Abstract
Objectives
To determine whether disease processes related to granulomatosis with polyangiitis (GPA) are reflected in gene expression profiles of nasal mucosa.
Methods
Nasal brushings of the inferior turbinate were obtained from 32 patients with GPA (10 with active nasal disease, 13 with prior nasal disease, 9 with no history of nasal disease) and a composite comparator group with and without inflammatory nasal disease (12 healthy people, 15 with sarcoidosis, 8 with allergic rhinitis). Differential gene expression was assessed between subgroups of GPA and comparators.
Results
339 genes were differentially expressed between the GPA and comparator groups (absolute fold change > 1.5; false discovery rate < 0.05). Top canonical pathways upregulated in nasal brushings from patients with GPA include granulocyte adhesion and diapedesis (p=8.6 E-22), agranulocyte adhesion and diapedesis (p=1.3 E-14), interleukin 10 signaling (3.0 E-11), and TREM1 signaling (9.0 E-11). A set of genes differentially expressed in GPA independent of nasal disease activity status included genes related to epithelial barrier integrity (fibronectin 1, desmosomal proteins) and several matricellular proteins (e.g. osteonectin, osteopontin). Significant overlap of differentially expressed genes was observed between active and prior nasal disease GPA subgroups. Peripheral blood neutrophil and mononuclear gene expression levels associated with GPA were similarly altered in the nasal gene expression profiles of patients with active or prior nasal disease.
Conclusions
Profiling the nasal transcriptome in GPA reveals gene expression signatures related to innate immunity, inflammatory cell chemotaxis, extracellular matrix composition, and epithelial barrier integrity. Airway-based expression profiling is feasible and informative in GPA.
Keywords: vasculitis, granulomatosis with polyangiitis (GPA Wegener’s), gene expression, ANCA-associated vasculitis, nasal mucosa
Nasal disease occurs in the majority of patients with granulomatosis with polyangiitis (GPA, Wegener’s) and is often a presenting feature of the disease[1]. Features of nasal disease in GPA include obstruction, crusting, ulceration, epistaxis, and cartilaginous/bony destruction with potential resultant saddle nose deformity[2]. The incidence of rhinosinusitis at time of diagnosis is estimated to be 75% in GPA, and 90% of patients with GPA will develop sinonasal disease at some point during the course of disease[1, 3]. For many patients with GPA, localized symptoms of upper airway involvement can precede the development of antineutrophil cytoplasmic antibodies (ANCA) and systemic disease by months to years[4].
There is an unmet need for novel diagnostic biomarkers and markers of nasal disease activity in GPA. When nasal histology for GPA is defined by the presence of small vessel vasculitis, granuloma, or extravascular necrosis, the diagnostic accuracy of nasal biopsies is only approximately 50%[5]. Patients with GPA often report persistent upper airway disease despite treatment and improvement in inflammation in other organ systems. In these settings, distinguishing symptoms of active nasal disease from symptoms related to chronic nasal damage is often challenging.
The objective of this study was to characterize the nasal transcriptome in GPA using samples collected with a minimally invasive brushing technique. Whole genome gene expression profiling of nasal brushings was used to identify differentially expressed genes in GPA versus a composite comparator group. Gene sets were identified within subsets of patients with GPA in association with active nasal disease, nasal damage, and independent of nasal disease activity status.
METHODS
Study Population
All patients were recruited from a single-center academic university hospital. The institutional review board approved the study, and all patients provided informed consent. Patients taking anticoagulants or with a known bleeding diathesis were excluded.
Granulomatosis with Polyangiitis Group: All patients with GPA fulfilled the 1990 American College of Rheumatology (ACR) Classification Criteria for Wegener’s granulomatosis[6]. To insure diagnostic accuracy, all patients were required to have documented anti-neutrophil cytoplasmic antibodies (ANCA) with specificity to either myeloperoxidase (MPO) or proteinase 3 (PR3) at some point during the disease course. Patients were classified into 3 groups based on nasal-related symptoms and disease status: 1) active nasal disease at the time of nasal brushing; 2) a history of prior nasal disease with inactive nasal disease at the time of nasal brushing; or 3) no known history of nasal symptoms attributed to GPA at any point during the disease course. Active nasal disease was defined as > 1 week ongoing symptoms of nasal obstruction, bloody nasal discharge, or nasal crusts attributed to active disease accompanied by visual conformation of nasal mucosal abnormalities on nasal speculum examination at the time of sample collection.
