Abstract
Background
Anhedonia represents a core symptom of major depression and may be a potential marker for melancholia. However, current understanding of this construct in depressive sub-types is limited.
Method
Participants were recruited from the Black Dog Institute (Sydney) and Massachusetts General Hospital (Boston). Diagnostic groups were derived on the basis of agreement between clinician and DSM-IV diagnosis from structured interviews. Currently depressed unipolar melancholic, non-melancholic and healthy control participants were administered a probabilistic reward task (PRT) to assess a behavioural correlate of anhedonia - blunted reward-based learning. Self-reported measures of anhedonia, approach and avoidance motivation were completed by the Sydney sample.
Results
Relative to healthy controls and non-melancholic participants, melancholic depressed participants had reduced response bias, highlighting blunted reward learning. Moreover, although non-melancholic participants were characterized by a delayed response bias, melancholic depressed participants failed to develop a bias throughout blocks. Response bias showed no associations with self-report measures of hedonic tone in depressed participants. Positive associations were observed between response bias, approach and avoidance motivation in non-melancholic participants only.
Limitations
Possible medication, fatigue and anxiety effects were not controlled; small sample sizes; inclusion criteria may have excluded those with severe melancholia and led to underestimation of group differences.
Conclusions
Melancholia is characterised by a reduced ability to modulate behaviour as a function of reward, and the motivational salience of rewarding stimuli may differ across depressive sub-types. Results support the view that melancholia is a distinct sub-type. Further exploration of reward system functioning in depressive sub-types is warranted.
Keywords: Melancholic, non-melancholic, depression, anhedonia, reward responsiveness, motivation
1. Introduction
The status of melancholia remains unclear. Dimensional models consider melancholia as a more severe expression of clinical depression, whilst binary models consider it as a separate entity (Parker and Hadzi-Pavlovic, 1996). In support of the latter , melancholia’s ascriptions include (i) biological determinants (e.g. dysfunction of the hypothalamic-pituitary axis), (ii) a set of over-represented clinical features (e.g. psychomotor disturbance), (iii) a more selective response to physical treatments, and (iv) a low placebo response rate (Parker and Hadzi-Pavlovic, 1996; Parker et al., 2010a). However, no feature to date has been identified as showing categorical specificity to melancholia.
Attempts to define and differentiate melancholia have been fraught with difficulty. As reviewed by Parker et al. (2013a), difficulties include: (i) self-rated symptoms being influenced by subjective factors including the tendency to ‘over-report’ or ‘under-report’, (ii) methodological limitations of measures adopting a dimensional vs. categorical approach to symptom ratings, (iii) observer biases, with individual clinicians referring to their own reference-base when judging symptom severity and (iv) limiting analyses to symptom data only. Whilst clinician ratings have tended to produce superior differentiation of melancholic and non-melancholic conditions (Parker et al., 2013b; Parker and Hadzi-Pavlovic, 1996), the level of precision continues to be sub-optimal. Clearer definition of core symptom constructs is therefore necessary. One such core symptom is anhedonia – a distinct reduction in the ability to experience pleasure or lack of reactivity to pleasurable stimuli (Meehl, 1975; APA, 2013).
Anhedonia represents a prominent symptom and potential trait marker of major depression (Loas, 1996). Whilst central to the definition of major depressive disorder (MDD; APA, 2013), anhedonia has traditionally been conceptualised as a core symptom of melancholia. Klein (1974) described the ‘endogenomorphic’ (melancholic) sub-type of depression as characterised by non-reactive and pervasive impairment of the capacity to experience or anticipate pleasure, later conceptualising the existence of two functional appetitive systems - consummatory and anticipatory pleasure (Klein, 1987). The former refers to pleasures linked with biological drive reduction (e.g. sexual, eating); the latter characterises pleasures associated with pursuit (e.g. hunting, searching). According to Klein, consummatory pleasure deficits are always associated with a melancholic depression (Loas and Boyer, 1996). Current proposals for a distinct melancholic depressive disorder weight pervasive anhedonia as one of several key features (Parker et al., 2010b; Taylor and Fink, 2008). ’Truly anhedonic’ depression (qua melancholia) was earlier hypothesised to be associated with a specific functional disorder of the CNS and to show a more favourable response to tricyclic antidepressants than other types of depression (Klein, 1974). More recent studies support linkages between melancholia and anhedonia. Perry et al. (1996) found that melancholic participants had a superior response to broad-action tricyclic antidepressants than narrow-action antidepressants (e.g. selective serotonin reuptake inhibitors; SSRIs), with the latter showing reduced efficacy for anhedonic symptoms such as motivational and reward-processing deficits in depression (Treadway and Zald, 2011).
