Abstract
Objective
Enhanced IL-6 expression plays an important role in the pathogenesis of osteoarthritis (OA). MCPIP1 is a novel post-transcriptional regulator of IL-6 expression and is targeted by miR-9. We investigated the MCPIP1 expression in OA cartilage and explored whether targeting of MCPIP1 by miR-9 contributes to enhanced IL-6 expression in OA.
Methods
Gene and protein expression in IL-1β-stimulated human OA chondrocytes/cartilage was determined by TaqMan assays and immunoblotting respectively. MCPIP1 and IL-6 mRNA expression at single cell level was analyzed using RNAScopeTM. MCPIP1 protein interaction with IL-6 mRNA was investigated using RNA immunoprecipitation (RIP). Transient transfections were used for siRNA mediated knockdown and overexpression of MCPIP1, its RNAse defective mutant, miR-9 or antagomir. Role of signaling pathways was evaluated using small molecule inhibitors. Binding of miR-9 with the “seed sequence” in the 3’UTR of MCPIP1 mRNA was investigated using a luciferase reporter assay.
Results
MCPIP1 mRNA expression was low but expression of miR-9 and IL-6 was high in the damaged OA cartilage. In IL-1β-stimulated OA chondrocytes expression of miR-9 and MCPIP1 was mutually exclusive and increase in miR-9 expression level correlated with reduced MCPIP1 expression and enhanced IL-6 expression. MCPIP1 protein directly binds with IL-6 mRNA and over-expression of wild type MCPIP1 destabilized the IL-6 mRNA. MCPIP1 expression was altered by overexpression or inhibition of miR-9. Transfection with miR-9 mimics inhibited the reporter activity and mutation of the “seed sequence” abolished the repression of reporter activity.
Conclusions
These studies implicate miR-9-mediated suppression of MCPIP1 in OA pathogenesis via upregulation of IL-6 expression in IL-1β-stimulated human OA chondrocytes.
Keywords: Osteoarthritis, IL-6, miR-9, MCPIP1, Chondrocytes
Introduction
Osteoarthritis (OA) is a multifactorial disease primarily caused due to an imbalance between the anabolic and catabolic factors in the articulating joint. It is a leading cause of disability in the elderly population with significant socioeconomic burden. OA is primarily characterized by the focal degradation of the cartilage but the affected joints also show synovial inflammation, osteophyte formation and sub-chondral bone sclerosis (1, 2). Aging and mechanical stress on the joint, including injury and joint instability, are important factors in the development of OA. Also it has been reported that women with OA have a 50% increased risk of fracture possibly because of postural instability, quadriceps weakness, joint pain and stiffness (3). Considerable evidence implicates high levels of IL-6 present in serum and synovial fluid of OA patients in the pathogenesis of the disease (4). A prospective population study on a cohort of British women showed a correlation of higher BMI and elevated serum levels of IL-6 with development of radiographic knee OA (5). IL-6 acts as a crucial mediator of MMP13 levels in combination with IL-1β and Oncostatin M (OSM) in human and bovine cartilage explants (6, 7) and in a mouse model of human OA (8, 9). IL-6 is also reported to inhibit the expression of type II collagen (10). However, mechanisms that post-transcriptionally regulate IL-6 expression in OA, and may be important in developing more effective OA therapies, are not generally well understood.
Post-transcriptional regulation of cytokine gene expression is tightly regulated in which both RNA binding proteins (11) and micro-RNAs (miRNAs), which are small endogenous non-coding RNAs, play a role (12). Biogenesis of miRNAs is tightly controlled at the transcription level to maintain homeostasis because miRNAs modify the expression of target mRNAs via interactions with the “seed sequence” generally located in the 3’UTR but may also be present in the 5’UTR or the coding region of the mRNAs (13-16). Important role of miRNAs in skeletal and cartilage homeostasis became evident from studies in Dicer knockout mice which exhibited severe skeletal growth defect (17). Deficiency of mir-140 causes age related OA like disease with loss of proteoglycan and fibrillation of articular cartilage in mice (18). Additionally, expression of miR-146, -9, -22, -27a and -27b, which are important regulators of OA related genes, is dysregulated in human chondrocytes (19).
RNA binding proteins interact with the AU rich elements (AREs) localized within the 3’UTR of the cytokine mRNA and are one of the most common determinants of mRNA stability in mammalian cells (11). A recently identified RNA binding protein monocyte chemo-attractant protein1-induced protein 1 (MCPIP1; ZC3H12A; NM_025079) possesses intrinsic RNAse activity and has been shown to be a novel post-transcriptional regulator of the expression of inflammatory cytokines (20). MCPIP1 does not require AREs for its activity and function through a stem-loop structure located in the 3’ UTR of the mRNA (20). MCPIP1 deficiency is lethal in mice and these animals show widely disseminated inflammation and high levels of IL-6 in blood (20). Expression of MCPIP1 is induced by MCP-1 and IL-1β in monocytes, macrophages and in fibroblast-like synovial (FLS) cells but not by TNF-α or IL-6 (21-23). Although, recent reports emphasize the importance of MCPIP1 in regulating inflammation, neither a specific role nor the molecular mechanisms of MCPIP1 action in OA pathogenesis have been determined.
In the present report we demonstrate that in human OA chondrocytes IL-1β-induced expression of IL-6 and MCPIP1 occurs simultaneously and increases in parallel for approximately 12 h after which MCPIP1 expression declines but expression of IL-6 surge. Down-regulation of MCPIP1 expression coincides with the increased expression of miR-9 which is initially expressed at low level. Upregulation of miR-9 expression coincide with the appearance of low levels of IL-6 protein in the chondrocyte culture medium. Using a luciferase reporter assay we show that miR-9 interact with the seed sequence in the 3’UTR of MCPIP1 mRNA. Overexpression of miR-9 was found to suppress the MCPIP1 mRNA translation and facilitate the enhanced expression of IL-6 mRNA and protein in IL-1β-stimulated human OA chondrocytes. Our findings thus suggest that under pathological conditions miR-9-mediated suppression of MCPIP1 act as a switch for enhanced expression of IL-6 in OA.
