Abstract
Background and Purpose
Prophylactic treatments that afford neuroprotection against stroke may emerge from the field of preconditioning. Resveratrol mimics ischemic preconditioning, reducing ischemic brain injury when administered two days prior to global ischemia in rats. This protection is linked to Sirt1 and enhanced mitochondrial function possibly through its repression of UCP2. BDNF is another neuroprotective protein associated with Sirt1. In this study we sought to identify the conditions of resveratrol preconditioning (RPC) that most robustly induce neuroprotection against focal ischemia in mice.
Methods
We tested four different RPC paradigms against a middle cerebral artery occlusion (MCAo) model of stroke. Infarct volume and neurological score were calculated 24 hours following MCAo. Sirt1-chromatin binding was evaluated by ChIP-qPCR. Percoll gradients were used to isolate synaptic fractions and changes in protein expression were determined via Western blot analysis. BDNF concentration was measured using a BDNF-specific ELISA assay.
Results
While repetitive RPC induced neuroprotection from MCAo, strikingly one application of RPC 14 days prior to MCAo showed the most robust protection, reducing infarct volume by 33% and improving neurological score by 28%. Fourteen days following RPC, Sirt1 protein was increased 1.5 fold and differentially bound to the UCP2 and BDNF promoter regions. Accordingly, synaptic UCP2 protein decreased by 23% and cortical BDNF concentration increased 26%.
Conclusions
RPC induces a novel extended window of ischemic tolerance in the brain that lasts for at least 14 days. Our data suggest that this tolerance may be mediated by Sirt1, through upregulation of BDNF and downregulation of UCP2.
Keywords: Resveratrol, Preconditioning, Cerebral Ischemia, Sirt1, BDNF, UCP2
Introduction
With few and limited clinically approved treatments, ischemic stroke remains a devastating pathology that plagues the world population1. Our ability to predict those who are predisposed to or at risk for stroke has significantly improved in recent years, making prophylactic treatment a viable option for neuroprotection. There is an identifiable population in the millions that are at an increased risk for stroke and are ideal candidates for prophylactic therapeutic intervention. Indeed, studies have demonstrated the immense potential of a prophylactic approach, utilizing forthcoming pharmacological agents derived from the field of ischemic preconditioning (IPC).
IPC refers to the ability of a brief, non-damaging ischemic episode to induce tolerance against a subsequent, damaging ischemic insult2. Ischemic tolerance is characterized to have an early window of protection which is activated immediately and lasts for several hours as well as a late window of protection which is active 2 days later2. Several pharmacological agents can mimic the effects of IPC, including resveratrol3. Resveratrol is a polyphenol synthesized naturally in several species of plants4. The therapeutic potential of resveratrol has been widely investigated in many pathological conditions5, although the investigation of resveratrol as an agent in stroke treatment is still in its infancy.
Similar to IPC, resveratrol preconditioning (RPC) induces ischemic tolerance, at least in part, by activation of silent information regulator 2 homologue 1 (Sirt1)3, 6. Sirt1 is a versatile NAD+-dependent deacetylase that acts upon both histone and non-histone proteins to regulate many biological processes. Additionally, Sirt1 has a host of neuroprotective properties in pathological states such as neurological disorders7. More recently, Sirt1 has been implicated in cerebral ischemia, fostering ischemic tolerance through common targets such as HIF, PGC1α and p538. Additional targets of Sirt1 include mitochondrial uncoupling protein 2 (UCP2), a proton channel found in the inner mitochondrial membrane that uncouples oxidative phosphorylation6 as well as brain-derived neurotrophic factor (BDNF)9, a growth factor involved in many neuronal processes including synaptic plasticity.
Our previous work demonstrates that RPC reduces ischemic brain injury when administered two days prior to cardiac arrest in rats6 as well as middle cerebral artery occlusion (MCAo) in mice (Narayanan et al. 2015, Stroke in Press). In terms of neuronal function, ischemic tolerance was associated with an increase in Sirt1 activity, a decrease in UCP2 and enhanced mitochondrial function in the hippocampus. The goal of this study was to identify the conditions of RPC that most robustly induce neuroprotection against focal cerebral ischemia in mice and investigate further the contribution of the Sirt1 signaling axis.
