Abstract
Objective
Systemic lupus erythematosus (SLE) is characterized by increased cardiovascular risk in adult-onset and childhood-onset SLE (cSLE). Type I interferons (IFNs) appear to play a prominent role in premature vascular damage in adult SLE, at least in part, by inducing impairments in the phenotype and function of endothelial progenitor cells (EPCs), thereby hampering vascular repair. It is not clear if EPC dysfunction is present in cSLE in association with a type I IFN signature.
Methods
Phenotype and numbers of EPCs were quantified in patients with cSLE, juvenile idiopathic arthritis (JIA), and matched healthy controls (HC). In a separate cohort of cSLE subjects, markers of subclinical atherosclerosis and endothelial dysfunction were quantified using standardized protocols, and analyzed for associations with type I IFN serum activity.
Results
EPC numbers and function were significantly decreased in cSLE as compared to JIA and HC. cSLE serum impaired HC EPC differentiation into mature endothelial cells, an effect blocked by type I IFN pathway inhibition. Type I IFN serum activity was not significantly associated with subclinical atherosclerosis and endothelial function in cSLE.
Conclusion
As in adults, cSLE is characterized by phenotypic and functional EPC abnormalities, likely triggered by type I IFNs. While cross-sectional analysis detected no global association between type I IFN signatures and vascular measures of subclinical atherosclerosis, longitudinal assessments are needed to evaluate if progression of vascular damage in cSLE is associated with type I IFNs as in the adult population.
Keywords: interferons, pediatric SLE, atherosclerosis, biomarker
Introduction
Increased cardiovascular (CV) morbidity and mortality occur in systemic lupus erythematosus (SLE) and cannot be explained by the Framingham risk equation (1, 2) Childhood-onset SLE (cSLE) patients carry a longer burden of disease due to younger age of onset, and often present with more severe clinical manifestations and major organ involvement (3). Subclinical atherosclerosis has been reported in cSLE, with significant increases in carotid intima media thickness (CIMT) (4, 5). While treatment with atorvastatin may decrease CIMT progression in a subset of cSLE, it did not reduce overall atherosclerosis risk (6), indicating that other factors involved in the pathophysiology of endothelial damage play a central vasculopathic role.
We and others have proposed that type I interferons (IFNs) play an important role in the pathogenesis of accelerated atherosclerosis in adult-onset SLE (aSLE) (7–9), through pleiotropic deleterious effects on the vasculature. Type I IFNs induce significant impairment in the capacity of endothelial progenitor cells (EPCs) to differentiate into mature endothelial cells (ECs) and repair the vasculature (7, 10). In murine models of lupus and atherosclerosis, type I IFN blockade abrogates endothelial dysfunction, prothrombotic phenotype, plaque formation and abnormal vasculogenesis (11, 12). We showed associations between type I IFN serum activity with vascular dysfunction and enhanced subclinical carotid and coronary atherosclerosis in aSLE (13). These observations suggest a crucial role for these cytokines in initiating and perpetuating atherosclerotic lesions in SLE.
A strong inverse correlation between CV risk factors and number and function of circulating EPCs has been reported in adults with various CV risk factors (7, 10). While little is known about EPCs and their role in atherogenesis in children, there is evidence that certain pediatric populations at risk for vascular disease have impaired EPC phenotype and function that correlates with endothelial dysfunction (14, 15).
We hypothesized that cSLE is also characterized by aberrant EPC phenotype and function secondary to enhanced type I IFN activity. We posit that these abnormalities may promote endothelial dysfunction and accelerated vascular damage in children. We analyzed EPC phenotype and function in cSLE and its association with type I IFN activity. We also assessed the association between type I IFN activity and vascular measures of early atherosclerosis.
Materials and Methods
Subject recruitment
Age- and gender-matched healthy controls (HC) and cSLE patients fulfilling American College of Rheumatology criteria were enrolled at Hospital for Sick Children (HSC, n=132 cSLE and n=170 HCs) and at the University of Michigan ((UM), n=25 SLE, n=29 HC)). Children fulfilling International League Against Rheumatism Criteria for juvenile idiopathic arthritis (JIA, n=21) were enrolled at UM. In the HSC SLE cohort, serum was obtained for type I-IFN activity and subclinical atherosclerosis and endothelial dysfunction were quantified by: a) CIMT using a Vivid 7 ultrasound machine (GE/Vingmed, Milwaukee, WI), a 12 MHz linear-array transducer and Carotid Analyzer (Vascular Tools, MIA LLC, Coralville, IA); b) brachial artery flow-mediated dilatation (FMD), using the same ultrasound system and Brachial Analyzer (Vascular Tools); c) pulse wave velocity (PWV) using a high-fidelity micromanometer (SPC-301, Millar Instruments, Houston, TX) and SphygmoCor® (AtCor Medical Inc, Itasca, IL). Vascular measures were compared with normative data acquired using the same standardized protocols (4). Institutional review boards approved study and all subjects signed informed consent.