Despite its recognition as a cardinal feature of depression and a potential marker of melancholia, measurement of anhedonia in depressive disorders has received minimal attention (Nakonezny et al., 2010). In one study comparing hedonic capacity in endogenomorphic (melancholic) and non-endogenomorphic (non-melancholic) participants, the former group was significantly more anhedonic, irrespective of depression severity (Loas and Boyer, 1996). This study focused on consummatory pleasure, and was the first to use operational definitions of anhedonia to test for differences in depressive sub-types. As previously overviewed (Loas and Boyer, 1996), earlier studies used indirect methods or unreliable definitions of anhedonia. For example, two studies (Fawcett et al., 1983; Hardy et al., 1986) reported a bimodal distribution of scores among depressive participants on the Fawcett-Clark Pleasure Capacity Scale (Fawcett et al., 1983) – primarily thought to measure appetitive pleasure (Klein, 1987). The bimodal distributions represented a normal range (88% of participants) and an extremely anhedonic distribution (12% of participants) in the Fawcett et al. (1983) study, providing some support for the existence of a qualitatively distinct subtype of depression (i.e. melancholia) characterised by pervasive anhedonia.
Over the years, research relying on self-reported anhedonia has been extended by studies involving laboratory-based measures of anhedonic behaviour (for a review, see Admon and Pizzagalli, in press). Blunted reward responsiveness (i.e., the inability to adapt behaviour in response to reward) has emerged as a key behavioural correlate of anhedonia (Pizzagalli et al., 2005). Reward system dysfunction is thought to directly contribute to anhedonic symptoms (Pizzagalli et al., 2005; Bogdan and Pizzagalli, 2006; Nestler and Carlezon, 2006; Treadway and Zald, 2011). Specific deficits in reward-related processes (e.g. the inability to anticipate or predict rewards, or the lack of motivation to perform the necessary actions to obtain rewards) may be globally interpreted as a loss of interest or pleasure, despite representing discrete processes (Der-Avakian et al., 2012). Clinical measures assessing multiple reward-related deficits are therefore required to more clearly conceptualise anhedonic behaviours. As reviewed previously (Der-Avakian et al., 2012), studies adopting this methodology have reported deficits in reward-based learning (i.e., failure to develop a biased response over time for a more frequently rewarded stimulus) in individuals with MDD or healthy individuals that either (i) have high trait anhedonia, (ii) a past history of MDD, (iii) are exposed to an acute stressor, or (iv) are administered a pharmacological challenge hypothesized to lower dopamine levels (Pizzagalli et al., 2008a; Bogdan and Pizzagalli, 2006; Pechtel et al., 2013; Pizzagalli et al., 2008b; Vrieze et al., 2013).
In addition to reward system dysfunction, motivational system dysfunction has been proposed to explain links between anhedonia and depression, with anhedonic individuals showing diminished motivation to engage in goal-directed behaviour and use information about potential reward when making decisions (Padrao et al., 2013). While studies in depressed samples have focused on reward system dysfunction, it is unclear whether anhedonia reflects alterations in motivational behaviour in melancholic and/or non-melancholic participants. Previous research has linked anticipatory pleasure with motivational processes that promote goal-directed behaviours aimed at achieving rewards (e.g. Carver, 2001; Dickinson & Balleine, 1995; Schultz, 2002), however to our knowledge this has not been directly examined in clinically depressed samples of melancholic and non-melancholic participants.
The current study therefore sought to assess hedonic capacity (consummatory and anticipatory) and motivational processes associated with reward in melancholic and non-melancholic participants, to determine whether these features assist with more precise delineation of depressive sub-types. These goals were pursued by combining two independent samples, one collected in Sydney, Australia and one in Boston, USA. We hypothesised that melancholic participants would exhibit more reward system dysfunction than non-melancholic participants and healthy controls, as manifested by a significantly reduced response bias toward a more frequently rewarded stimulus in a laboratory task. In the Sydney sample, we further sought to explore whether melancholic and non-melancholic participants would differ on self-reported anticipatory vs. consummatory anhedonia, hypothesising that the former group would show greater hedonic deficits on both aspects. Moreover, for this sample, we included a measure of approach and avoidance motivation – the Behavioral Inhibition and Behavioral Activation Scales (BIS/BAS; Carver and White, 1994) – in order to determine how these constructs relate to motivational processes promoting goal-directed behaviour towards reward. We hypothesised that approach motivation would be associated with reward-based learning – quantified by high BAS scores showing positive correlations with response bias. Finally, we expected that self-reported anhedonia would be associated with reward-based learning in melancholic and non-melancholic groups, as manifested by negative associations between anhedonia scores and response bias.