Materials and Methods
Full descriptions of the methods sections can be found in the online supplement. Please see http://stroke.ahajournals.org
Animals and treatments
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Miami and were in accordance with institutional regulations. 8–12 week old C57Bl/6J male mice obtained from The Jackson Laboratory (n=108) were randomly assigned to different treatment groups. Mice were injected i.p. with 10 mg/kg trans-resveratrol (3,4′,5-Trihydroxy-trans-stilbene, Sigma) in 1.5% dimethyl sulfoxide (DMSO, Sigma) 0.9% saline (Hospira Inc.) (RPC) or the control 1.5% DMSO, 0.9% saline (Veh). All injections, experimental procedures and analysis throughout this study were conducted in a blinded fashion.
Focal cerebral ischemia by reversible middle cerebral artery occlusion (MCAo)
Right-side MCAo was produced as previously described10. Cerebral blood flow was monitored using a Laser-Doppler (Perimed System, Stockholm, Sweden) probe. MCAo was induced by inserting a silicone-coated 8-0 monofilament nylon surgical suture into the internal carotid artery and circle of Willis via the proximal external carotid. After 60 min of MCAo the suture was removed.
Neurological scoring and infarct volume
24 hours after MCAo, animals were scored based on a neurobehavioral battery10 and infarct volume was quantified by TTC staining11 as previously described.
Chromatin immunoprecipitation and subsequent quantitative PCR
Mouse brain cortices were dissected, frozen, pulverized in liquid nitrogen and resuspended in cross-linking solution (1% formaldehyde). Nuclei were lysed, chromatin was sonicated to 200–500 bp fragments, centrifuged and diluted in ChIP dilution buffer. The sonicated, cleared chromatin supernatant was used for immunoprecipitation with 5 µg of anti-Sirt1 antibody or control IgG overnight at 4°C. Beads were washed sequentially with 1 ml each of: Low salt wash, High salt wash, LiCl wash and TE wash. Beads were resuspended in elution buffer and cross-linking was reversed. DNA was purified by phenol-chloroform extraction and EtOH precipitation. Quantitative real-time PCR was carried out using SYBR Green reagent (Roche) in the LightCycler® 480 II (Roche Applied Science) and analyzed with the ΔΔCT method. DNA-relative enrichment was determined by normalizing to input genomic DNA and pre-serum IgG as background.
Isolation of synaptosomes via Percoll gradient fractionation
Percoll gradients were used to isolate synaptosomes according to Dunkley and colleagues12 with slight modifications. Mice were decapitated under isoflurane anesthesia and their cortices immersed in isolation medium at 4°C. Tissue was chopped, homogenized, diluted to give 10% w/v and centrifuged at 500 × g for 5 min (Sorvall RC5 centrifuge, Newton CT). The supernatant was layered on a Percoll (Sigma) gradient and centrifuged at 32,500 × g for 5 min. Synaptosomes were collected between 23-15% and 15-10% Percoll junctions, washed once with isolation medium by centrifugation at 15,000 × g for 10 min and once with 0.25 M sucrose. The final pellet was resuspended in RIPA buffer and processed for protein analysis according to the Western blot protocol.
Western blotting
Mouse cortices were homogenized in RIPA buffer. Protein concentration was measured by Bradford assay (BioRad). Equal amounts of protein were run on 12% SDS-polyacrylamide gels and transferred to a nitro-cellulose membrane (BioRad). The next steps in order were: block 2 hours at room temperature; probe with primary antibody overnight at 4°C; incubate secondary antibody for 1 hour at RT; addition of substrate for 3 minutes; protein bands detected with x-ray film (Denville Scientific). Films were analyzed densitometrically using Image J. Two normalized values (for the same sample) from different blots were averaged to give the values presented.
BDNF ELISA assay
BDNF concentration was quantified using a ChemiKineTM BDNF Sandwich ELISA Kit (Millipore) according to the manufacturer’s instructions.