Composite Comparator Group: Healthy volunteers, patients with sarcoidosis, and patients with allergic rhinitis were recruited into a composite comparator group. Sarcoidosis was included because it is a multi-system granulomatous disease with features that overlap GPA including nasal involvement. All patients with sarcoidosis had biopsy-confirmed disease. Patients with allergic rhinitis were recruited from an allergy clinic with diagnosis confirmed by clinical features and specific IgE testing. All patients with allergic rhinitis had ongoing nasal symptoms at the time of sample collection.
Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss) Group: A second comparator group comprised of patients with eosinophilic granulomatosis with polyangiitis (EGPA) was studied because EPGA is a small-vessel vasculitis that shares many features with GPA, including a high prevalence of nasal disease. All patients with EGPA fulfilled the 1990 American College of Rheumatology (ACR) Classification Criteria for Churg Strauss Syndrome[7].
Sample Collection and Processing
Using a nasal speculum, brushings were obtained from the right inferior turbinate with a standard cytology brush (Medical Packaging Company, Camarillo, CA) as previously described[8]. Brushings were placed in RNA lysis buffer and frozen at −80C until use. RNA was isolated via Qiagen RNeasy Mini Kits per manufacturer’s protocol. The integrity and purity of RNA were assessed using an Agilent Bioanalyzer and spectrophotometric analysis, respectively. An average of 3μg of high-quality total RNA was obtained from each collection. Visual inspection by light microscopy of representative slides prepared from nasal brushings confirmed that samples included 80–90% epithelial cells per high-powered field with a small fraction of hematopoietic cells included in each brushing sample.
Microarray Data Acquisition and Preprocessing
100ng of total RNA from nasal brushings was processed, labeled, and hybridized to Affymetrix GeneChip Human Gene 1.0 ST Arrays each containing 19,658 probe sets, as previously described[9]. Samples were processed in 3 separate batches, and samples from patients with EGPA were only included in the third batch.
Microarray data were analyzed using Partek Genomics Suite software (Partek Inc., St. Louis, MO). Probe summarization and normalization were performed using Robust Multichip Average and Log2 transformation (Affymetrix Chip Definition File Human Gene 1.0 ST v1.r4). For comparisons involving microarray data from patients with EGPA, only the batch 3 samples were analyzed. Data quality was assessed using principal components analysis and sample histogram plots, and no samples were excluded. Differential gene expression was identified using mixed linear regression models adjusting for treatment status and batch effect. Treatment status was defined as “on treatment” versus “off treatment” based upon use of glucocorticoids or other immunosuppressant agents at the time of nasal sampling.
Microarray Data Analysis
Prior to sample processing for microarrays, patients with GPA were selected and matched to the composite comparator group on the basis of age, sex, and race. Differential gene expression was compared between patients with GPA and the composite comparator group. A 1.5 fold-change and a false discovery rate (FDR) < 0.05 defined differential expression. As a secondary analysis, gene expression was compared between each of the three GPA subgroups (active nasal, prior nasal, never nasal) and the composite comparator group. For these analyses given the smaller sample sizes of the subgroups, a fold change of >1.5 and p-value < 0.05 without FDR correction defined differential gene expression. Pathway analysis of relevant gene sets was examined using default settings in Ingenuity Pathway Analysis (IPA, QIAGEN Redwood City). Gene Set Enrichment Analysis (GSEA) was used to compare differentially expressed genes derived from nasal brushings to published transcriptomic data sets in GPA derived from blood (Supplementary Material).
Validation of Candidate Genes by real time PCR
Gene expression of selected candidate genes was validated by quantitative RT-PCR. The analysis was performed using SYBR Green-based RT2 qPCR Primer Assays (Qiagen, Valencia, CA). GAPDH was used as a housekeeping gene to normalize all samples. All real-time PCR experiments were carried out in triplicate on each sample.