Materials and Methods
Reagents
Media and other reagents for cell culture were purchased from Life Technologies (Carlsbad, CA, USA). Pronase, Collagenase and Complete Protease inhibitor tablets were from Roche Diagnostics (Indianapolis, IN, USA). Recombinant human IL-1β, IL-1 receptor antagonist (RA) and IL-6 were from R&D Systems. Antibodies specific for ZC3H12A were purchased from GeneTex (Irvine, CA, USA) and anti-IL-6 antibodies were from Cell Signaling Technology (Danvers, MA) and anti-β-Actin and anti-MMP13 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Actinomycin D was purchased from Sigma Chemical Company (St. Louis, MO, USA). IL-6 specific ELISA kit was from Booster Biological Technology (Fremont, CA, USA). Kits and other supplies for the preparation of total RNA were from Qiagen (Valencia, CA, USA). Trisilencer 27 mer MCPIP1 targeting and non-targeting siRNAs were from Origene (Rockville, MD, USA). miR-9 mimics and antagomirs were from Ambion (Life Technologies).
Cartilage samples and chondrocytes preparation
The study protocol to use de-identified, discarded human cartilage samples was reviewed and approved as “non-human subject study under 45 CFR” by the Institutional Review Board of North East Ohio Medical University, Rootstown, OH and SUMMA Health Systems, Akron, OH. Human cartilage samples were obtained from the hip joints of OA patients aged 45–72 years (n=9, mean age, 59 ± 11.6 years) who underwent total joint arthroplasty at Summa St. Thomas Hospital, Akron, OH. Macroscopically unaffected and affected cartilage pieces from the femoral head were resected from smooth and damaged areas of the cartilage respectively. A portion of isolated cartilage pieces was immediately stored in liquid nitrogen and was used later for the preparation of total RNA. Remaining cartilage pieces were used for the preparation of OA chondrocytes and were sequentially digested with Pronase (1mg/ml) and Collagenase (1mg/ml) and the isolated OA chondrocytes were plated in complete medium as described previously (16, 24-27).
In situ mRNA Expression Analysis in Human OA Chondrocytes
In situ mRNA expression was determined using RNAScope (Advanced Cell Diagnostics, Hayward, CA, USA) according to the instructions provided. In brief, human OA chondrocytes were seeded in 4-chambered slides (Fisher Scientific, Waltham, MA). After treatment with IL-1β, chondrocytes were fixed on slide and digested with protease followed by hybridization with the fluorophor labeled IL-6 and MCPIP1 target specific probes. Amplifications were performed using the kit-supplied reagents, coverslips were mounted using the anti-fade mounting media with DAPI (Vector Laboratories, Burlingame, CA, USA). Images were acquired using an inverted Olympus IX 70 confocal microscope FV300 (Olympus Corporation, Tokyo, Japan).
Total RNA isolation and Real time PCR
Total RNA from frozen cartilage and isolated chondrocytes was prepared essentially as previously described (16, 24-27). For mRNA expression analysis cDNA was synthesized from 1 µg of total RNA using High-Capacity cDNA Reverse Transcription Kit (Life Technologies) and mRNA expression was quantified using TaqMan Gene Expression Assays as previously described (16, 24-27). Expression levels were determined in one plate for all samples simultaneously and normalized to the corresponding amounts of β-Actin or RNU6B cDNA measured within the same plate. Relative expression levels were calculated using the 2−ΔΔCT method (28).
Chondrocyte Treatment and Transfections
For each treatment primary human OA chondrocytes were seeded in 35 cm dishes in complete medium and treated with IL-1β or other agents as previously described (16, 24-27). After treatment, chondrocytes were washed and RNA or protein was prepared immediately or were stored at −80°C for later use. Culture supernatants were collected and stored in −80°C and were used to quantify IL-6 levels by ELISA. To study the effect of siRNA-mediated depletion of MCPIP1 on IL-6 mRNA stability, chondrocytes were transfected with MCPIP1 targeting siRNA or non-targeting siRNA at a final concentration of 100nM using Amaxa Nucleofactor System (Lonza AG, Walkersville, MD) according to the manufacturer’s instructions. Briefly, 4×106 chondrocytes were seeded into 10 cm culture dishes and two to three days later were digested with pronase and collagenase. siRNA was diluted in 100 µl of nucleofactor solution and chondrocytes were transfected using P01 program, transferred to complete medium and seeded into 6 well plates. To study the effect of overexpression of MCPIP1 on IL-6 mRNA, human chondrocytes were transfected as above with the wild type MCPIP1 or its mutant (in which PIN domain which possess RNAse catalytic activity was deleted) expression constructs (23) using 5µg plasmid DNA. Chondrocytes with depleted MCPIP1 expression or overexpression of wild type MCPIP1 or its mutant form were first stimulated with IL-1β for 2 h and then treated with Actinomycin D (4μM) to halt transcription. IL-6 mRNA levels at different time points were assessed by TaqMan assay.
RNA Immunoprecipitation (RIP)
Human OA chondrocytes (6×106) were stimulated with IL-1β for 24 hr and then fixed in 1% formaldehyde for 15 min at 25°C. Glycine was added to quench the crosslinking for 5 min and chondrocytes were washed in ice cold PBS twice, scrapped and resuspended in 1ml RIP lysis buffer (50 mM HEPES pH 7.5, 140 mM NaCl, 1 mM EDTA, 1% triton X-100, 0.1% deoxycholate, 1X Complete protease inhibitors cocktail) containing RNase inhibitor (50 U/500 μl buffer), and then nucleic acids were fragmented by extensive sonication (Fisher Scientific, Model FB705). Immunoprecipitation was performed by adding the control IgG or anti-MCPIP1 antibody to the mixture and incubated at 4°C overnight. Reactions were cleared by centrifugation at 12,000 rpm for 15 min, and 25 μl protein A/G agarose slurry (equilibrated in RIP lysis buffer containing RNase inhibitor (Santa Cruz) was added to the supernatant and incubated for additional 2 hr and then the agarose beads were washed extensively in RIP lysis buffer containing RNase inhibitor. The eluted fraction was resuspended in 200mM final concentration of NaCl, before adding 20 μg proteinase K. Mix was incubated at 42°C for 1 hr and then at 65°C for overnight. Finally RNA was extracted and purified using RNeasy Mini Spin column. Immumoprecipitated RNA was reverse transcribed and IL-6 and MCPIP1 mRNAs were quantified using TaqMan Assays. Data were expressed as fold enrichment of anti-MCPIP1 antibody relative to IgG.