Statistical analysis
All data are represented as mean ± SEM. For statistical analysis, Student’s t-tests were used to compare differences between 2 groups. Differences were consider significant at the 95% confidence interval (* = p<0.05, ** = p<0.01).
Results
Repetitive RPC treatment confers ischemic tolerance against focal ischemia
Based on our previous findings that RPC induces ischemic tolerance when administered 2 days prior to injury3, 6, we sought to evaluate RPC in the context of focal cerebral ischemia utilizing the MCAo mouse model. Our first hypothesis was that repetitive RPC over an extended period of time induces ischemic neuroprotection. To this end, mice were given RPC or Veh every other day for 14 days prior to MCAo, mimicking the standard preconditioning paradigm repeated 7 times (Fig. 1A). Animals that did not display a ≥80% reduction in cerebral blood flow (CBF) were excluded – full documentation of CBF, exclusion and mortality can be found in Table 1. Compared to Veh, RPC significantly reduced infarct volume by 20% (p<0.01, Veh n=12, RPC n=12) (Fig. 1B). Next, we hypothesized that increasing the frequency of RPC treatment could enhance this protection. Thus, mice were given RPC or Veh every day for 14 days prior to MCAo (Fig. 1C). Similar to every other day treatment, everyday treatment significantly reduced infarct volume by 27% compared to Veh (p<0.01, Veh n=12, RPC n=14) (Fig. 1D). From this we conclude that repetitive RPC induces ischemic tolerance against focal ischemia.
Figure 1. Repetitive RPC induces ischemic tolerance against focal ischemia.
A, every other day treatment schematic. B, every other day RPC reduces infarct volume. Veh n=12; RPC n=12; ** = p<0.01 compared to Veh. C, every day treatment schematic. D, every day RPC reduces infarct volume similar to every other day RPC. Veh n=12; RPC n=14; ** = p<0.01 compared to Veh. Individual arrows indicate a RPC or Veh treatment; MCAo is indicated by the black square; TTC is indicated by the checkered square. Representative TTC stained brain sections are shown on the left.
Table 1.
| Group | Baseline CBF |
Ischemia CBF |
Reperfusion CBF |
Excluded (n) |
Mortality (n) |
|---|---|---|---|---|---|
| Every Other Day Veh | 100 | 11.9±4.46 | 62.2±25.32 | 0 | 6 |
| Every Other Day RPC | 100 | 10.0±4.09 | 75.4±56.28 | 4 | 2 |
| Every Day Veh | 100 | 11.9±4.89 | 72.2±23.93 | 3 | 2 |
| Every Day RPC | 100 | 9.7±3.89 | 59.4±36.87 | 2 | 2 |
| Intermittent Veh | 100 | 11.3±3.17 | 67.4±28.34 | 2 | 6 |
| Intermittent RPC | 100 | 11.5±3.76 | 68.5±28.14 | 4 | 4 |
| Single Veh | 100 | 11.61±3.36 | 65.43±17.33 | 1 | 0 |
| Single RPC | 100 | 11.35±5.32 | 71.24±22.27 | 0 | 1 |
| Total | 16 | 23 | |||
Laser-Doppler flowmetry was used to measure CBF for inclusion/exclusion. Measurements are taken 1) baseline prior to occlusion, 2) ischemia immediately after occlusion is initiated and 3) reperfusion immediately after occlusion is removed. Values are represented as % of baseline±SD.
Mice were excluded the study if they did not reach ≤80% drop in blood flow from baseline.
Mortality figures illustrate mice who died <24 hours following MCAo.
