Abstract
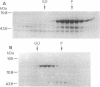
A protein of apparent molecular mass of approximately 59 kDa of the FK506-binding protein class (FKBP59) has been found associated with the heat shock protein hsp90 included in nontransformed steroid receptor complexes and termed FKBP59-HBI (HBI for Heat shock protein 90 Binding Immunophilin). Further data analysis has revealed that this immunophilin also belongs to the tetratricopeptide repeat family of proteins. In this work, we describe the hsp90-binding domain of FKBP59-HBI. Density gradient centrifugation, gel filtration, and immunoadsorption analyses failed to demonstrate a stable association between FKBP59-HBI and hsp90 in the rabbit reticulocyte lysate. Using a gel-retardation assay, we provide evidence for a specific ATP-independent interaction between highly purified wild-type rabbit FKBP59-HBI and human hsp90 beta. This interaction was not affected by the immunosuppressants FK506 and rapamycin. Examination of the behavior of several mutants led us to conclude that the tetratricopeptide motifs localized in the C-terminal part of FKBP59-HBI are necessary for hsp90 binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossard M. J., Bergsma D. J., Brandt M., Livi G. P., Eng W. K., Johnson R. K., Levy M. A. Catalytic and ligand binding properties of the FK506 binding protein FKBP12: effects of the single amino acid substitution of Tyr82 to Leu. Biochem J. 1994 Jan 15;297(Pt 2):365–372. doi: 10.1042/bj2970365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberger A., Schröder H., Büttner M., Valencia A., Bukau B. A conserved loop in the ATPase domain of the DnaK chaperone is essential for stable binding of GrpE. Nat Struct Biol. 1994 Feb;1(2):95–101. doi: 10.1038/nsb0294-95. [DOI] [PubMed] [Google Scholar]
- Callebaut I., Renoir J. M., Lebeau M. C., Massol N., Burny A., Baulieu E. E., Mornon J. P. An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambraud B., Rouvière-Fourmy N., Radanyi C., Hsiao K., Peattie D. A., Livingston D. J., Baulieu E. E. Overexpression of p59-HBI (FKBP59), full length and domains, and characterization of PPlase activity. Biochem Biophys Res Commun. 1993 Oct 15;196(1):160–166. doi: 10.1006/bbrc.1993.2229. [DOI] [PubMed] [Google Scholar]
- Czar M. J., Owens-Grillo J. K., Dittmar K. D., Hutchison K. A., Zacharek A. M., Leach K. L., Deibel M. R., Jr, Pratt W. B. Characterization of the protein-protein interactions determining the heat shock protein (hsp90.hsp70.hsp56) heterocomplex. J Biol Chem. 1994 Apr 15;269(15):11155–11161. [PubMed] [Google Scholar]
- Goebl M., Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991 May;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kinoshita N., Morikawa K., Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell. 1990 Jan 26;60(2):319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Honoré B., Leffers H., Madsen P., Rasmussen H. H., Vandekerckhove J., Celis J. E. Molecular cloning and expression of a transformation-sensitive human protein containing the TPR motif and sharing identity to the stress-inducible yeast protein STI1. J Biol Chem. 1992 Apr 25;267(12):8485–8491. [PubMed] [Google Scholar]
- Hutchison K. A., Brott B. K., De Leon J. H., Perdew G. H., Jove R., Pratt W. B. Reconstitution of the multiprotein complex of pp60src, hsp90, and p50 in a cell-free system. J Biol Chem. 1992 Feb 15;267(5):2902–2908. [PubMed] [Google Scholar]
- Hutchison K. A., Czar M. J., Scherrer L. C., Pratt W. B. Monovalent cation selectivity for ATP-dependent association of the glucocorticoid receptor with hsp70 and hsp90. J Biol Chem. 1992 Jul 15;267(20):14047–14053. [PubMed] [Google Scholar]
- Hutchison K. A., Scherrer L. C., Czar M. J., Ning Y., Sanchez E. R., Leach K. L., Deibel M. R., Jr, Pratt W. B. FK506 binding to the 56-kilodalton immunophilin (Hsp56) in the glucocorticoid receptor heterocomplex has no effect on receptor folding or function. Biochemistry. 1993 Apr 20;32(15):3953–3957. doi: 10.1021/bi00066a015. [DOI] [PubMed] [Google Scholar]
- Joab I., Radanyi C., Renoir M., Buchou T., Catelli M. G., Binart N., Mester J., Baulieu E. E. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. 1984 Apr 26-May 2Nature. 308(5962):850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- Kieffer L. J., Seng T. W., Li W., Osterman D. G., Handschumacher R. E., Bayney R. M. Cyclophilin-40, a protein with homology to the P59 component of the steroid receptor complex. Cloning of the cDNA and further characterization. J Biol Chem. 1993 Jun 15;268(17):12303–12310. [PubMed] [Google Scholar]
- Le Bihan S., Renoir J. M., Radanyi C., Chambraud B., Joulin V., Catelli M. G., Baulieu E. E. The mammalian heat shock protein binding immunophilin (p59/HBI) is an ATP and GTP binding protein. Biochem Biophys Res Commun. 1993 Sep 15;195(2):600–607. doi: 10.1006/bbrc.1993.2088. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Herrick J., Faber L. E., Renoir J. M., Radanyi C., Baulieu E. E. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992 Mar 5;267(7):4281–4284. [PubMed] [Google Scholar]
- Massol N., Lebeau M. C., Renoir J. M., Faber L. E., Baulieu E. E. Rabbit FKBP59-heat shock protein binding immunophillin (HBI) is a calmodulin binding protein. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1330–1335. doi: 10.1016/0006-291x(92)90448-t. [DOI] [PubMed] [Google Scholar]