2. Methods and Materials
2.1. Participants
This was a two-site study, involving participants recruited in Sydney (Sample 1) and Boston (Sample 2).
Sample 1 (Sydney)
Patients referred to the Black Dog Institute Depression Clinic were invited to participate in research. Written informed consent was obtained as per University of New South Wales Ethics Committee requirements. Prior to consultation with a psychiatrist, participants underwent a structured diagnostic interview – the MINI (Sheehan et al., 1998) – assessing DSM-IV criteria for MDD and melancholia. An independent clinical interview conducted by a psychiatrist was undertaken to derive a diagnosis of unipolar melancholic or non-melancholic depression. Clinical diagnosis broadly respects DSM-IV criteria, but weights psychomotor disturbance as a specific feature of melancholia. Inclusion criteria were: aged 18 – 70, currently depressed according to the MINI, a QIDS-SR (Quick Inventory of Depressive Symptoms – Self Report; Rush et al., 2003) score of 11 or higher (indicating moderate to severe depression), good English comprehension and ability to provide informed consent to participate in research. To ensure a rigorously defined group, participants required a concordant MINI and clinician diagnosis of current unipolar melancholic or non-melancholic depression. Participants were excluded if they met DSM-IV criteria for Bipolar Disorder, Schizoaffective Disorder or Schizophrenia. Other exclusion criteria were significant current cognitive impairment, current substance use/dependence, psychosis, or organic disorders. The majority (71.4%) were medicated (with 4 taking multiple medications). Medication classes were predominantly Selective Serotonin Reuptake Inhibitors (52.2%), followed by Selective Noradrenaline Reuptake Inhibitors (34.8%), Tricyclics (17.4%), and with a minority taking atypical antipsychotics, Monoamine Oxidase Inhibitors and Noradrenaline-Serotonin Specific Antidepressants (8.7%, 4.3% and 4.3% respectively). Eligible participants completed self-report questionnaires and the probabilistic reward task (PRT), detailed below.
Sample 2 (Boston)
Participants were derived from a larger database of community participants tested with the PRT (detailed below) in previous research (Pizzagalli et al., 2007, 2008a). Healthy controls were selected solely on the basis of having the identical age of a participant from the Sydney site. This allowed us to identify 19 healthy controls [14 of these participants had been included in the Pizzagalli et al. (2008a) study; the remaining 5 from the Pizzagalli et al. (2007) study]. Additionally, 11 unmedicated MDD participants tested in the Pizzagalli et al. (2008a) study who met criteria for melancholic (n = 7) or non-melancholic (n = 4) depression were included. Healthy controls were recruited from the community using flyers/advertisements; MDD participants were recruited from treatment studies at the Depression Clinical and Research Program at Massachusetts General Hospital (for more details see Pizzagalli et al., 2007, 2008a). Participants who met inclusion criteria after a phone screen were invited to the laboratory for diagnostic interviews, conducted by trained psychiatrists or Masters-level clinical interviewers. Inclusion criteria for the MDD sample included: (1) DSM-IV diagnosis of MDD (APA, 1994), as assessed by the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002); (2) score ≥17 on the 21-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960); (3) no psychotropic medications for at least 2 weeks (6 months for dopaminergic drugs, 6 weeks for fluoxetine, and 4 weeks for neuroleptics and benzodiazepines); (4) no current or past history of MDD with psychotic features; (5) no other Axis I diagnosis, with the exception of anxiety disorders; and (6) no ECT in the previous 6 months. Inclusion criteria for controls included no medical or neurological illness, no current or past psychopathology (SCID, Non-patient Edition), and no current psychotropic medications. MDD participants performed the PRT before starting antidepressant treatment. The study was approved by the Committee on the Use of Human Subjects in Research at Harvard University and the Partners Human Research Committee. Participants were compensated with $10/hour. All Boston site participants completed the Beck Depression Inventory II (BDI-II; Beck et al., 1996) while only depressed participants completed the HRSD.