RPC induces a novel extended window of ischemic tolerance against focal ischemia
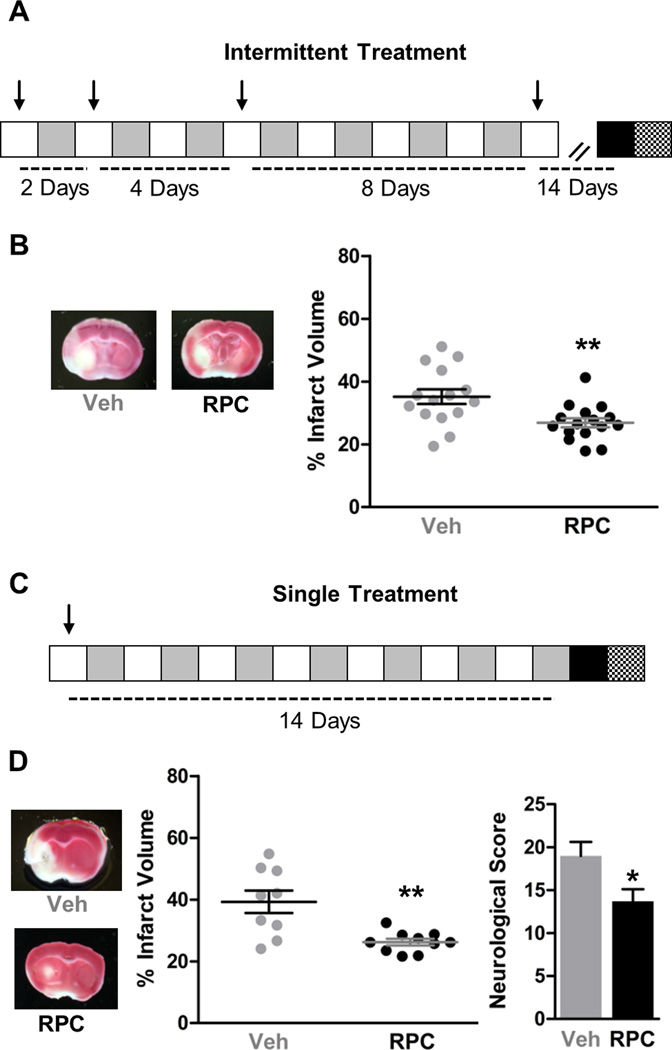
Since there was no significant difference between the protection afforded by every other day and every day treatment, we rationalized that perhaps less frequent RPC could also provide ischemic tolerance. Our previous studies show that IPC induces ischemic tolerance that lasts for at least 4 days in organotypic slice cultures3. Therefore, our next hypothesis was that intermittent RPC could induce an extended window of ischemic tolerance. To test this hypothesis, we used a geometrical growth pattern of intermittent RPC consisting of 4 applications at intervals of 1, 2, 4 and 8 days with 14 days between the last treatment and MCAo (Fig. 2A). Strikingly, compared to Veh, intermittent RPC significantly reduced infarct volume by 24% (p<0.01, Veh n=15, RPC n=16) (Fig. 2B). Before any conclusions can be drawn from this intermittent RPC paradigm, an important distinction to make is whether it is the culmination of 4 applications of RPC that give rise to this long-lasting ischemic tolerance or if simply one application of RPC can induce ischemic tolerance for this extended period of time. To test this, mice were given RPC or Veh once, 14 days prior to MCAo (Fig. 2C). Remarkably, one application of RPC 14 days prior to MCAo significantly reduced infarct volume by 33% compared to Veh (p<0.01, Veh n=9, RPC n=10) (Fig. 2D). To confirm that this reduction in infarct volume was accompanied by improved neurological performances, we calculated neurological score 24 hours following MCAo and found that compared to Veh, RPC significantly improved this functional outcome measure by 28% (p<0.05) (Fig. 2D) From these results we conclude that a single application of RPC induces a novel extended window of ischemic tolerance that lasts for at least 14 days.
Figure 2. RPC induces a novel extended window of ischemic tolerance against focal ischemia.
A, intermittent treatment schematic. B, intermittent RPC reduces infarct volume. Veh n=15; RPC n=16; ** = p<0.01 compared to Veh. C, single treatment schematic. D, a single application of RPC reduces infarct volume and improves neurological score. Veh n=9; RPC n=10; * = p<0.05; ** = p<0.01 compared to Veh. Individual arrows indicate a RPC or Veh treatment; MCAo is indicated by the black square; TTC and neurological scoring is indicated by the checkered square. Representative TTC stained brain sections are shown on the left.