RESULTS
Patient Characteristics
Nasal brushings of the inferior turbinate were obtained from 32 patients with GPA (10 with active nasal disease, 13 with prior nasal disease, 9 with never nasal disease) and 35 controls with and without inflammatory nasal disease (12 healthy people, 15 with sarcoidosis, 8 with allergic rhinitis). There were no significant clinical or demographic differences between patients with GPA and the composite comparator group, except more patients with GPA were taking glucocorticoids and other immunosuppressant agents compared to the composite comparator group (Supplemental Table 1). Five of 15 (33%) patients with sarcoidosis had active or prior nasal disease and 5 of 12 (42%) patients with EGPA had active nasal disease at the time of sample collection.
Differential Expression in the GPA versus Composite Comparator Groups
There were 393 probesets (339 genes) differentially expressed between GPA and the composite comparator group (see Supplemental Table 2 for complete list). Visualization of differential expression is displayed in Supplemental Figures 1&2. A list of the most differentially expressed genes by fold change is provided in Table 1. Differential expression of proteinase 3 (PR3) and myeloperoxidase (MPO), the major auto-antigens in GPA, was not observed between GPA and the comparator group. Upregulation of genes related to epithelial structural proteins was observed in GPA, including keratin and small proline-rich proteins. Of the 339 differentially expressed genes, the top 5 canonical pathways upregulated in nasal brushings from GPA patients were granulocyte adhesion and diapedesis (ratio=29/177; p=8.6 E-22), agranulocyte adhesion and diapedesis (ratio=23/189; p=1.3 E-14), interleukin (IL) 10 signaling (ratio=13/69; p=3.0 E-11), LXR/RXR activation (ratio=16/121; p=4.3 E-11), and TREM1 signaling (ratio=13/75; p=9.0 E-11). Of the 393 probes differentially expressed between GPA and the composite comparator group, there was concordant differential expression in 270 (69%) of the probes between GPA and EGPA, including most of the top differentially expressed genes displayed in Table 1.
TABLE 1.
Top Differentially Expressed Nasal Genes in GPA
| Gene Symbol | Description | Fold Change (GPA vs Comp) | P value (GPA vs Comp) | Fold Change (GPA vs EGPA) | P value (GPA vs EPGA) |
|---|---|---|---|---|---|
| Top 20 Genes Upregulated in GPA | |||||
| TREM1 | Triggering receptor expressed on myeloid cells 1 | 3.06 | 4.8 E-04 | 2.56 | 0.03 |
| AQP9 | Aquaporin 9 | 3.02 | 1.2 E-03 | 2.44 | 2.9 E-03 |
| CXCR1 | Chemokine (C-X-C motif) receptor 1 | 3.02 | 1.1 E-03 | 2.49 | 4.9 E-0.3 |
| IL1B | Interleukin 1, beta | 2.97 | 1.4 E-03 | 3.31 | 0.01 |
| IL1R2 | Interleukin 1 receptor, type II | 2.90 | 2.4 E-04 | 2.31 | 0.04 |
| HBB | Hemoglobin, beta | 2.77 | 9.4 E-03 | 1.35 | 0.14 |
| FCGR2A | Fc fragment of IgG, low affinity IIa, receptor (CD32) | 2.74 | 5.3 E-04 | 2.09 | 0.06 |
| CXCR2 | Chemokine (C-X-C motif) receptor 2 | 2.70 | 5.8 E-04 | 2.12 | 6.2 E-03 |
| FPR2 | Formyl peptide receptor 2 | 2.57 | 2.5 E-03 | 2.43 | 0.04 |