2.2 Self-report Questionnaires
The following self-report measures of anhedonia and motivation were administered in the Sydney sample (the scales being unavailable in the Boston sample).
Snaith-Hamilton Pleasure Scale (SHAPS)
The 14-item SHAPS (Snaith et al., 1995) is a commonly used measure of state hedonic capacity, assessing four domains of hedonic experience: interest/pastimes, social interaction, sensory experience, and food/drink. The measure assesses pleasure experienced in the “last few days” on a 4-point Likert scale (definitely agree to definitely disagree). Psychometric properties evaluated in an adult outpatient sample diagnosed with MDD , supported the SHAPS as a reliable and valid unidimensional measure of hedonic capacity with high internal consistency (α = 0.91) (Nakonezny et al., 2010). Scoring was undertaken as per Nakonezny et al. (2010) whereby ‘disagree’ responses receive a score of 1 and ‘agree’ responses receive a score of 0 (score range 0 to 14). Higher total SHAPS score indicates a higher level of state anhedonia.
Temporal Experience of Pleasure Scale (TEPS)
The 18-item TEPS (Gard et al., 2006) measures two components of pleasure - anticipatory (pleasure in anticipation of future activities) and consummatory (pleasure experience in the moment). The TEPS has demonstrated good internal consistency, test/retest reliability, and convergent and discriminant validity (Gard et al., 2006).
Behavioral Inhibition and Behavioral Activation Scales (BIS/BAS)
The 24-item BIS/BAS scales (Carver and White, 1994) assess individual differences in temperament related to two physiological self-regulatory systems as hypothesised by Gray (1981). The Behavioral Activation System (BAS) captures sensitivity to reward and goal-directed pursuits (trait approach motivation) - a tendency to seek positive experiences or rewards. Three BAS sub-scales (Drive, Fun Seeking and Reward Responsiveness) measure differing sensitivity aspects of this system. BAS-Drive measures persistence in pursuing desired goals; BAS-Fun Seeking reflects the desire and willingness to approach a potentially rewarding event; BAS Reward Responsiveness measures positive responses to the occurrence or anticipation of rewarding activities.
The Behavioral Inhibition System (BIS) represents sensitivity to punishment and non-reward (trait avoidance motivation) and the tendency to avoid possible negative events. BIS/BAS scales allow for observation of differences in anticipatory behaviour when faced with potential reward by considering risk patterns of individuals and evaluating trial-by-trial adjustments in motivational behaviour according to history of reward or non-reward (Padrao et al., 2013).
2.3 Probabilistic Reward Task (PRT)
The PRT is a computerised 25-minute task that provides an objective measure of reward responsiveness by measuring shifts in response toward a differentially rewarded stimulus (Pizzagalli et al., 2005). Participants were instructed prior to the task that the aim was to win as much money as possible by identifying which of two stimuli (short or long mouth on a schematic face) was presented on a computer screen. Participants were given their task ‘earnings’ (on average, between $5 and $7) following completion.
A practice trial was first completed to ensure task comprehension, followed by 3 blocks of 100 trials, with short rest breaks (30 sec) between blocks. Each trial commenced with the presentation of an asterisk for 500 msec, serving as a fixation point in the middle of the screen. Next, a mouthless cartoon figure appeared, followed by one of two perceptually similar stimuli – a short mouth (11.5 mm) or a long mouth (13 mm), which were presented very briefly (100 ms). To induce a response bias, correct identification (via key-stroke) of one stimulus was rewarded (“Correct!! You won 5 cents”) three times more frequently (n = 30 per block) than that of the other stimulus (n = 10 per block). The stimulus reinforced most frequently is the “rich” stimulus; the less reinforced stimulus is defined as the “lean” stimulus. Selection of which stimulus would be the “rich” stimulus was performed by the investigator prior to task commencement and was counterbalanced within participant groups. The PRT was run using E-Prime software (version 2.0).
2.4 Data Processing and Statistical Analyses
PRT data quality was ascertained as per previously established procedures (Pizzagalli et al., 2005, 2008a,b). This involved identification of outlying data points within trial blocks (e.g., reaction time shorter than 150 ms, participants performing at chance levels) and across participants, which are subsequently excluded from analyses. Following data processing, a total of 5 melancholic and 3 non-melancholic patients from the Sydney sample were excluded from analyses, yielding a total sample of 35 participants (15 non-melancholic, 20 melancholic).