Sirt1 and its targets are augmented 14 days following RPC
Given that Sirt1 is a well-established target of resveratrol and that in our previous studies blockade of Sirt1 ablates ischemic tolerance3,6, we investigated whether changes in Sirt1 expression occurred in this extended window of ischemic tolerance. 14 days following a single application of RPC, cortical Sirt1 levels were significantly increased 1.5-fold of Veh (p<0.01, n=10) (Fig. 3A). To assess whether this increase had functional implications, we turned our attention to putative chromatin binding sites for Sirt1. Based on known interactions with Sirt113, 14 and our previous studies implicating BDNF9 and UCP26 in ischemic tolerance, we evaluated Sirt1 binding to promoter regions within the BDNF and UCP2 genes. Chromatin immunoprecipitation and subsequent qPCR 14 days following a single application of RPC revealed that Sirt1 binding to both the BDNF and UCP2 promoters was significantly enhanced, 2.66-fold and 1.14-fold, respectively (BDNF p<0.01, UCP2 p<0.05, n=3) (Fig. 3B). This enhancement was not seen in Veh treated mice. Accordingly, we found that cortical BDNF levels were significantly increased by 27% (p<0.05, n=7) and synaptic UCP2 levels were significantly decreased by 23% (p<0.05, n=6) compared to Veh 14 days following a single application of RPC (Fig 3C and D). These data suggest that Sirt1 may be modulating BDNF and UCP2 expression at 14 days where ischemic tolerance is observed.
Figure 3. Sirt1 and its targets are differentially regulated 14 days following a single application of RPC.
A, cortical Sirt1 protein was increased 14 days following a single application of RPC. Representative bands for Sirt1 are shown below the quantification graph. ⸗ = non-adjacent bands from the same blot. n=10; ** = p<0.01 compared to Veh. B, ChIP-qPCR analysis revealed Sirt1 binding to BDNF and UCP2 promoter regions was enhanced 14 days following a single application of RPC. n=3; * = p<0.05; ** = p<0.01. C, cortical BDNF levels were increased 14 days following a single application of RPC. Values are expressed as pg/mg of protein. n=7; * = p<0.05. D, synaptic UCP2 protein levels were decreased 14 days following a single application of RPC. Values were normalized to the mitochondrial marker voltage VDAC. The functional UCP2 dimer (70 kDa) was used for quantification, no 35 kDa monomer band was detected. Representative band are shown below the quantification. n=6; * = p<0.05.
Discussion
The major finding of this study – that a single application of RPC can protect against focal ischemia 14 days later – is both novel and intriguing. To our knowledge, this is the first evidence that a single pharmacological preconditioning treatment can induce a state of long-lasting ischemic tolerance. This is a new extended window of preconditioning beyond the 2–3 day time point or classical late window commonly studied.
In work prior to this study, evidence hinted that an extended window may exist. In organotypic slices, we showed that one application of IPC affords protection that lasts at least 4 days3. Additionally, a paradigm of repetitive hypoxia preconditioning (varying O2 exposure over 2 weeks) conferred protection from focal ischemia for up to 2 months after treatment15. Elucidating the long term effects of preconditioning will provide a framework for the development of novel clinical applications in the future. The major argument for resveratrol’s immense potential is that it has already been deemed safe for clinical use in humans16.
Resveratrol either directly or indirectly activates and/or upregulates Sirt117, 18. Previously we have shown increased Sirt1 to be a common mechanism of preconditioning-mediated protection, where protection was lost in the presence of the Sirtuin inhibitor Sirtinol3. The proposed acute mechanisms for Sirt1-mediated ischemic tolerance include modulating the neuroinflammatory response via NF-κB19, reducing/inhibiting pro-apoptotic factors such as p5320 as well as enhancing mitochondrial function via PGC1α21 and UCP26. Here, we found Sirt1 to be upregulated at 14 days following a single RPC treatment. A chronic upregulation of Sirt1 could promote changes in gene expression that are maintained overtime that give rise to this extended window of ischemia tolerance. In line with this notion, 14 days following RPC we find enhanced Sirt1 binding to UCP2 and BDNF promoter regions.