EPC quantification
For the UM HC, SLE and JIA cohorts, PBMCs were obtained and EPCs quantified by fluorescence-activated cell sorting (FACS) analysis as described (7, 8, 11–13) on a FACSCalibur (BD Biosciences, San Jose, CA); 10,000 events were quantified and results were analyzed by FlowJo software. EPCs were characterized as described by our group (CD34+CD133+CD4−CD3−CD56−CD79b−).
Assessment of EC differentiation
PBMCs were plated on fibronectin-coated plates (BD Biosciences, 4 million cells/well) and cultured for 2 weeks in endothelial growth media with 20% FBS (GibcoBRL) or 20% allogeneic serum from HC, JIA or cSLE patients. Media was changed every three days. At 14 days, differentiation into mature ECs was quantified by assessing coexpression of Texas red-acetylated LDL (Ac-LDL) (Biomedical Technologies, Ward Hill, MA) and FITC-Ulex Europaeus Agglutinin 1 (UEA-1) (Vector Laboratories, Burlingame, CA) by fluorescent microscopy (Leica DMIRB inverted) using CellC cell counting software (12). Seven microphotographs were obtained per field, and reported as mean ± SEM per sample. For studies using allogeneic human serum, cells were cultured in the presence or absence of 2 µg/ml neutralizing anti-human IFN α/β receptor (IFNα/βR) (PBL, Piscataway, NJ) or IgG2a isotype (Abcam, Cambridge, MA). Antibody was added at each media change every 2–3 days and cells were quantified as above.
Type I IFN serum activity
This bioassay has been described elsewhere (13). In brief, HeLa cells were incubated with DMEM/10%FBS medium (negative control), 1 KU/ well recombinant IFN-α (positive control) (Invitrogen, Carlsbad, CA), 50% SLE sera (by volume), or 50% control sera for 6 hours. RNA was extracted using RNeasy (Qiagen, Venlo, Netherlands) and reverse transcribed to cDNA (Invitrogen). Real-time PCR was performed an ABI PRISM 7900HT (Applied Biosystems, Foster City, CA) using 2x SYBR Green supermix (Bio-Rad, Hercules, CA), in triplicate, to quantify five type I IFN-inducible genes (IFIG) - myxovirus resistance-1 (MX1), double-stranded RNA-activated protein kinase (PRKR), IFN-induced protein with tetratricopeptide repeats (IFIT1), IFN-induced protein-44 (IFI44), and IFN-induced protein 44-like (C1orf-29) – and the housekeeping gene hypoxanthine guanine phosphoribosyltransferase 1 (HPRT1). Primers were obtained from Integrated DNA Technologies (Coralville, IA). Results were averaged, normalized to the housekeeping gene, and plotted as fold-induction against 25 age- and gender-matched HC.
Statistical analysis
Multivariable linear regression analyses were used to determine associations between individual IFIGs and vascular markers (CIMT, FMD, and PWV), adjusting for age, gender, and medications. A principal components analysis (PCA) was applied to assess differences in the profiles of IFIGs to vascular measures, as described in aSLE (13). Difference of the means was analyzed using Student's t-test for EPC quantification and differentiation. Clinical data were adjusted for disease activity. To examine associations between clinical and demographic variables and vascular dysfunction, a Mann –Whitney test of medians was used for categorical data, as outcomes were not normally distributed, and simple linear regression was used for continuous variables. Demographic data between the cohorts was assessed for significance by using Chi-square or Fischer’s Exact test for categorical variables and one-way ANOVA for continuous variables.
Results
Patient characteristics
Demographic and clinical characteristics between HC, JIA and cSLE patients are shown in Table 1. The mean age of UM cSLE patients was older than HSC cSLE, as the minimum age of enrollment was 12 years rather than 8 years due to blood requirements. The older UM cSLE cohort had a longer mean duration of disease than the HSC cSLE cohort, and UM JIA cohort had more long-standing disease at the time of enrollment. Most cSLE patients were taking hydroxychloroquine, half were on a steroid-sparing agent, and most were on no prednisone or less 0.5 mg/kg prednisone daily.