Immunoblotting
After treatments, OA chondrocytes were harvested, washed with cold PBS and lysed in ice-cold protein lysis buffer (50 mM Tris pH 7.6, 400 mM NaCl, 0.5% NP-40, 1 mM PMSF and 1 × protease inhibitor cocktail (Roche) as described previously (29, 30). Lysates were clarified by centrifugation (15000g for 15 min at 4°C) and the supernatant was analyzed immediately or stored at −80°C. Equivalent amounts of lysate protein (25 µg) were resolved by 10% SDS–PAGE and transferred to a PVDF membrane (Hybond P, Amersham Biosciences, Piscataway, New Jersey) and the blots were incubated with diluted anti-IL-6 (1:1000), or anti-MCPIP1 (1:1000) or anti-β-Actin (1:5000) primary antibodies in TBST overnight at 4°C. Blots were then incubated with horseradish peroxidase-conjugated secondary antibody and the antibody reactive proteins were visualized and analyzed using the Syngene Pxi imager and the Syngene Image analysis software respectively.
IL-6 ELISA Assay
IL-6 levels in culture supernatants from different time points were determined using a human IL-6-specific ELISA kit according to manufacturer’s instructions (Booster Immunoleader). Values were derived using a standard curve prepared using recombinant human IL-6.
Luciferase Reporter Assay
MCPIP1 mRNA’s 3’UTR was amplified by PCR using primer pairs 5’- GTCAACTAG TCTCTCCTACAAGTCCCAGCA-3’ and 5’-TGACAAGCTTTTGAAAGGGCTCACAATG AT-3’. Amplified product was digested and cloned into HindIII-SpeI site of the pMIR-REPORT vector (Ambion/Life Technologies) to generate MCPIP1-pMIR-REPORT vector. QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) was used to mutate the miR-9 seed sequence located in the 3’UTR of MCPIP1 mRNA using oligo 5’-GAAACCCACAAAGATTTGATACTG TAGGATTG-3’ (mutated nucleotides are in bold) to generate mutant MCPIP1 3’UTR luciferase reporter construct mMCPIP1-pMIR-REPORT vector. Reporter vectors containing the wild type or mutated 3’UTRs were transfected alone or co-transfected with miR-9 mimic as described above and 24 h post-transfection Luciferase activity was measured using the dual reporter system (Promega, Madison, WI).
Statistical Analyses
The values are presented as the Mean ± SD and the statistically significant difference between the experimental groups and controls was determined using Student’s t-test. Each experiment was repeated three times using three independent patients samples. P<0.05 was considered to be statistically significant.
Results
MCPIP1 expression was low in damaged OA cartilage and was induced by IL-1β in OA chondrocytes
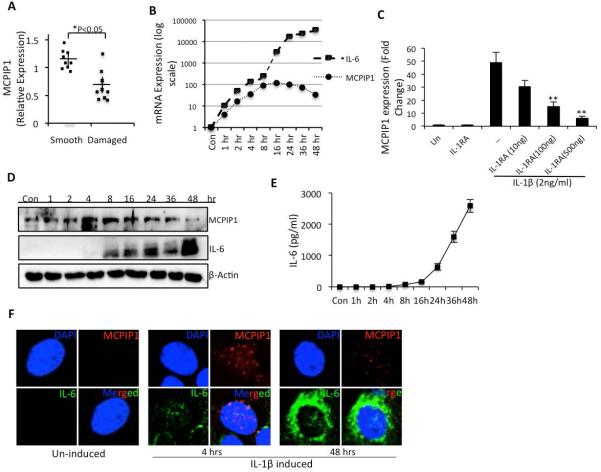
Expression of MCPIP1 mRNA (NM_025079) was compared between the smooth and damaged cartilage of the same patient and between the cartilage samples obtained from different patients who underwent total hip arthroplasty. In all of the samples analyzed, expression of MCPIP1 mRNA was significantly low in damaged cartilage compared to smooth cartilage (Figure 1A). We determined the expression of IL-6 and IL-1β in all of the samples used. However in case of IL-1β, in three cartilage samples we were unable to detect the expression but in the remaining samples expression of IL-1β was not detected in smooth cartilage but was readily detectable in the damaged cartilage. Similarly, in two samples we were not able to detect the expression of IL-6, in one sample no difference was noted in the smooth and damaged cartilage and in the rest expression of IL-6 was undetectable in the smooth cartilage. In IL-1β-stimulated OA chondrocytes, expression of MCPIP1 mRNA and IL-6 mRNA (NM_000600) followed a similar pattern of induction but expression of IL-6 mRNA was significantly accelerated 12 hr post-stimulation and peaked to more than ten thousand fold at 48 hr post-stimulation with IL-1β (Figure 1B). On the other hand expression of MCPIP1 mRNA was 140 fold higher compared to controls between 12-16 hr post-stimulation with IL-1β and then declined (Figure 1B). IL-1β mediated downstream effect was abolished when cells were treated with IL-1R antagonist in a dose dependent manner, suggesting specificity of the IL-1β driven induction of MCPIP1 expression (Figure 1C).
Figure 1. MCPIP1 expression is decreased in OA cartilage and induced upon IL-1β treatment in chondrocytes.