- Nakao K., Myers J. E., Faber L. E. Development of a monoclonal antibody to the rabbit 8.5S uterine progestin receptor. Can J Biochem Cell Biol. 1985 Jan;63(1):33–40. doi: 10.1139/o85-005. [DOI] [PubMed] [Google Scholar]
- Peattie D. A., Harding M. W., Fleming M. A., DeCenzo M. T., Lippke J. A., Livingston D. J., Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanyi C., Lombès M., Renoir J. M., Delahaye F., Baulieu E. E. A novel monoclonal anti-rabbit hsp90 antibody: usefulness for studies on hsp90-steroid receptor interaction. J Steroid Biochem Mol Biol. 1992 Sep;42(8):863–874. doi: 10.1016/0960-0760(92)90095-z. [DOI] [PubMed] [Google Scholar]
- Radanyi C., Renoir J. M., Sabbah M., Baulieu E. E. Chick heat-shock protein of Mr = 90,000, free or released from progesterone receptor, is in a dimeric form. J Biol Chem. 1989 Feb 15;264(5):2568–2573. [PubMed] [Google Scholar]
- Ratajczak T., Carrello A., Mark P. J., Warner B. J., Simpson R. J., Moritz R. L., House A. K. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59). J Biol Chem. 1993 Jun 25;268(18):13187–13192. [PubMed] [Google Scholar]
- Renoir J. M., Le Bihan S., Mercier-Bodard C., Gold A., Arjomandi M., Radanyi C., Baulieu E. E. Effects of immunosuppressants FK506 and rapamycin on the heterooligomeric form of the progesterone receptor. J Steroid Biochem Mol Biol. 1994 Jan;48(1):101–110. doi: 10.1016/0960-0760(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Baulieu E. E. Un effet des immunosuppresseurs FK506 et rapamycine sur le fonctionnement du récepteur de la progestérone: la protéine "p59-HBI", un carrefour de l'immunologie et de l'endocrinologie? C R Acad Sci III. 1992;315(11):421–428. [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Faber L. E., Baulieu E. E. The non-DNA-binding heterooligomeric form of mammalian steroid hormone receptors contains a hsp90-bound 59-kilodalton protein. J Biol Chem. 1990 Jun 25;265(18):10740–10745. [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Yang C. R., Baulieu E. E. Antibodies against progesterone receptor from chick oviduct. Cross-reactivity with mammalian progesterone receptors. Eur J Biochem. 1982 Sep;127(1):81–86. doi: 10.1111/j.1432-1033.1982.tb06840.x. [DOI] [PubMed] [Google Scholar]
- Rexin M., Busch W., Gehring U. Protein components of the nonactivated glucocorticoid receptor. J Biol Chem. 1991 Dec 25;266(36):24601–24605. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Scherrer L. C., Hutchison K. A., Sanchez E. R., Randall S. K., Pratt W. B. A heat shock protein complex isolated from rabbit reticulocyte lysate can reconstitute a functional glucocorticoid receptor-Hsp90 complex. Biochemistry. 1992 Aug 18;31(32):7325–7329. doi: 10.1021/bi00147a017. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Boguski M. S., Goebl M., Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990 Jan 26;60(2):307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Michaud W. A., Wootton J. C., Boguski M. S., Connelly C., Hieter P. TPR proteins as essential components of the yeast cell cycle. Cold Spring Harb Symp Quant Biol. 1991;56:663–673. doi: 10.1101/sqb.1991.056.01.075. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith D. F. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993 Nov;7(11):1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Stensgard B. A., Welch W. J., Toft D. O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem. 1992 Jan 15;267(2):1350–1356. [PubMed] [Google Scholar]
- Smith D. F., Sullivan W. P., Marion T. N., Zaitsu K., Madden B., McCormick D. J., Toft D. O. Identification of a 60-kilodalton stress-related protein, p60, which interacts with hsp90 and hsp70. Mol Cell Biol. 1993 Feb;13(2):869–876. doi: 10.1128/mcb.13.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Chang H., Albers M. W., Schreiber S. L., Toft D. O., Faber L. E. P59 (FK506 binding protein 59) interaction with heat shock proteins is highly conserved and may involve proteins other than steroid receptors. Biochemistry. 1993 Aug 31;32(34):8842–8847. doi: 10.1021/bi00085a015. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Maeda Y., Nakao K., Wakim N. G., Duhring J. L., Faber L. E. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986 Sep 9;25(18):5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Hung S., Chan H. K., Marcy A., Martin M., Calaycay J., Boulton D., Sigal N., Kincaid R. L., Siekierka J. J. Characterization of high molecular weight FK-506 binding activities reveals a novel FK-506-binding protein as well as a protein complex. J Biol Chem. 1992 Oct 25;267(30):21753–21760. [PubMed] [Google Scholar]
- Yem A. W., Reardon I. M., Leone J. W., Heinrikson R. L., Deibel M. R., Jr An active FK506-binding domain of 17,000 daltons is isolated following limited proteolysis of chicken thymus hsp56. Biochemistry. 1993 Nov 30;32(47):12571–12576. doi: 10.1021/bi00210a004. [DOI] [PubMed] [Google Scholar]
- Yem A. W., Tomasselli A. G., Heinrikson R. L., Zurcher-Neely H., Ruff V. A., Johnson R. A., Deibel M. R., Jr The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13. J Biol Chem. 1992 Feb 15;267(5):2868–2871. [PubMed] [Google Scholar]