Table 1.
Demographic and clinical characteristics
| HSC cSLE (N=132) |
HSC healthy controls (N=178) |
UM cSLE (N=25) |
UM JIA (N=21) |
UM healthy controls (N=29) |
p value+ | |
|---|---|---|---|---|---|---|
| Age, years (mean ± SEM) | 14.8 ± 2.7 | 14.4 ± 2.1 | 18.2 ± 2.7 | 15.4 ± 2.3 | 14.5 ± 4.7 | <0.001* |
| Gender M:F | 23:109 | 84:94 | 3:22 | 8:13 | 8:21 | 0.078** |
| Mean duration of disease, years | 2.1 ± 2.1 | N/A | 4.0 ± 3.0 | 6.0 ± 5.4 | N/A | <0.01*** |
| Range of disease duration, years | 0.2 – 13.3 | N/A | 0 – 9.0 | 0 – 15.0 | N/A | |
| Current Medications (n(%)) | ||||||
| NSAID | 14 (11) | 10 (40) | 16 (76) | ****<0.001 | ||
| ACE- or ARB | 25 (19) | 15 (60) | None* | <0.001 | ||
| Prednisone | 82 (62) | 13 (52) | 1 (5) | 0.377 | ||
| Hydroxychloroquine (Plaquenil) | 114 (86) | 24 (96) | 12 (57) | 0.008 | ||
| Mycophenolate | 24 (18) | 11 (44) | None | 0.008 | ||
| Azathioprine | 42 (32) | 3 (12) | None | 0.054 | ||
| Cyclophosphamide | 17 (13) | 1 (4) | None | 0.312 | ||
| Methotrexate | 7 (5) | 1 (4) | 5 (24) | 0.008 | ||
| Cyclosporine | 3 (2) | 2 (8) | 1 (5) | 0.323 | ||
| IVIG | 4 (3) | 0 (0) | None | 1 | ||
|
Vascular markers (mean ± SEM) |
||||||
| CIMT, mm | 0.410 ± 0.050 | 0.439 ± 0.045 | <0.001 | |||
| FMD, % change | 8.2 ± 3.9 | 7.5 ± 3.2 | 0.808 | |||
| PWV, m/s | 5.4 ± 1.0 | 5.1 ± 0.9 | 0.328 | |||
Data shown as mean ± standard deviation or N (%).
Medications primarily used for treatment of SLE were only compared between the two cSLE cohorts as these are not used in JIA.
One-way ANOVA used for multiple comparisons of continuous variables and Chi-square used for multiple comparisons of categorical variables.
ACE: angiotensin converting enzyme inhibitors; ARB: angiotensin II receptor blockers; CIMT: carotid intima-media thickness; cSLE: childhood-onset systemic lupus erythematosus; F: female; FMD: flow-mediated dilatation; HSC: Hospital for Sick Children; IVIG: intravenous immunoglobulins; JIA: juvenile idiopathic arthritis; M: male; NS: not significant; NSAIDS: nonsteroidal anti-inflammatory drugs; PWV: pulse wave velocity; UM: University of Michigan.
Statistics used was one-way ANOVA for multiple comparisons, differences evident through uncorrected Fisher’s LSD
comparing HcSLE with UMcSLE, other comparisons in age not significant;
chi square analysis for all groups;
when comparing HcSLE with UMSLE;
comparing HSC cSLE with UM SLE
Vascular tests
CIMT in HC was 0.439 ± 0.04 mm, and was significantly higher that in cSLE (0.410 ± 0.050 mm, p < 0.001) . FMD values were higher among cSLE than HC (8.2 ± 3.9% vs. 7.5 ± 3.2%) but this was not statistically significant (p=0.8). PWV values assessing arterial stiffness did not differ between cSLE and HC (5.4 ± 1.0 m/s versus 5.1 ± 0.9 m/s, respectively , p=0.328). Less than < 2% of cSLE patients undergoing vascular tests had results that deviated > 2 SD from the HC (4/127 for CIMT, 4/126 for PWV, and 0/119 for FMD). As such, the baseline data in the cSLE cohort did not suggest significant vascular dysfunction in cSLE.