Following prior procedures (Pizzagalli et al., 2005, 2008a,b; Bogdan and Pizzagalli, 2006; AhnAllen et al., 2012), task performance was analysed in relation to four variables: response bias, discriminability, accuracy (i.e., hit rates) and reaction time (RT). Response bias provides an index of the participant’s systematic preference for the stimulus paired with the more frequent reward, and is thus the main variable of interest. Discriminability captures task difficulty or the ability to perceptually differentiate between the two stimuli, and thus represents an important control variable. Accuracy (= Hit rates = [(number of hits)/(number of hits + number of misses)] and RT provide additional information about task performance, but are considered secondary variables because they are imperfect measures of performance in the context of a response bias (Macmillan and Creelman, 1991). Accuracy and RT findings are therefore reported in the Supplementary Material. In addition to examining response bias as a function of blocks, we also tested reward learning, which was operationalized as the change of the response bias over time – calculated as the difference score between blocks 3 and 1 (ΔResponse Bias).
Computations for response bias and discriminability are based on the behavioural model of signal detection (e.g. McCarthy and Davison, 1979; Tripp & Alsop, 1999). Formulae are as follows:
.
A high response bias emerges when a participant shows a high number of correct identifications for the rich stimulus, and the converse for a lean stimulus. By contrast, discriminability is a measure of the participant’s ability to perceptually distinguish between the two stimuli (and thus provides a measure of task difficulty). To allow computations for conditions linked to 100% accuracy, 0.5 was added to each cell in the calculation matrix.
T-tests and chi-square tests were performed on demographic data, and self-report measures. Two sets of analyses were performed on PRT data. In the first set, mixed model repeated-measures ANOVAs with Site (Sydney, Boston), Block (1,2,3) and MDD Subgroup (melancholic, non-melancholic) were performed on response bias and discriminability scores. For accuracy and reaction time, the additional within-subjects factor of Stimulus (rich, lean) was added to the model. In the second set, and after confirming the absence of site differences, Group × Block (for response bias and discriminability scores) and Group × Block × Stimulus (for accuracy and reaction time) ANOVAs were re-run by including healthy controls. For brevity, for ANOVA considering three groups (healthy controls, non-melancholic, melancholic), only effects involving the factor Group are reported. Throughout the ANOVAs, Greenhouse-Geisser corrections were used where applicable. For the Sydney sample, Pearson correlations (two-tailed) were computed for the melancholic and non-melancholic groups to investigate relationships between response bias and scores on self-reported measures.
3. Results
3.1 Sample Characteristics
Demographic and clinical information are presented in Table 1. Across sites, the three groups did not differ with respect to age [F(2, 62)= 1.40, p > 0.25], gender [Chi squared (2) = 1.45, p > 0.40], education [Chi squared (2) = 3.33, p > .15] or marital status [Chi squared (8) = 10.06, p > 0.25]. Nevertheless, because it has been suggested that the phenotype of melancholia emerges with increasing age (Hyett et al., 2008; Parker et al., 2001), all analyses were repeated by entering age as a covariate. No significant differences between melancholic and non-melancholic groups emerged on measures of depression at either site (measured with the BDI and HRSD in the Boston sample and the QIDS in the Sydney sample). All Boston site participants completed the BDI regardless of depression status with significant differences between the depressed and non-depressed groups significant in Bonferroni-corrected post hoc analyses [F(2, 27) = 43.10, p < 0.001]. Across sites, the mean age of MDD onset in the depressed samples was 26 years (range: 21–30), with no difference between melancholic and non-melancholic MDD participants [F(1, 26) = 0.21, p > 0.60].
Table 1.