As mentioned earlier, Sirt1 is known to negatively regulate UCP2 by binding directly to its promoter14. This is supported in our current study as we observed a decrease in synaptic UCP2 protein levels. In our previous studies we observed a reduction in UCP2 two days after RPC in rats where protection from cardiac arrest was seen. Neuroprotection and decreased UCP2 were also linked to enhanced mitochondrial efficiency6. In another line of evidence, UCP2 knockout mice were found to be resistant to permanent focal ischemia22. In these mice, protection was attributed to increased antioxidant defenses and a reduction in oxidative damage. It should be noted that UCP2’s effects in regards to cerebral ischemia are still somewhat controversial. Other groups have shown that overexpression of UCP2 is also protective against cerebral ischemia23 and that the same UCP2 knockout mice mentioned above were actually more susceptible to a model of transient focal ischemia24. Discrepancies could be due to variation across species, genetic compensation in knockout mouse models, the type of ischemia model employed or choice of preconditioning agent.
Sirt1 is also known to upregulate BDNF expression; the two proposed mechanisms are indirect and include Sirt1 deacetylation of MeCP2 (which regulates expression of BDNF by binding at its promoter)25, and Sirt1 inhibition of miR-134 expression (a micro-RNA that downregulates expression of BDNF)13. In addition, binding of Sirt1 to the BDNF promoter has been identified in the mouse cerebellum, suggesting that a direct interaction might also occur26. Moreover, BDNF was decreased in the brains of Sirt1 null mice 27. BDNF is an important growth factor that promotes survival and growth of existing neurons as well as the differentiation of new ones. It also impacts synaptic plasticity and learning and memory28. Our previous work shows enhanced BDNF as a mechanism for ischemia tolerance following IPC or protein kinase C epsilon (PKCε)-induced preconditioning9, in which increased BDNF was associated with a delay in anoxic depolarization. Another group shows that intravenous BDNF administration following ischemia improves recovery possibly through a mechanism centered on neurogenesis29. Given that all of these changes appear to occur homogeneously throughout the cortex, we expect them to impact both the core and penumbral regions of the infarct.
The fact that one dose of resveratrol has protective effects that are apparent 2 weeks later suggests a promising prophylactic avenue. The validity of a preconditioning approach in the clinic was nicely described by Dirnagl and colleagues30 who defined clinical scenarios where a preconditioning approach could have a direct impact. Resveratrol also protects against stroke when administered post injury. Although not tested as a postconditioning paradigm, future studies are needed to test whether resveratrol could be used in a postconditioning setting. Also future studies can test if pre and post resveratrol treatment in tandem can enhance neuroprotection.
Resveratrol has been used in several clinical trials and deemed safe for use in humans at doses up to a few grams per day. Our finding that tachyphylaxis to RPC did not occur when administered on an everyday basis suggests that multiple administrations are feasible, if required. Additionally, oral administration of resveratrol as a neuroprotective intervention in rodents has been validated by several groups and appears similar to that of i.p or i.v. administration31. Taken together, our current findings build upon the body of preclinical evidence for resveratrol as a potential treatment in the stroke clinic.
Supplementary Material
Acknowledgements
Sources of funding
NIH Grants NS45676, NS054147, NS34773 (MAPP), 1F31NS089356-01 (KBK), 5R01NS073779-04 (KRD) and AHA Grant 13POST16720001 (JTN).