cSLE displays abnormal phenotype and function of EPCs triggered by type I IFNs
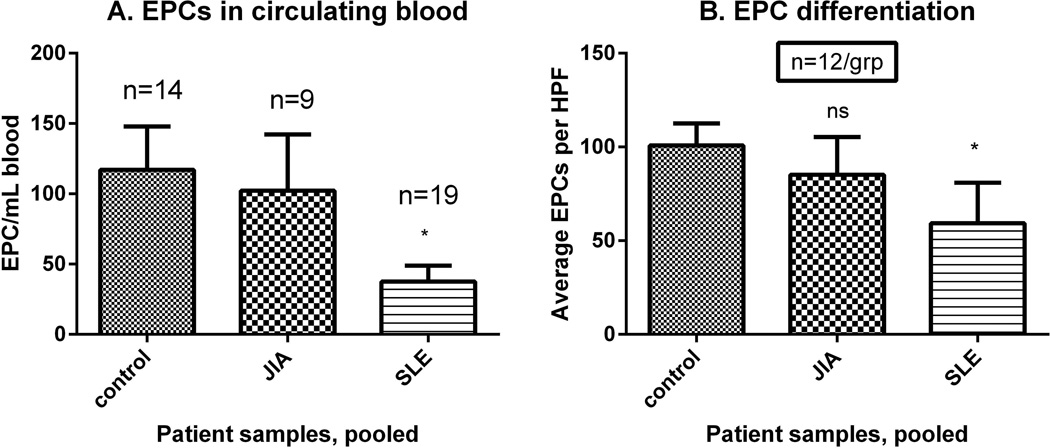
cSLE had significantly decreased circulating EPCs when compared to HC and JIA, which did not correlate with age (p=0.391), disease duration (p=0.692), or immunosuppressive medications (p=0.062 for mycophenolate, p=0.442 for prednisone) (Figure 1A). In comparison with HC or JIA, EPCs, cSLE EPCs displayed significantly decreased capacity to differentiate into mature ECs (p=0.01) (Figure 1B). Increasing age appeared to be associated with decreased differentiation, as determined by linear regression (p=0.034), while disease duration was not (p=0.242). There was no association between use of mycophenolate and azathioprine and number or function of EPCs (p=0.399 and 0.167, respectively when comparing to patients not on these drugs). Insufficient numbers of patients were on high dose prednisone (1 mg/kg/day or more) or cyclophosphamide to test for changes in EPC function. There was no correlation between cSLE EPC levels and SLEDAI, BMI, systolic blood pressure, creatinine, ESR, CRP and lipid profile (p > 0.05).
Figure 1. EPCs are decreased in cSLE when compared to JIA and healthy controls.
(A) The number of patients in each group is listed above the corresponding bar. EPCs are decreased in circulating blood of cSLE as compared to JIA or controls. (B) EPC differentiation was assessed by fluorescent microscopy (n=12/group). Coexpression of acetylated-LDL (Texas Red) and UEA-1-Agglutinin (FITC) identifies mature ECs. Fewer EPCs in cSLE underwent differentiation as compared to JIA or controls.
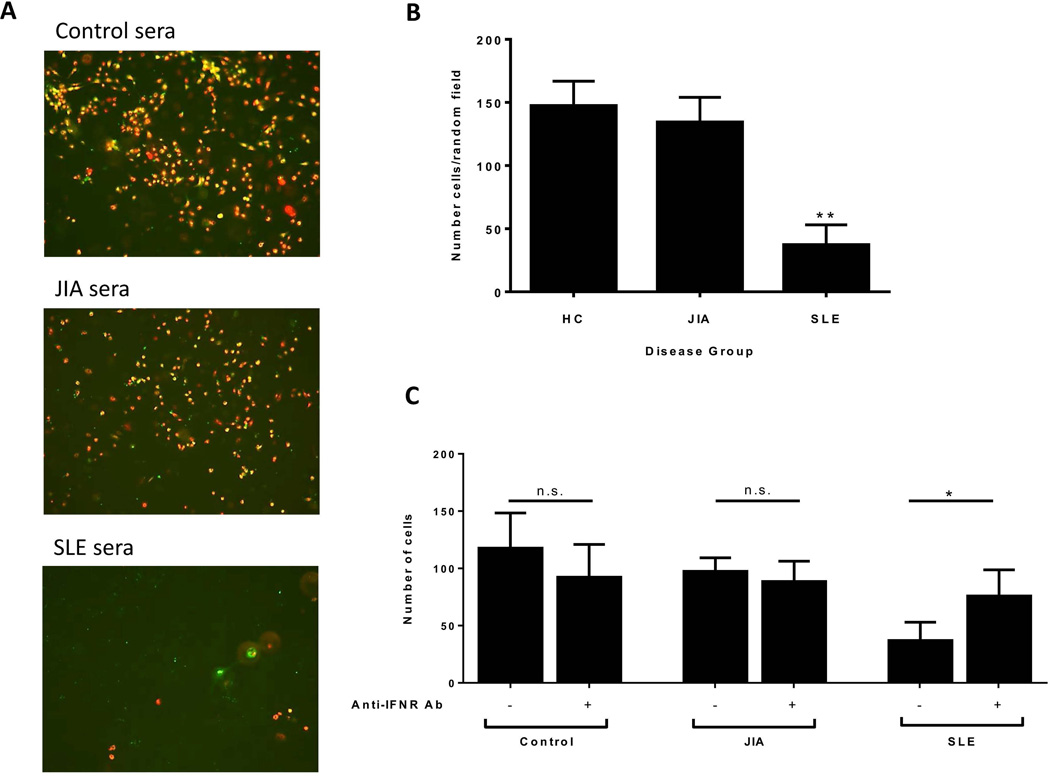
To assess the role of circulating type I IFNs in inhibition of endothelial differentiation in cSLE, similar to what we reported in aSLE, HC EPCs were differentiated into mature ECs in 20% allogeneic HC or patient serum under proangiogenic stimulation as described (12) (Figure 2A). cSLE serum, but not JIA serum, significantly hampered EC differentiation when compared to HC serum (p < 0.010) (Figure 2B). The detrimental effect of cSLE serum on EC differentiation was significantly attenuated by neutralizing antibodies to IFNα/βR (p < 0.05). Neutralizing Abs to IFNα/βR had no significant effect on HC EPCs exposed to JIA or HC serum (Figure 2C).
Figure 2. IFNα in cSLE serum reduces EPC survival.