Sociodemographic and mood data in control (n = 19), non-melancholic MDD (n = 19), and melancholic MDD (n = 27) participants.
| Control | Non-melancholic | Melancholic | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 38.26 | 12.13 | 44.05 | 11.70 | 43.52 | 12.25 |
| Gender ratio (female/male) | 9/10 | N/A | 6/13 | N/A | 13/14 | N/A |
| Education (% bachelor’s degree of above) | 64.3 | N/A | 33.3 | N/A | 40.0 | N/A |
| Marital status (% married or defacto) | 14.3 | N/A | 27.8 | N/A | 32.0 | N/A |
| BDI-II (Boston sample only)** | 5.05 | 5.06 | 33.00 | 9.42 | 28.43 | 10.47 |
| HRSD (17-item) (Boston sample only) | N/A | N/A | 19.50 | 0.71 | 18.17 | 2.04 |
| QIDS-SR (Sydney sample only) | N/A | N/A | 16.18 | 3.49 | 20.00 | 17.53 |
| Age of depression onset | N/A | N/A | 24.77 | 10.17 | 26.87 | 13.31 |
Significant difference between depressed groups and the healthy control group using follow up tests and Bonferroni correction p<0.001
3.2 Self-reported anhedonia, approach and avoidance motivation data (Sydney sample)
Data are presented in Table 2. Contrary to our hypotheses, scores for non-melancholic and melancholic MDD participants did not differ significantly (all p > 0.20) on any measure: SHAPS [t(33) = 0.08], TEPS-Anticipatory [t(33) = 0.75], TEPS-Consummatory [t(33) = 1.07], BAS-Drive [t(33) = 0.05], BAS-Reward Responsiveness [t(33) = 0.87], BAS-Fun Seeking [t(33) = 0.22], BIS [t(33) = 1.22].
Table 2.
Self-reported anhedonia, approach and avoidance motivation in non-melancholic (n = 15) and melancholic (n = 20) MDD participants (Sydney sample).
| Measure | Non-melancholic | Melancholic | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| SHAPS | 6.6 | 4.2 | 6.5 | 3.2 |
| TEPS | ||||
| Anticipatory | 35.2 | 8.8 | 32.7 | 10.3 |
| Consummatory | 32.8 | 8.0 | 29.9 | 7.9 |
| BIS/BAS | ||||
| BAS-Drive | 8.3 | 3.2 | 8.3 | 2.6 |
| BAS-Reward Responsiveness | 15.3 | 2.3 | 14.5 | 2.5 |
| BAS-Fun Seeking | 10.1 | 2.2 | 9.1 | 2.6 |
| BIS | 24.9 | 2.7 | 23.3 | 4.4 |
3.3 PRT Results
Response Bias
The MDD Subgroup (melancholic, non-melancholic) × Site (Sydney, Boston) × Block (Block 1, 2, 3) revealed a significant Group × Block interaction (F(2, 84) = 5.55, p < 0.009, Greenhouse-Geisser ε = 0.81), indicating that the two MDD subgroups differed in their response bias development. Critically, all effects involving Site were not significant (all p > 0.29).
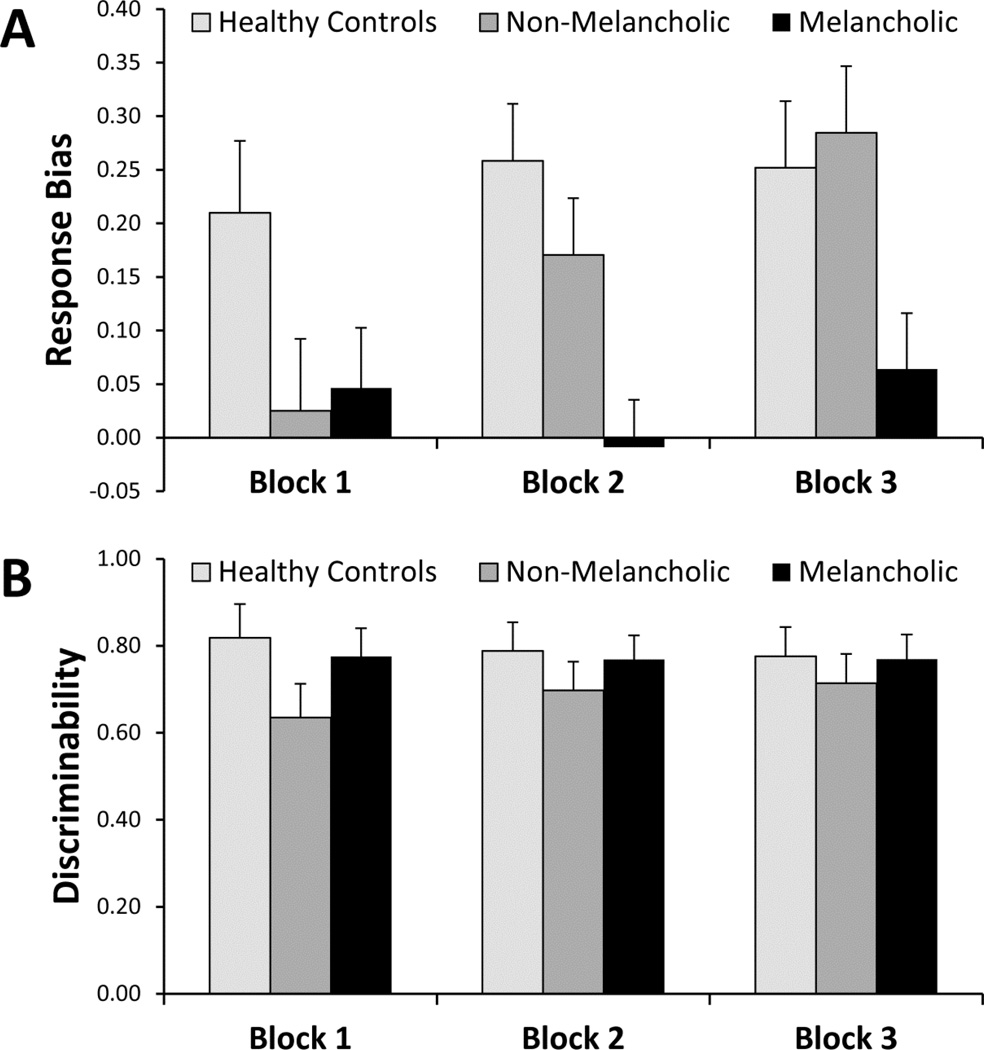
