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr., Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias PK, Elias MF, D’Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosomatic Medicine. 2005;67:24–30. doi: 10.1097/01.psy.0000151745.67285.c2. [DOI] [PubMed] [Google Scholar]
- 16.Zeki Al Hazzouri A, Haan MN, Neuhaus JM, Pletcher M, Peralta CA, Lopez L, et al. Cardiovascular risk score, cognitive decline, and dementia in older mexican americans: the role of sex and education. Journal of the American Heart Association. 2013;2:e004978. doi: 10.1161/JAHA.113.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeki Al Hazzouri A, Haan MN, Yingzi D, Neuhaus JM, Yaffe K. Reduced heart rate variability is associated with worse cognitive performance in elderly Mexican Americans Hypertension. 2014;63:181–187. doi: 10.1161/HYPERTENSIONAHA.113.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeki Al Hazzouri A, Newman AB, Simonsick E, Sink KM, Sutton Tyrrell K, Watson N, et al. Pulse Wave Velocity and Cognitive Decline in Elders: The Health, Aging, and Body Composition Study. Stroke; a journal of cerebral circulation. 2013;44:388–393. doi: 10.1161/STROKEAHA.112.673533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. The Lancet. Neurology. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 20.Launer LJ. The epidemiologic study of dementia: a life-long quest? Neurobiology of aging. 2005;26:335–340. doi: 10.1016/j.neurobiolaging.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto M, Inoue K, Moriki A. Associations of brachial-ankle pulse wave velocity and carotid atherosclerotic lesions with silent cerebral lesions. Hypertension research : official journal of the Japanese Society of Hypertension. 2007;30:767–773. doi: 10.1291/hypres.30.767. [DOI] [PubMed] [Google Scholar]
- 23.Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PH, et al. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–170. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 24.Hunt KJ, Evans GW, Folsom AR, Sharrett AR, Chambless LE, Tegeler CH, et al. Acoustic shadowing on B-mode ultrasound of the carotid artery predicts ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke; a journal of cerebral circulation. 2001;32:1120–1126. doi: 10.1161/01.str.32.5.1120. [DOI] [PubMed] [Google Scholar]
- 25.Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke; a journal of cerebral circulation. 2008;39:800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr., et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of clinical epidemiology. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 27.Carotid Ultrasound. Sonographer Manual Version 1.1. Ultrasound Reading Center. New England Medical Center; [Accessed March 30, 2015]. Coronary Artery Risk Development in Young Adults. http://www.cardia.dopm.uab.edu/images/more/pdf/mooy20/Y20%20Ultrasound%20Manual.pdf. [Google Scholar]
- 28.Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. Journal of clinical psychology. 1984;40:785–787. doi: 10.1002/1097-4679(198405)40:3<785::aid-jclp2270400325>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III) Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 30.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 31.Peralta CA, Vittinghoff E, Bansal N, Jacobs D, Jr., Muntner P, Kestenbaum B, et al. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:261–266. doi: 10.1053/j.ajkd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 33.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke; a journal of cerebral circulation. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 34.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. The Lancet. Neurology. 2007;6:1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 35.Myredal A, Gan LM, Osika W, Friberg P, Johansson M. Increased intima thickness of the radial artery in individuals with prehypertension and hypertension. Atherosclerosis. 2010;209:147–151. doi: 10.1016/j.atherosclerosis.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Ozaki K, Kubo T, Imaki R, Shinagawa H, Fukaya H, Ohtaki K, et al. The anti-atherosclerotic effects of lipid lowering with atorvastatin in patients with hypercholesterolemia. J Atheroscler Thromb. 2006;13:216–219. doi: 10.5551/jat.13.216. [DOI] [PubMed] [Google Scholar]
- 37.Yu CM, Zhang Q, Lam L, Lin H, Kong SL, Chan W, et al. Comparison of intensive and low-dose atorvastatin therapy in the reduction of carotid intimal-medial thickness in patients with coronary heart disease. Heart. 2007;93:933–939. doi: 10.1136/hrt.2006.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]