Footnotes
Disclosures
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nature reviews. Neuroscience. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 3.Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- 4.Carrizzo A, Forte M, Damato A, Trimarco V, Salzano F, Bartolo M, et al. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;61:215–226. doi: 10.1016/j.fct.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health--a comprehensive review of human clinical trials. Molecular nutrition & food research. 2011;55:1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 6.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F, Wang S, Gan L, Vosler PS, Gao Y, Zigmond MJ, et al. Protective effects and mechanisms of sirtuins in the nervous system. Progress in neurobiology. 2011;95:373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: Role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann JT, Thompson JW, Raval AP, Cohan CH, Koronowski KB, Perez-Pinzon MA. Increased bdnf protein expression after ischemic or pkc epsilon preconditioning promotes electrophysiologic changes that lead to neuroprotection. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:121–130. doi: 10.1038/jcbfm.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-l-lysine: Neurological and histological validation. Brain Res. 1999;833:181–190. doi: 10.1016/s0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- 11.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, et al. Toll-like receptor 9: A new target of ischemic preconditioning in the brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunkley PR, Jarvie PE, Robinson PJ. A rapid percoll gradient procedure for preparation of synaptosomes. Nature protocols. 2008;3:1718–1728. doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, et al. A novel pathway regulates memory and plasticity via sirt1 and mir-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, et al. Sirt1 regulates insulin secretion by repressing ucp2 in pancreatic beta cells. PLoS biology. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stowe AM, Altay T, Freie AB, Gidday JM. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Annals of neurology. 2011;69:975–985. doi: 10.1002/ana.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Annals of the New York Academy of Sciences. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 17.Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, et al. Sirt1 activation by small molecules: Kinetic and biophysical evidence for direct interaction of enzyme and activator. The Journal of biological chemistry. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S, Liu B, Zhou Z. Resveratrol activates sirt1 in a lamin a-dependent manner. Cell cycle. 2013;12:872–876. doi: 10.4161/cc.24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of nf-kappab-dependent transcription and cell survival by the sirt1 deacetylase. The EMBO journal. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahtola E, Louhelainen M, Forsten H, Merasto S, Raivio J, Kaheinen P, et al. Sirtuin1-p53, forkhead box o3a, p38 and post-infarct cardiac remodeling in the spontaneously diabetic goto-kakizaki rat. Cardiovascular diabetology. 2010;9:5. doi: 10.1186/1475-2840-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu HR, Wang ZY, Zhu XL, Wu XX, Li EG, Xu Y. Icariin protects against brain injury by enhancing sirt1-dependent pgc-1alpha expression in experimental stroke. Neuropharmacology. 2010;59:70–76. doi: 10.1016/j.neuropharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 22.de Bilbao F, Arsenijevic D, Vallet P, Hjelle OP, Ottersen OP, Bouras C, et al. Resistance to cerebral ischemic injury in ucp2 knockout mice: Evidence for a role of ucp2 as a regulator of mitochondrial glutathione levels. Journal of neurochemistry. 2004;89:1283–1292. doi: 10.1111/j.1471-4159.2004.02432.x. [DOI] [PubMed] [Google Scholar]
- 23.Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nature medicine. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- 24.Haines BA, Mehta SL, Pratt SM, Warden CH, Li PA. Deletion of mitochondrial uncoupling protein-2 increases ischemic brain damage after transient focal ischemia by altering gene expression patterns and enhancing inflammatory cytokines. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1825–1833. doi: 10.1038/jcbfm.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zocchi L, Sassone-Corsi P. Sirt1-mediated deacetylation of mecp2 contributes to bdnf expression. Epigenetics : official journal of the DNA Methylation Society. 2012;7:695–700. doi: 10.4161/epi.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacIsaac KD, Lo KA, Gordon W, Motola S, Mazor T, Fraenkel E. A quantitative model of transcriptional regulation reveals the influence of binding location on expression. PLoS computational biology. 2010;6:e1000773. doi: 10.1371/journal.pcbi.1000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, et al. Sirt1 activates mao-a in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bramham CR, Messaoudi E. Bdnf function in adult synaptic plasticity: The synaptic consolidation hypothesis. Progress in neurobiology. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke; a journal of cerebral circulation. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 30.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: From experimental strategies to clinical use. The Lancet. Neurology. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh N, Agrawal M, Dore S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS chemical neuroscience. 2013;4:1151–1162. doi: 10.1021/cn400094w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.