(A) RNA was isolated from damaged or smooth cartilage and MCPIP1 mRNA levels were determined by TaqMan assay. Each dot represents the value from a single patient sample. (B) Chondrocytes were serum starved for 12 hr and then stimulated with 2ng/ml IL-1β for the indicated time pointsand MCPIP1 and IL-6 mRNA level was measured by TaqMan assay with expression of β-Actin as endogenous control. (C) Chondrocytes were treated with increasing doses of IL-R antagonist in presence or absence of IL-1β. MCPIP1 expression was measured after 16 hrs incubation as above. (D) Cell lysates were prepared and IL-6 or MCPIP1 protein levels were determined by Western blotting using antibodies specific for IL-6 and MCPIP1. Blots were probed with anti β-Actin antibody to ensure equal loading. (E) Supernatants from the control or stimulated cells were analyzed by a sandwich ELISA to determine the secreted IL-6 concentration. (F) For In-situ hybridization chondrocytes were induced for the indicated time points and RNA hybridization was performed on formalin fixed cells using IL-6 or MCPIP1 specific probes. Fluorescence was visualized using Olympus confocal microscope. Data are represented as mean ±SD of three experiments performed in duplicates using at least three patients samples. *p< 0.05, **p<0.005.
Of interest are the findings that appearance of low levels of IL-6 protein in OA chondrocytes and secretion in the culture medium coincided with the decline in MCPIP1 mRNA and protein expression (Figure 1D and 1E). Gene expression and localization of IL-6 and MCPIP1 transcripts at the level of single chondrocytes showed that initially after stimulation with IL-1β, MCPIP1 mRNA was abundantly induced and localized in the cytoplasm with little detectable expression of IL-6 mRNA (Figure 1F). At later time points, IL-6 transcripts were abundant, primarily localized in the cytoplasm and the expression of MCPIP1 mRNA was barely detectable in OA chondrocytes (Figure 1F). These data are in agreement with the protein expression data (Figure 1D) and indicate a potential role of MCPIP1 in IL-6 regulation under pathological conditions in OA chondrocytes.
MCPIP1 is a post-transcriptional regulator of IL-6 expression in OA chondrocytes
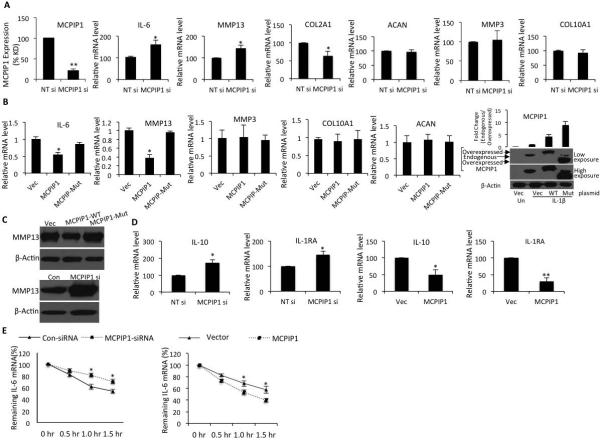
In OA chondrocytes, effective knockdown of MCPIP1 mRNA expression by MCPIP1 targeting siRNAs relative to non-targeting siRNAs was confirmed by TaqMan assay (Figure 2A). The effect of MCPIP1 depletion on IL-6 expression was examined after stimulation with IL-1β. MCPIP1 depletion led to significantly increased IL-6 expression compared to control chondrocytes (Figure 2A, p<0.005). No difference in mRNA expression of chondrocyte-specific marker genes aggrecan (ACAN, NM_013227) MMP3 (NM_002422) and type-X collagen (Col10A1, NM_000493) was observed in OA chondrocytes with depleted MCPIP1 expression (Figure 2A). Expression of COL2A1 mRNA (X16711) was significantly reduced upon knock-down of MCPIP1 possibly due to higher levels of IL-6 which is known to suppress the expression of type-II collagen in chondrocytes (10). However, expression of MMP13 mRNA (NM_002427) and protein was significantly higher in chondrocytes with depleted MCPIP1 expression indicating that either MCPIP1 also regulate the expression of MMP-13 or this could be a reflection of enhanced IL-6 expression which is known to induce the expression of MMP-13 (Figure 2A and C) (31, 32). When OA chondrocytes overexpressing wild type MCPIP1 were stimulated with IL-1β, expression of IL-6 was substantially and significantly reduced but no reduction in IL-6 expression in OA chondrocytes transfected with RNase inactive mutant of MCPIP1 was observed (Figure 2B) indicating that the intrinsic RNase activity of MCPIP1 is required for reducing IL-6 mRNA levels in OA chondrocytes. Endogenous expression and overexpression of MCPIP1 wild type or mutant form in chondrocytes was confirmed by Western blot using anti-MCPIP1 antibody (Figure 2B). Expression of MMP13 was also low in OA chondrocytes overexpressing wild type MCPIP1 (Figure 2B and C) again suggesting that MCPIP1 may also be a regulator of MMP-13 expression. IL-6 is a pleiotropic cytokine, which can elicit inflammatory or anti-inflammatory response by inducing anti-inflammatory cytokine IL-10 and IL-1RA (33). Expression of IL-10 or IL1RA was significantly high in chondrocytes with reduced expression of MCPIP1 while overexpression of MCPIP1 downregulated the IL-10 or IL-1RA expression (Figure 2E). These data indicate that MCPIP1 also plays a role in regulating the expression of these genes albeit indirectly.
Figure 2. MCPIP1 knockdown or overexpression alters IL-6 expression in OA chondrocytes.
(A) Chondrocytes were transfected with non-targeted siRNA or siRNA against MCPIP1. Twenty-four hr post-transfection chondrocytes were induced by IL-1β for 8 hr and then the expression of MCPIP1, IL-6, MMP13, COL2A1, ACAN, MMP3 or COL10A1 was measured by TaqMan Assay. (B) Wild type or mutant MCPIP1 encoding cDNA constructs were transfected by electroporation into primary chondrocytes and 24 hr later were stimulated for 12 hr and then the expression of indicated genes was determined. Overexpression of Wild type and the mutant form is shown by Western blot using anti-MCPIP1 antibody. (C) Endogenous MMP13 expression was measured by Western blot upon knockdown or overexpression of MCPIP1 as in B. (D) Chondrocytes were transfected as in A and B and induced by IL-1β and gene expression of IL-10 or IL-1RA was measured by TaqMan assay. (E) Primary chondrocytes were transfected separately by non-targeted siRNA, siRNA against MCPIP1, vector control or wild type MCPIP1 cDNA construct and treated as above and then challenged with 4μM ActD for the indicated time points. IL-6 mRNA expression is represented as percentage of remaining IL-6 mRNA. Data are mean ±SD of three experiments performed in duplicates using at least three patient samples. *p< 0.05, **p<0.005.