| CSF3R | Colony stimulating factor 3 receptor | 2.56 | 8.8 E-04 | 2.31 | 0.04 |
| KIAA0226L | KIAA0226-like | 2.47 | 6.0 E-04 | 2.44 | 0.02 |
| SLC11A1 | Solute carrier family 11 | 2.47 | 1.9 E-04 | 2.45 | 0.01 |
| OSM | Oncostatin M | 2.42 | 9.4 E-04 | 2.41 | 0.02 |
| FPR1 | Formyl peptide receptor 1 | 2.41 | 1.6 E-03 | 2.18 | 0.05 |
| GLT1D1 | Glycosyltransferase 1 domain containing 1 | 2.39 | 8.2 E-04 | 2.36 | 0.03 |
| RGS2 | Regulator of G-protein signaling 2, | 2.39 | 1.1 E-03 | 2.04 | 0.03 |
| PLEK | Pleckstrin | 2.37 | 3.3 E-03 | 2.49 | 0.03 |
| HCAR3 | Hydroxycarboxylic acid receptor 3 | 2.34 | 1.4 E-03 | 2.11 | 0.01 |
| THBS1 | Thrombospondin 1 | 2.33 | 1.5 E-05 | 1.90 | 0.03 |
| IL18RAP | Interleukin 18 receptor accessory protein | 2.32 | 3.2 E-03 | 1.85 | 7.5 E-03 |
| Top 10 GenesDownregulated in GPA | |||||
| NTS | Neurotensin | −2.51 | 7.6 E-05 | −1.90 | 0.09 |
| SPARCL1 | SPARC-like 1 (hevin) | −2.00 | 4.1 E-06 | −1.42 | 0.13 |
| UGT2A1 | UDP glucuronosyltransferase 2 family, polypeptide A1, | −1.87 | 7.8 E-04 | −1.83 | 0.05 |
| GALNT13 | UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosa | −1.80 | 4.9 E-05 | −1.25 | 5.6 E-03 |
| C11orf70 | Chromosome 11 open reading frame 70 | −1.69 | 8.8 E-03 | −1.98 | 0.03 |
| IL33 | Interleukin 33 | −1.68 | 3.6 E-05 | −1.87 | 1.9 E-03 |
| UGT2B7 | UDP glucuronosyltransferase 2 family, polypeptide B7 | −1.65 | 9.6 E-04 | −1.35 | 8.0 E-03 |
| C14orf45 | chromosome 14 open reading frame 45 | −1.63 | 6.8 E-03 | −2.06 | 0.01 |
| SERPINB10 | Serpin peptidase inhibitor, clade B (ovalbumin), member 10 | −1.63 | 4.5 E-03 | −1.16 | 0.55 |
| FMO6P | Flavin containing monooxygenase 6 pseudogene | −1.63 | 2.2 E-04 | −1.63 | 0.05 |
Differentially Expressed Genes in GPA Subgroups
Intersection of genes that were differentially expressed between the 3 GPA subgroups (active nasal disease, prior nasal disease, no prior history of nasal disease) and the composite comparator group are displayed in Figure 1. 26 probes (18 genes) were differentially expressed in all three GPA subgroups versus the composite comparator group, including upregulation of genes involved in cellular adhesion [fibronectin 1 (FN1), desmoglein-3 (DSG3), and desmocollin 2 (DSC2)] and matricullular proteins [osteopontin (SPP1), osteonectin (SPARC)]. Differential expression of other key matricellular proteins was observed throughout the dataset. Thrombospondin 1 and 2 (THBS1, THBS2) were upregulated (fold change = 2.33/1.24; p=1.4E-03/4.4E-04) and hevin (SPARCL1) was downregulated (fold change −2.00, p= 4.1E-06) in nasal mucosa from patients with GPA versus comparators.
FIGURE 1.
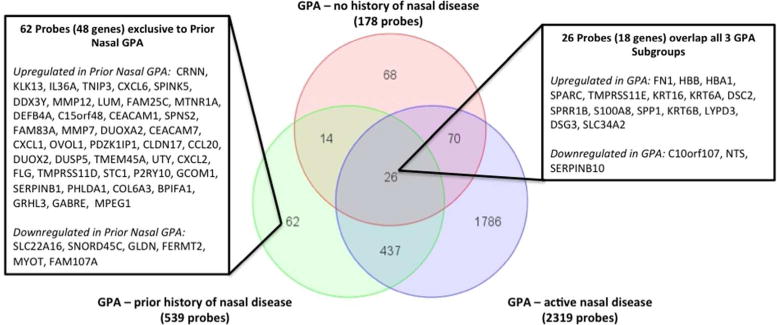
Venn diagram showing the number and overlap of differentially expressed probes between the 3 GPA subgroups and the composite comparator group. There were 26 probes that were differentially expressed in all 3 GPA subgroups versus the comparator group, and these genes are listed in an insert. There were 62 probes that were uniquely differentially expressed between subjects with GPA and prior nasal disease versus the comparator group, and these genes are listed in an inset.