(A) Representative microphotographs of healthy EPCs cultured for 14 days in 20% patient or healthy control serum. Cells were labeled with Dil-Ac-LDL (red) and UEA-1-FITC (green). MATURE ECS coexpress both markers (yellow). Representative images from serum of n=5 control, n=5 JIA, and n=7 cSLE across n=4 PMBC donors (x10 magnification). (B) Serum from cSLE but not JIA significantly reduces survival of healthy EPCs at day 15. Columns show mean ± SE number of cells in random fields (1-way ANOVA with Dunnett’s multiple comparisons test, **p < 0.01). (C) Blockade of IFNα signaling restores EC differentiation in control cells exposed to SLE, but not control or JIA, serum. Columns show mean ±SE number of cells with n=5 control, n=4 JIA, n=7 cSLE (paired t test, *p < 0.05).
Type I IFN signatures and vascular assessments in cSLE
Type I IFN serum activity was significantly elevated in cSLE (supplementary figure 1). IFN serum activity did not correlate with BMI, systolic blood pressure, creatinine, CRP, ESR, or lipid profiles. Post-hoc analysis showed a trend for an association between higher expression of IFN-inducible genes PRKR and C1orf-29 in those cSLE patients with CIMT values that deviated > 1 SD from the HC mean (p=0.053 and p=0.074, respectively).
Discussion
Type I IFNs appear to play important roles in SLE pathogenesis and in the accelerated atherosclerosis characteristic of this disease (8, 9, 11–13). In aSLE, type I IFN activity has been linked to decreased vascular function, higher CIMT and coronary calcification (13). Murine and human studies have shown that type I IFNs have significant pleiotropic effects that are deleterious to the vasculature, from promoting endothelial damage and impaired endothelial repair, to facilitating foam cell formation and enhancing thrombosis (11, 12). Indeed, type I IFNs are directly cytotoxic to EPCs and impair their function (7, 10, 14). It is well recognized that cSLE is associated with an elevated type I IFN signature (9); however, it is unknown if children are equally susceptible to undergo EPC impairments and accelerated vascular damage by type I IFNs. Decreased EPCs and impaired function have been described in other pediatric autoimmune diseases, such as type I diabetes mellitus (15), and obese children display decreased circulating EPCs in association with vascular dysfunction (14). We found decreased numbers and function of circulating EPCs from cSLE, similar to what we and others described in aSLE. Increasing age was the only variable that significantly correlated with reduced EPC differentiation. Immunosuppression did not appear to impact EPC differentiation, although too few patients were on high dose prednisone or cyclophosphamide to analyze these contributions.