To determine whether MCPIP1 influenced IL-6 mRNA stability, IL-6 transcript levels were quantified in OA chondrocytes with depleted MCPIP1 expression. Depletion of MCPIP1 increased the IL-6 mRNA stability (t1/2 120 min) while overexpression of wild type MCPIP1 decreased the IL-6 mRNA stability by almost 50% (t1/2 60 min) indicating that MCPIP1 down-regulate IL-6 mRNA expression by affecting the stability of IL-6 mRNA transcripts in OA chondrocytes under pathological conditions (Figure 2E). Direct interaction of MCPIP1 protein with IL-6 mRNA in OA chondrocytes under pathological condition was confirmed using RIP assay (Figure 3). Taken together these data indicate that in OA chondrocytes MCPIP1 is a post-transcriptional regulator of IL-6 mRNA expression and exert this effect by destabilizing the IL-6 transcripts through direct binding and degradation by the intrinsic RNAse activity of the MCPIP1 protein as reported previously (20).
Figure 3. MCPIP1 interact with IL-6 mRNA in OA chondrocytes.
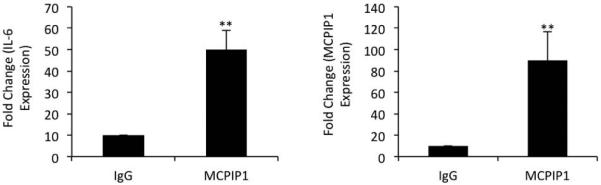
Primary chondrocytes were stimulated for 8 hr with IL-1β in vitro. Cell lysates were subjected to RIP (RNA immune-precipitation assay) using IgG control antibody or anti-MCPIP1 antibody. Immunoprecipitated RNA was reverse transcribed and was used to measure the expression of IL-6 or MCPIP1 mRNA by TaqMan assay. Data are represented as mean ±SD of three experiments performed in duplicates using at least three patients samples. **p<0.005.
Expression of miR-9 was high in damaged OA cartilage and was up-regulated under pathological conditions in OA chondrocytes
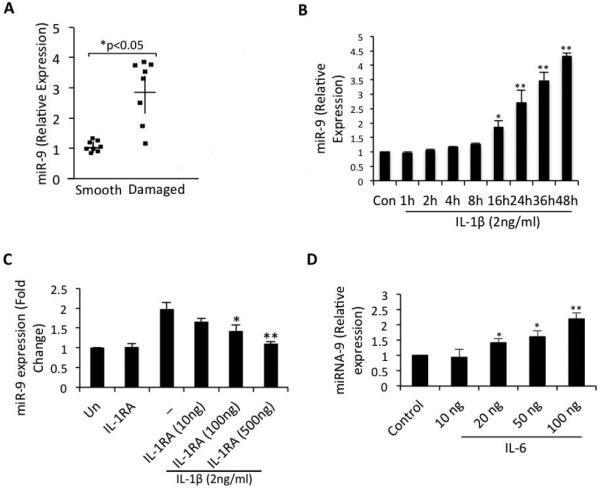
In previous studies it was shown that miR-9 was differentially expressed in OA cartilage (34) and target MCPIP1 mRNA in neuroprogenitor cells (35). However, whether MCPIP1 is expressed in OA cartilage and in OA chondrocytes and whether the expression of miR-9 is induced under pathological conditions and inhibits the expression of MCPIP1 has not been reported. We examined the expression of miR-9 and MCPIP1 directly in damaged and smooth cartilage from several patients (n=9) and in all cases, except in one, expression of miR-9 was high in damaged OA cartilage (Figure 4A) as reported previously (34) but the expression of MCPIP1 was low in the same region in the samples analyzed (Figure 1A). In OA chondrocytes stimulated with IL-1β, expression of miR-9 was low initially but showed a gradual and significant increase in a time dependent manner with significantly high expression levels noted at 16 hr beyond post-stimulation (Figure 4B). However, expression of miR-9 was inhibited upon treatment of OA chondrocytes with IL-1RA (Figure 4C). Indeed, increased expression levels of miR-9 at the time points analyzed correlated with the corresponding decline in MCPIP1 expression and enhanced expression of IL-6 (Figure-1B, D). Stimulation of OA chondrocytes with low doses of IL-6 (20-100 ng/ml) induced the expression of miR-9 (Figure 4D). Taken together these data suggests that miR-9 levels sufficient to suppress MCPIP1 expression and facilitate enhanced IL-6 expression require stimulation by IL-6 as well. This also indicates that IL-6 itself acts to remove the block in its expression by upregulating the expression of miR-9 in an inflammatory response.
Figure 4. Expression of miR-9 was upregulated in damaged cartilage and induced in chondrocytes upon IL-1β or IL-6 stimulation.
(A) miR-9 expression was directly assessed in damage or smooth cartilage. Each dot represents the value from a single patient sample. (B) Chondrocytes were serum starved for 12 hr and then stimulated with 2ng/ml IL-1β for the indicated time points in the presence or absence of IL-1RA (C) or (D) IL-6 for 24 hr and then RNA was extracted and miR-9 expression was measured by TaqMan Assay. Data are represented as Mean ±SD of three experiments performed in duplicates using at least three patients samples. **p<0.005.