Among genes differentially expressed in GPA with prior nasal disease versus the composite comparator group, 463 of 539 probes (86%) were also differentially expressed in GPA with active disease. There were 62 probes (48 genes) that were uniquely differentially expressed between GPA with prior nasal disease and the composite comparator group, which may reflect changes in gene expression related to nasal damage.
Selected Candidate Genes and Validation by RT-PCR
Five candidate genes with increased expression in nasal brushings from patients with GPA were selected for validation by RT-PCR. These genes included: triggering receptor expressed on myeloid cells 1 (TREM1), IL-8, osteopontin (SSP1), small proline-rich protein 1B (SPRR1B), and alpha-1 antitrypsin (SERPINA1). Candidate genes were selected on the basis of degree of differential expression in the dataset and any known associations with GPA. Gene expression of each candidate gene as measured by microarray is displayed by disease subgroup (Figure 2). Differential expression of each gene was confirmed in a subset of patients with GPA and comparators by RT-PCR (data not shown).
FIGURE 2.
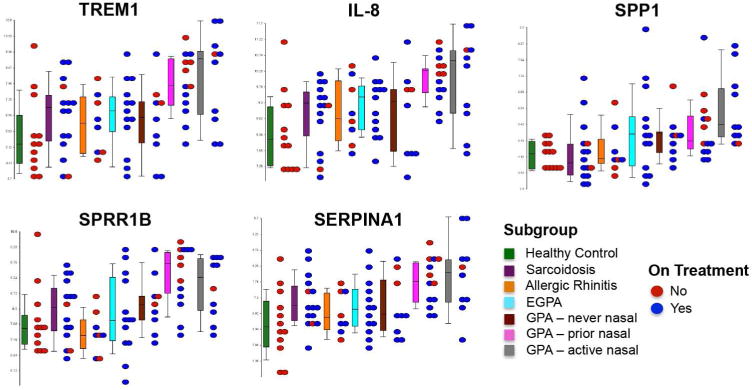
Dot plots illustrating microarray-based gene expression values of 5 selected candidate genes across all of the different subgroups in this study. The boxplots show the range of gene expression for each gene. The dots show individual patient data within each subgroup and are color coded to reflect treatment status [red = not on treatment for disease; blue = patient receiving either oral glucocorticoids, nasal glucocorticoids, or other immunosuppressant agents]. Significantly increased expression in patients with GPA with active or prior nasal disease was observed for all candidate genes (p<0.001). There was a trend of increased SPP1 and SPRR1B expression in all subgroups of patients with GPA relative to the composite comparator groups with highest expression of SPP1 observed in the GPA subgroup with active nasal disease and the highest expression of SPRR1B observed in the GPA subgroup with prior nasal disease.
DISCUSSION
This report details the first whole-genome gene expression profiling study of airway tissue from patients with GPA. Using a minimally invasive nasal brushing technique, gene expression signatures were defined in association with disease activity status. Because rhinosinusitis is an early, prevalent, and characteristic feature of GPA, gene expression pathways identified by profiling nasal mucosa from patients with GPA may provide insight into disease pathogenesis and disease susceptibility. Histologically, necrotizing vasculitis and inflammation consisting of histiocytes, lymphocytes, plasma cells, and multinucleated giant cells surrounding geographic areas of basophilic necrosis with granuloma formation is characteristic of nasal involvement in GPA[10]. Differentially expressed genes identified in the nasal mucosa of patients with GPA suggest the importance of innate immunity, granulocyte and agranulocyte migration and diapedesis, extracellular matrix composition, and epithelial barrier integrity in the pathogenesis of nasal disease in GPA. These findings parallel results from a previous study that measured expression values of 49 selected transcripts in nasal mucosa from patients with GPA versus controls and found differential expression of a genes related to antimicrobial defense (defensins, S1000A7), innate immunity (TLR4, MYD88), and epithelial barrier integrity (fibronectin 1, thrombospondin receptor)[11].