Unpaired t-tests confirmed that, relative to non-melancholic participants, melancholic participants had significantly lower learning from Block 1 to Block 3 (0.018±0.34 vs. 0.26±0.29, t(44) = 2.52, p < 0.025) as well as from Block 1 to Block 2 (−0.06±0.23 vs. 0.15±0.24, t(44) = 2.88, p < 0.06). In addition, relative to non-melancholic participants, melancholic participants had significantly lower response bias in both Block 2 and 3 (all p < 0.020) (Figure 1A). For non-melancholic participants, response bias in Block 1 was significantly lower than in Block 2, which was in turn significantly lower than in Block 3 (all t(18) > 2.15, all p < 0.045), whereas no differences among blocks emerged for melancholic participants (all p > 0.12).
Figure 1.
Analyses including three groups
The Group (healthy controls, non-melancholic depressed, melancholic depressed) × Block ANOVA confirmed a significant Group × Block interaction (F(4, 124) = 3.46, p < 0.015, ε = 0.87), and revealed a main effect of Group (F(2, 62) = 5.02, p < 0.010). [These effects were confirmed when entering age as a covariate; Group × Block: F(4, 122) = 3.87, p < 0.008; Group: (F(2, 61) = 4.81, p < 0.015].
Relative to controls, both non-melancholic (t(36)=1.98, p = 0.055) and melancholic (t(44) = 1.85, p = 0.072) depressed participants tended to show lower response bias in block 1 (Figure 1A). In addition, relative to controls, melancholic participants had lower response bias scores in both Block 2 and 3 (both t(44) > 2.21, all p < 0.032). Finally, separate one-sample t-tests indicated that response bias in healthy controls was significantly greater than zero in all three blocks [all t(18) > 3.14, all p < 0.006], whereas non-melancholic participants showed such effect only in blocks 2 and 3 [all t(18) > 3.30, all p < 0.004]. In contrast, for melancholic participants, response bias was not significantly different from zero in any block [all t(26) < 0.99, all p > 0.33].
Discriminability
No significant effects emerged from the MDD Subgroup × Site × Block ANOVA (all p > 0.063), indicating that groups or sites did not differ in task difficulty (Figure 1B).
Analyses including three groups
No effects emerged when all three groups were considered (Figure 1B), regardless whether age was considered as a covariate or not (all p > 0.29).
Accuracy (Hit Rates) and RT. See Supplementary Material.
3.4 Associations between PRT performance and self-reported anhedonia (Sydney sample)
Correlations were calculated for self-reported anhedonia and two aspects of response bias: (i) response bias at the end of the experiment (Block 3) and ii) reward learning [i.e., ΔResponse Bias = Response Bias(Block 3) – Response Bias(Block 1)]. Correlations in the total sample were examined first, followed by correlations within the melancholic and non-melancholic groups (see Table 3).