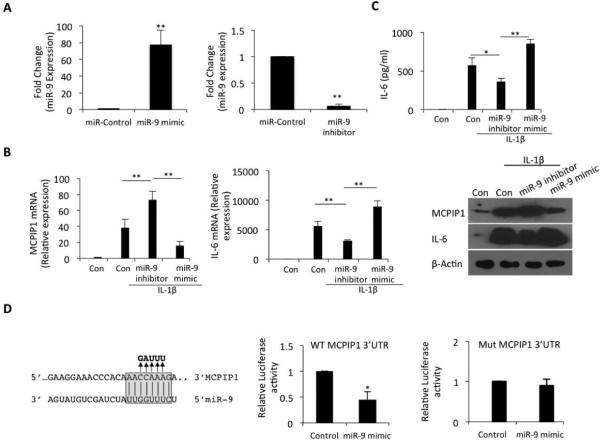
Over-expression or inhibition of miR-9 alters MCPIP1 and IL-6 expression in OA chondrocytes
Transient transfection assays were used to directly confirm the effect of overexpression or inhibition of miR-9 (Figure 5A) on MCPIP1 and IL-6 expression in OA chondrocytes stimulated with IL-1β. OA chondrocytes transfected with miR-9 inhibitor showed a significant up-regulation of MCPIP1 expression and a concomitant dramatic decline in IL-6 expression (Figure 6B, P< 0.005) under pathological conditions. Overexpression of miR-9 was sufficient to suppress the expression of MCPIP1 mRNA and these cells also showed a sharp increase in the expression of IL-6 mRNA (Figure 5B). This effect was also reflected in the protein levels of IL-6 and MCPIP1 detected by Western blotting using specific antibodies (Figure 5B). IL-6 protein levels in culture supernatants mirrored the pattern of IL-6 mRNA expression in OA chondrocytes under the same conditions (Figure 5C). Co-transfection of a reporter construct containing the 3’UTR of MCPIP1 mRNA with miR-9 mimic significantly suppressed the luciferase activity which was abolished when the miR-9 binding site was mutated (Figure 5D). Taken together these results demonstrate that miR-9 acts as a direct suppressor of MCPIP1 and facilitate enhanced IL-6 expression in OA chondrocytes under pathological conditions.
Figure 5. miR-9 directly targets the seed sequence in the 3’UTR of MCPIP1 mRNA.
(A) Overexpression or inhibition of miR-9 expression was measured by TaqMan assay. Expression of RNU6B was used as endogenous control. (B) Primary OA chondrocytes were transfected with 100 nM each of miR-9 mimic or miR-9 inhibitor by electroporation. Cells were induced for 36 hr and expression of miR-9, MCPIP1 and IL-6 was quantified by TaqMan assays. Protein level was measured by Western blotting using anti-MCPIP1 or anti-IL-6 antibody. Expression of β-Actin was used as an internal control. (C) Culture supernatant from (B) was used to determine the IL-6 protein level by sandwich ELISA. (D) miR-9 seed sequence are indicated in gray box which is complement to the position 148-155 of MCPIP1 3’ UTR. Arrows indicate nucleotide changes (in bold) present in the MCPIP1 mutant 3’UTR. MCPIP1 3’UTR luciferase reporter construct (WT-left panel or mutant-right panel) was transfected in OA chondrocytes alone or co-transfected with miR-9 mimic and 24 hr post-transfection Luciferase activity was measured using the dual reporter assay. Renilla luciferase was co-transfected for normalization purpose. Data are represented as Mean ±SD of three experiments performed in duplicates using at least three patients samples. *p<0.05, **p<0.005.
Discussion
The hallmark of OA is the focal degradation of the cartilage elicited by increased expression of matrix degrading proteases. There is enhanced expression of inflammatory cytokines including IL-6 in the affected joints and IL-6 has been shown to activate chondrocytes, the only cell type present in the cartilage, to produce matrix metalloproteinases and aggrecanases in addition to other factors suggesting an association of IL-6 expression with OA pathogenesis (36). Over expression of IL-6 in OA joints is indicative of the failure of mechanisms that normally regulate IL-6 expression at the transcription and/or post-transcriptional level.
In the present study we for the first time show that enhanced IL-6 expression correlates with the up-regulation of miR-9 and down-regulation of MCPIP1 mRNA and protein expression in human OA cartilage and in OA chondrocytes under pathological conditions. Indeed, using a luciferase reporter assay we demonstrate the direct interaction of miR-9 with the “seed sequence” in the 3’UTR of MCPIP1 mRNA in human OA chondrocytes. This suggests that in human OA chondrocytes miR-9 binds to the 3’UTR of MCPIP1 mRNA as the luciferase activity was responsive to miR-9 overexpression indicating that miR-9 directly targets MCPIP1 mRNA and regulates protein expression by inducing translational suppression. Our data also provide unique and novel information that IL-6 is also an inducer of miR-9 expression in human OA chondrocytes and thus facilitate its own enhanced expression by inducing a suppressor of MCPIP1 expression in an inflammatory response. This positive feedback loop has not previously been reported and may be important in IL-6-mediated induction of IL-10 and IL-1RA and/or mediators of cartilage degradation in OA (33). However this needs to be investigated further.