Neutrophils are a critical component of the innate immune system and are a key pathogenic cell in ANCA-associated vasculitis[12]. Differential expression of genes related to neutrophil chemotaxis (IL-8, CXCR1, CXCR2, FPR1, FPR2), inflammation (IL1, IL1R2) and toll-like receptors (TLR2, TLR4, TLR8) was observed in nasal mucosa in GPA compared to healthy and disease controls. The most upregulated gene in nasal mucosa in GPA was TREM1. TREM1 is a signaling receptor on neutrophils and monocytes that amplifies pro-inflammatory cytokine production, degranulation, and phagocytosis in response to bacterial stimuli[13]. Increased serum levels of soluble TREM1 have been associated with disease activity in AAV[14]. TREM1 may serve as an important mediator between the nasal microbiome and disease pathogenesis in GPA.
Expression of SERPINA1 was increased in the nasal mucosa of patients with GPA during active and prior nasal disease. SERPINA1 encodes for alpha-1 antitrypsin, which is a serine protease inhibitor of PR3. Point mutations in the SERPINA1 gene have been associated with development of GPA [15], and polymorphisms in the SERPINA1 gene have been associated with severe treatment-refractory rhinosinusitis[16]. Increased SERPINA1 expression in nasal mucosa of patients with GPA raises the possibility of a protease-antiprotease imbalance in the nasal environment that could play a causal role in the development of GPA.
A set of differentially expressed genes in the nasal mucosa of patients with GPA compared to healthy and disease controls was identified independent of nasal disease activity status, including matricellular proteins. Matricellular proteins (eg osteopontin, osteonective, hevin, and thrombospondins) are secreted into the extracellular matrix and have been implicated in granuloma formation, a defining phenotypic feature of GPA[17][18].
The strengths of this study include use of a novel, minimally invasive tissue sampling method to perform comprehensive whole-genome transcriptomics in a highly relevant organ in GPA. Profiling of a tissue directly involved with disease offers an advantage for understanding pathways involved in the disease. A composite comparator group comprised of healthy and relevant disease controls and an additional vasculitis comparator group were included to increase the likelihood that identified gene expression signatures were specific to GPA and not generic to nasal inflammation.
There are some potential limitations of this study to consider. Nasal brushings collect a heterogeneous cell population comprised primarily of epithelial cells with some contribution from hematopoietic cells. Several interesting epithelial-cell specific candidate genes emerged from these analyses including SPPR1, DSC1, and DSG3. Epithelial cells, neutrophils, or macrophages can express other candidate genes, such as SERPINA1, SPP1, and IL-8. Future studies to assess these candidate genes in purified cell populations from patients with GPA are warranted. Comparisons of expression signatures identified from nasal brushings with signatures derived from deeper nasal biopsies with confirmatory immunohistochemistry studies in nasal biopsy tissue are needed. Patients did not undergo naso-laryngoscopy which may have been useful to define the extent of upper respiratory involvement and direct the site of tissue sampling beyond brushing of the inferior turbinate. Results from this study suggest potential for interaction between the nasal microbiome and nasal innate immunity in GPA; however, the nasal microbiome was not assessed in this cohort. More patients with GPA compared to the other study groups were taking glucocorticoids or other immunosuppressant agents at the time of sampling; however, differential gene expression was assessed in regression models that adjusted for treatment status. Finally, there were small numbers of patients within each subgroup, increasing the potential for false discovery in the secondary analyses.
Profiling of the nasal transcriptome in GPA reveals gene expression signatures related to innate immunity, inflammatory cell chemotaxis, epithelial barrier integrity, and extracellular matrix composition. By studying gene expression in association with nasal disease activity, sets of genes related to active disease, damage, and disease susceptibility were identified. Future studies to validate and investigate these candidate biomarkers in the context of disease pathogenesis in GPA are warranted.
Supplementary Material
Acknowledgments
Financial supports of conflicts disclosure: This research was supported through the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the Vasculitis Research Fund at Boston University. Dr. Grayson received support from a Rheumatology Scientist Development Award from the Research and Education Foundation of the American College of Rheumatology and from a Boston University CTSI microarray initiative grant (UL1 RR025771).