Previous studies in aSLE have shown that IFIGs were associated with decreased endothelial function, increased CIMT, arterial stiffness, and severity of coronary calcification (13). We could not confirm these observations in cSLE, even though type I IFNs were increased in cSLE as compared to HC, and CIMT was in fact lower in cSLE than in HC. The lack of significant differences in vascular measurements between cSLE and HC controls is likely a main factor for the lack of correlation with type I IFNs. Indeed, only a minority of cSLE patients had abnormal values, unlike in the adult study. Furthermore, a post-hoc exploratory analysis showed a trend for an association of specific IFIGs and higher values of CIMT. The significance of these findings needs to be confirmed in an independent cohort where more abnormal values are present. As such, the differences between the adult and pediatric SLE studies regarding associations between type I IFN responses and functional/anatomical evidence of CVD are likely related to lack of prominent vascular damage in this cSLE cohort. Longer follow-up will be needed to assess the role of these cytokines in progression of CVD in cSLE.
The results of this study suggest that, similar to aSLE, cSLE is characterized by impaired EPC phenotype and function, likely driven by type I IFNs. Future longitudinal studies should systematically assess whether these abnormalities contribute to severity and/or progression of premature vascular damage and CV events in cSLE.
Supplementary Material
Acknowledgments
We thank the University of Michigan Center for Statistical Consultation and Research for assistance with statistical analysis, and Keri Gisslen for assistance with recruitment.
Funding
Supported by the Intramural Research Program at NIAMS/NIH and by the Heart and Stroke Foundation of Canada. SM was supported by NIH PHS grant T32HD7513-15. JR was supported by NW England MRC Clinical Pharmacology and Therapeutics Training Scheme grant G1000417/94909.
Footnotes
Conflicts of Interest
JB received funding for a lupus fellowship in part by GlaxoSmithKline. All other authors reported no conflicts of interest.
References
- 1.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MJ. Premature vascular damage in systemic lupus erythematosus. Autoimmunity. 2009;42:580–586. doi: 10.1080/08916930903002479. [DOI] [PubMed] [Google Scholar]
- 3.Stichweh D, Arce E, Pascual V. Update on pediatric systemic lupus erythematosus. Curr Opin Rheumatol. 2004;16:577–587. doi: 10.1097/01.bor.0000137852.42270.0f. [DOI] [PubMed] [Google Scholar]
- 4.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 5.Barsalou J, Bradley TJ, Silverman ED. Cardiovascular risk in pediatric-onset rheumatological diseases. Arthritis Research Therapy. 2013;15:212. doi: 10.1186/ar4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardoin SP, Schanberg LE, Sandborg CI, Barnhart HX, Evans GW, Yow E, et al. Secondary analysis of APPLE study suggests atorvastatin may reduce atherosclerosis progression in pubertal lupus patients with higher C reactive protein. Ann Rheum Dis. 2014;73:557–566. doi: 10.1136/annrheumdis-2012-202315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopalan S, Somers EC, Brook RD, Kehrer C, Pfenninger D, Lewis E, et al. Endothelial cell apoptosis in systemic lupus erythematosus: A common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–3683. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 8.Denny M, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ, et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110:2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak K, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. NEJM. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 11.Thacker S, Zhao W, Smith C, Luo W, Wang H, Vivekanandan-Giri A, et al. Type I interferons modulate endothelial function, repair, thrombosis and plaque severity in murine models of lupus and atherosclerosis. Arthritis Rheum. 2012;64:2975–2985. doi: 10.1002/art.34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thacker S, Berthier C, Mattinzoli D, Rastaldi MP, Kretzler M, Kaplan MJ. The detrimental effects of interferon-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J Immunology. 2010;185:4457–4469. doi: 10.4049/jimmunol.1001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somers EC, Zhao W, Lewis EE, Wang L, Wing JJ, Sundaram B, et al. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PLOS One. 2012;7:e37000. doi: 10.1371/journal.pone.0037000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruyndonckx L, Hoymans VY, Frederix G, De Guchtenaere A, Franckx H, Vissers DK, et al. Endothelial progenitor cells and endothelial microparticles are independent predictors of endothelial function. J Pediatr. 2014;165:300–305. doi: 10.1016/j.jpeds.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Hörtenhuber T, Rami-Mehar B, Satler M, Nagl K, Höbaus C, Höllerl F, et al. Endothelial progenitor cells are related to glycemic control in children with type 1 diabetes over time. Diabetes Care. 2013;36:1647–1653. doi: 10.2337/dc12-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.