MicroRNA-9 is an interesting example of controlling the balance between neural stem cell proliferation and differentiation (37). In human gastric adenocarcinoma miR-9 acts to suppress proliferation by post-transcriptional inhibition of NF-κB1 expression (38). In micro-vascular endothelial cells miR-9 reduce SOCS5 levels and activate JAK-STAT pathway leading to cell migration and angiogenesis (39). Therefore we speculate that miR-9 might be altering multiple genes expression and regulating complex sets of signaling pathways in OA chondrocytes. However, whether miR-9 facilitates enhanced expression of IL-6 in OA by targeting its suppressor MCPIP1 in chondrocytes or any other cell type has not been reported previously. Our data of miR-9 expression directly in OA cartilage is in agreement with the results reported by Jones et al (34) but our studies go further as we also show that expression levels of miR-9 in OA cartilage negatively correlated with the expression of MCPIP1 and IL-6. Our analysis at single chondrocyte level revealed that expression of MCPIP1 and IL-6 was almost mutually exclusive upon IL-1β stimulation at the time points analyzed. Second, overexpression of wild type MCPIP1 was sufficient to suppress IL-1β-induced IL-6 expression, which was abolished in human OA chondrocytes with overexpression of a mutant form of MCPIP1 that lacks RNAse activity (23). Overexpression of wild type MCPIP1 in human OA chondrocytes altered the IL-6 mRNA stability and expression as IL-6 mRNAs showed a reduced half-life compared to OA chondrocytes transfected with control vector. Inhibition of miR-9 resulted in increased expression of MCPIP1 and suppression of IL-1β-induced expression of IL-6 in human OA chondrocytes. Post-transcriptional regulation of IL-6 expression by MCPIP1 and suppression of MCPIP1 expression by miR-9 in human OA chondrocytes is not known and also there is little information about post-transcriptional regulation of IL-6 in OA. Our data thus provides an insight into the disruption of post-transcriptional regulation of IL-6 by showing that expression of MCPIP1 is suppressed by a highly conserved microRNA, miR-9, to allow high levels of IL-6 expression in human OA chondrocytes under pathological conditions.
IL-6 is a pleiotropic cytokine which has pro- and anti-inflammatory properties (33). It activates the transcription of its target genes via formation of an IL-6 receptor complex involving a membrane bound IL-6 receptor (IL-6R), soluble IL-6R (sIL-6R) and gp130 followed by activation of STAT1/STAT3 pathway (40). Our results demonstrate that enhanced expression of MCPIP1 down-regulates the expression of IL-10 and IL1RA. IL-10 is known to induce IL-1RA expression in monocytes and neutrophils (41). Therefore in human OA chondrocytes upregulation of IL-1RA upon knock down of MCPIP1 may not be surprising since this might be the direct consequence of IL-10 upregulation. IL-10 stimulates cartilage proteoglycan synthesis (42) and in OA chondrocytes expression of IL-10 remains elevated compared to normal chondrocytes (43) and thus may be beneficial in controlling the inflammatory response in OA. Low levels of MCPIP1 expression in damaged OA cartilage correlates with the high levels of IL-6 expression in OA chondrocytes. This is supported by our data as change in IL-10 expression levels that correlated with MCPIP1 expression levels under pathological condition is suggestive of an attempt to counteract the inflammatory and degenerative response elicited by IL-6 in osteoarthritis. Our data also indicate that disruption of the anti-inflammatory mechanisms in OA may be important in the progression of the disease. Therefore, it is tempting to speculate that MCPIP1 occupies a central position where it balances the levels of inflammatory and anti-inflammatory cytokines in OA.
There is a direct correlation between IL-6 expression level and clinical outcome of rheumatoid arthritis and blocking of IL-6 function has emerged as a new therapeutic strategy for RA and related inflammatory disease (44). Our data thus suggest that blocking IL-6 may be an attractive option for inhibiting OA progression as well. The destabilization of IL-6 mRNA by AU-rich element binding proteins has been studied (45, 46), however, dysregulation of IL-6 by RNA modifying protein in OA to our knowledge is not shown. In the present study for the first time we have shown IL-6 regulation by MCPIP1 in human OA chondrocytes under pathological conditions and thus provide strong evidence of MCPIP1 being an important post-transcriptional regulator of IL-6 expression in a terminally differentiated cell type. Recently, Masuda et al identified Arid5a which enhances IL-6 mRNA stability by binding through the 3’UTR of IL-6 (47). Arid5a and MCPIP1 have partially overlapping binding sequence in the 3’ UTR of IL-6 and it has been found that Arid5a opposes MCPIP1 binding. Interestingly, IL-1β also induces Arid5a mRNA in macrophages but whether Arid5a is also induced in OA chondrocytes under pathological conditions is not known and will be studied in future. Regulation of IL-6 expression indirectly by miR-9 through the down-regulation of MCPIP1 expression in OA chondrocytes is a novel finding and suggests that miR-9 may be an important player in the pathophysiology of OA.
In summary, our data for the first time provide evidence of miR-9/MCPIP1 axis being active in OA cartilage and chondrocytes and that miR-9-mediated suppression of MCPIP1 expression acts as a switch for the over expression of IL-6 under pathological conditions. Therefore targeting of miR-9/MCPIP1/IL-6 axis may prove to be a useful strategy for potential therapeutic applications in OA.