References
- 1.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Annals of internal medicine. 1992;116(6):488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 2.Jones NS. Nasal manifestations of rheumatic diseases. Annals of the rheumatic diseases. 1999;58(10):589–90. doi: 10.1136/ard.58.10.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grayson PC, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, Koening CL, et al. New features of disease after diagnosis in 6 forms of systemic vasculitis. The Journal of rheumatology. 2013;40(11):1905–12. doi: 10.3899/jrheum.121473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennings CR, Jones NS, Dugar J, Powell RJ, Lowe J. Wegener’s granulomatosis–a review of diagnosis and treatment in 53 subjects. Rhinology. 1998;36(4):188–91. [PubMed] [Google Scholar]
- 5.Del Buono EA, Flint A. Diagnostic usefulness of nasal biopsy in Wegener’s granulomatosis. Human pathology. 1991;22(2):107–10. doi: 10.1016/0046-8177(91)90030-s. [DOI] [PubMed] [Google Scholar]
- 6.Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis and rheumatism. 1990;33(8):1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 7.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis and rheumatism. 1990;33(8):1094–100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 8.Sridhar S, Schembri F, Zeskind J, Shah V, Gustafson AM, Steiling K, et al. Smoking-induced gene expression changes in the bronchial airway are reflected in nasal and buccal epithelium. BMC genomics. 2008;9:259. doi: 10.1186/1471-2164-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spira A, Beane J, Pinto-Plata V, Kadar A, Liu G, Shah V, et al. Gene expression profiling of human lung tissue from smokers with severe emphysema. American journal of respiratory cell and molecular biology. 2004;31(6):601–10. doi: 10.1165/rcmb.2004-0273OC. [DOI] [PubMed] [Google Scholar]
- 10.Aubry MC. Necrotizing granulomatous inflammation: what does it mean if your special stains are negative? Modern pathology : an official journal of the United States and Canadian Academy of Pathology Inc. 2012;25(Suppl 1):S31–S8. doi: 10.1038/modpathol.2011.155. [DOI] [PubMed] [Google Scholar]
- 11.Laudien M, Hasler R, Wohlers J, Bock J, Lipinski S, Bremer L, et al. Molecular signatures of a disturbed nasal barrier function in the primary tissue of Wegener’s granulomatosis. Mucosal immunology. 2011;4(5):564–73. doi: 10.1038/mi.2011.9. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber A, Kettritz R. The neutrophil in antineutrophil cytoplasmic autoantibody-associated vasculitis. Journal of leukocyte biology. 2013;94(4):623–31. doi: 10.1189/jlb.1012525. [DOI] [PubMed] [Google Scholar]
- 13.Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. Journal of immunology. 2003;170(7):3812–8. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 14.Daikeler T, Regenass S, Tyndall A, Gencay MM, Roth M, Christ-Crain M, et al. Increased serum levels of soluble triggering receptor expressed on myeloid cells-1 in antineutrophil cytoplasmic antibody-associated vasculitis. Annals of the rheumatic diseases. 2008;67(5):723–4. doi: 10.1136/ard.2007.076547. [DOI] [PubMed] [Google Scholar]
- 15.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. The New England journal of medicine. 2012;367(3):214–23. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilty SJ, Bosse Y, Cormier C, Endam LM, Desrosiers MY. Polymorphisms in the SERPINA1 (Alpha-1-Antitrypsin) gene are associated with severe chronic rhinosinusitis unresponsive to medical therapy. American journal of rhinology & allergy. 2010;24(1):e4–e9. doi: 10.2500/ajra.2010.24.3429. [DOI] [PubMed] [Google Scholar]
- 17.Rotta G, Matteoli G, Mazzini E, Nuciforo P, Colombo MP, Rescigno M. Contrasting roles of SPARC-related granuloma in bacterial containment and in the induction of anti-Salmonella typhimurium immunity. The Journal of experimental medicine. 2008;205(3):657–67. doi: 10.1084/jem.20071734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh Y, Oh I, Morimoto J, Uede T, Morimoto A. Osteopontin has a crucial role in osteoclast-like multinucleated giant cell formation. Journal of cellular biochemistry. 2014;115(3):585–95. doi: 10.1002/jcb.24695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