Acknowledgements
This work was supported in part by National Institutes of Health grants (RO1-AT-005520; RO1-AT-007373; R21-AR-064890) and funds from the Northeast Ohio Medical University. We also thank Dr Jolanta Jura, Department of General Biochemistry, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, 30-387 Krakow, Poland, for graciously and generously providing the MCPIP1 wild type and mutant expression constructs and the permission to use them in our studies.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bian Q, Wang YJ, Liu SF, Li YP. Osteoarthritis: genetic factors, animal models, mechanisms, and therapies. Front Biosci (Elite Ed) 2012;4:74–100. doi: 10.2741/361. [DOI] [PubMed] [Google Scholar]
- 2.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan MY, Center JR, Eisman JA, Nguyen TV. Bone mineral density and association of osteoarthritis with fracture risk. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 5.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009;60(7):2037–45. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawston TE, Curry VA, Summers CA, Clark IM, Riley GP, Life PF, et al. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998;41(10):1760–71. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Rowan AD, Koshy PJ, Shingleton WD, Degnan BA, Heath JK, Vernallis AB, et al. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheum. 2001;44(7):1620–32. doi: 10.1002/1529-0131(200107)44:7<1620::AID-ART285>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6(2):71–9. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 9.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31(6):784–8. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 10.Poree B, Kypriotou M, Chadjichristos C, Beauchef G, Renard E, Legendre F, et al. Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem. 2008;283(8):4850–65. doi: 10.1074/jbc.M706387200. [DOI] [PubMed] [Google Scholar]
- 11.White EJ, Brewer G, Wilson GM. Post-transcriptional control of gene expression by AUF1: mechanisms, physiological targets, and regulation. Biochim Biophys Acta. 2013;1829(6-7):680–8. doi: 10.1016/j.bbagrm.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asirvatham AJ, Magner WJ, Tomasi TB. miRNA regulation of cytokine genes. Cytokine. 2009;45(2):58–69. doi: 10.1016/j.cyto.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310(5755):1817–21. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 15.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 16.Akhtar N, Makki MS, Haqqi TM. MicroRNA-602 and MicroRNA-608 Regulate Sonic Hedgehog Expression via Target Sites in the Coding Region in Human Chondrocytes. Arthritis Rheumatol. 2015;67(2):423–34. doi: 10.1002/art.38952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi T, Lu J, Cobb BS, Rodda SJ, McMahon AP, Schipani E, et al. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc Natl Acad Sci U S A. 2008;105(6):1949–54. doi: 10.1073/pnas.0707900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y, Yokoyama S, et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24(11):1173–85. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumatol. 2012;8(9):543–52. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, et al. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458(7242):1185–90. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98(9):1177–85. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koga T, Yamasaki S, Migita K, Kita J, Okada A, Kawashiri S, et al. Post-transcriptional regulation of IL-6 production by Zc3h12a in fibroblast-like synovial cells. Clin Exp Rheumatol. 2011;29(6):906–12. [PubMed] [Google Scholar]
- 23.Mizgalska D, Wegrzyn P, Murzyn K, Kasza A, Koj A, Jura J, et al. Interleukin-1-inducible MCPIP protein has structural and functional properties of RNase and participates in degradation of IL-1beta mRNA. Febs j. 2009;276(24):7386–99. doi: 10.1111/j.1742-4658.2009.07452.x. [DOI] [PubMed] [Google Scholar]
- 24.Haseeb A, Makki MS, Haqqi TM. Modulation of ten-eleven translocation 1 (TET1), Isocitrate Dehydrogenase (IDH) expression, alpha-Ketoglutarate (alpha-KG), and DNA hydroxymethylation levels by interleukin-1beta in primary human chondrocytes. J Biol Chem. 2014;289(10):6877–85. doi: 10.1074/jbc.M113.512269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasheed Z, Akhtar N, Haqqi TM. Pomegranate extract inhibits the interleukin-1beta-induced activation of MKK-3, p38alpha-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res Ther. 2010;12(5):R195. doi: 10.1186/ar3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss FR, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res Ther. 2009;11(3):R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62(5):1361–71. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Makki MS, Heinzel T, Englert C. TSA downregulates Wilms tumor gene 1 (Wt1) expression at multiple levels. Nucleic Acids Res. 2008;36(12):4067–78. doi: 10.1093/nar/gkn356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahidul Makki M, Cristy Ruteshouser E, Huff V. Ubiquitin specific protease 18 (Usp18) is a WT1 transcriptional target. Exp Cell Res. 2013;319(5):612–22. doi: 10.1016/j.yexcr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahlgren J, Maisi P, Sorsa T, Sutinen M, Tervahartiala T, Pirila E, et al. Expression and induction of collagenases (MMP-8 and -13) in plasma cells associated with bone-destructive lesions. J Pathol. 2001;194(2):217–24. doi: 10.1002/path.854. [DOI] [PubMed] [Google Scholar]
- 32.Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthritis Cartilage. 2009;17(11):1513–8. doi: 10.1016/j.joca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 34.Jones SW, Watkins G, Le Good N, Roberts S, Murphy CL, Brockbank SM, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17(4):464–72. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Chao J, Kook YH, Gao Y, Yao H, Buch SJ. Involvement of miR-9/MCPIP1 axis in PDGF-BB-mediated neurogenesis in neuronal progenitor cells. Cell Death Dis. 2013;4:e960. doi: 10.1038/cddis.2013.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146(3):185–96. doi: 10.1016/j.clim.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denli AM, Cao X, Gage FH. miR-9 and TLX: chasing tails in neural stem cells. Nat Struct Mol Biol. 2009;16(4):346–7. doi: 10.1038/nsmb0409-346. [DOI] [PubMed] [Google Scholar]
- 38.Wan HY, Guo LM, Liu T, Liu M, Li X, Tang H. Regulation of the transcription factor NF-kappaB1 by microRNA-9 in human gastric adenocarcinoma. Mol Cancer. 2010;9:16. doi: 10.1186/1476-4598-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. Embo j. 2012;31(17):3513–23. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong Z, Wen Z, Darnell JE., Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins JK, Malyak M, Arend WP. The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine Cytokine Res. 1994;13(1):47–54. [PubMed] [Google Scholar]
- 42.van Roon JA, van Roy JL, Gmelig-Meyling FH, Lafeber FP, Bijlsma JW. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996;39(5):829–35. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]
- 43.Iannone F, De Bari C, Dell'Accio F, Covelli M, Cantatore FP, Patella V, et al. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin Exp Rheumatol. 2001;19(2):139–45. [PubMed] [Google Scholar]
- 44.Ding C, Jones G. Anti-interleukin-6 receptor antibody treatment in inflammatory autoimmune diseases. Rev Recent Clin Trials. 2006;1(3):193–200. doi: 10.2174/157488706778250168. [DOI] [PubMed] [Google Scholar]
- 45.Zhao W, Liu M, D'Silva NJ, Kirkwood KL. Tristetraprolin regulates interleukin-6 expression through p38 MAPK-dependent affinity changes with mRNA 3' untranslated region. J Interferon Cytokine Res. 2011;31(8):629–37. doi: 10.1089/jir.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paschoud S, Dogar AM, Kuntz C, Grisoni-Neupert B, Richman L, Kuhn LC. Destabilization of interleukin-6 mRNA requires a putative RNA stem-loop structure, an AU-rich element, and the RNA-binding protein AUF1. Mol Cell Biol. 2006;26(22):8228–41. doi: 10.1128/MCB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masuda K, Ripley B, Nishimura R, Mino T, Takeuchi O, Shioi G, et al. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci U S A. 2013;110(23):9409–14. doi: 10.1073/pnas.1307419110. [DOI] [PMC free article] [PubMed] [Google Scholar]