Table 3.
Correlations between (1) response bias variables and (2) self-report measures of anhedonia, approach and avoidance motivation
| Measure | Total sample (n = 35) |
Non-melancholic (n = 15) |
Melancholic (n = 20) |
|||
|---|---|---|---|---|---|---|
| RB block 3 | ΔRB | RB block 3 | ΔRB | RB block 3 | ΔRB | |
| SHAPS | .03 | .12 | −.28 | .27 | .24 | −.06 |
| TEPS | ||||||
| Anticipatory | −.06 | .10 | −.06 | .32 | −.13 | −.08 |
| Consummatory | .05 | −.06 | .29 | .02 | −.17 | −.15 |
| BIS/BAS | ||||||
| BAS-Drive | −.33 | .03 | −.44 | .10 | −.33 | −.06 |
| BAS-Reward Responsiveness | .19 | .10 | .57* | .44 | −.06 | −.19 |
| BAS-Fun Seeking | .11 | −.07 | .09 | .19 | .02 | −.30 |
| BIS | .09 | .13 | .54* | .08 | −.15 | .16 |
p < 0.05 RB = Response Bias
Contrary to our hypotheses, no significant relationships were observed between self-reported anhedonia scores (SHAPS and TEP scores) and response bias in Block 3 or reward learning. Null findings emerged for the total sample as well as within the melancholic and non-melancholic subgroups. Similarly, in the total sample, no significant relationships were observed between BAS or BIS scores, response bias and reward learning. However, significant positive correlations were observed between BAS-Reward Responsiveness score, BIS score and response bias in the non-melancholic group – and with no such associations observed in the melancholic group.
4. Discussion
Anhedonia may assist in providing more exact delineation of depressive disorders in biological research and clinical practice, thus requiring more precise definition and assessment (Snaith, 1993; Pizzagalli, 2014). This study presents a multi-method investigation of anhedonia in clearly defined clinical samples of melancholic and non-melancholic patients versus controls in an attempt to advance this aim.
Compared to controls, both depressed groups showed a lower response bias in block 1. However, over subsequent blocks, non-melancholic participants increased their response bias while melancholic participants had a response bias non-significantly different from 0 across all blocks. This suggests, firstly, that depressed participants – irrespective of depressive sub-type - showed initial deficits in reward-based learning, consistent with previous research in this area in independent samples (Vrieze et al., 2013). Second, and consistent with our hypotheses, melancholic participants did not develop a bias toward the more rewarded stimulus, and differed from both non-melancholic patients and controls. This suggests that melancholic participants exhibited a more pronounced deficit in developing behaviour to maximise reward. Critically, the deficit in reward-based learning emerged in the absence of any general impairment in task performance, as manifested by a lack of group differences in discriminability, suggesting that the impairment was not due to global cognitive impairments in the sample. Further highlighting a lack of global abnormalities, melancholic participants were actually more accurate than their non-melancholic counterparts for the lean stimulus (see Supplemental Material), consistent with the notion that their behaviour was not affected by the differential reinforcement schedule.
Deficits in reward-based learning in melancholic patients may reflect dopaminergic dysfunction. As noted earlier, reward-based learning deficits were reported in healthy controls who were administered a pharmacological challenge assumed to lower dopamine levels due to pre-synaptic auto-receptor activation (Pizzagalli et al., 2008b). Dopaminergic dysfunction is implicated in melancholia (Parker and Hadzi-Pavlovic, 1996), playing a crucial role in incentive motivation, reward learning and guiding reward prediction processes (Berridge, 2004; Schultz, 2013). Furthermore, dopaminergic pathways from the ventral tegmental area to the nucleus accumbens and prefrontal cortex guide motivation to pursue incentives (Depue and Collins, 1999). Accordingly, mood and motivational disturbances in melancholia may be related to dysfunction in the limbic loop linking medial prefrontal structure with the ventral striatum, and cognitive deficits to disturbances in the prefrontal loop linking lateral prefrontal structures to the caudate nucleus (Parker and Hadzi-Pavlovic, 1996). Striatal dysfunction has previously been implicated in both the anticipatory and consummatory phase of reward processing in MDD (Pizzagalli et al., 2009), however depressive sub-types were not examined. The caudate and anterior cingulate cortex (ACC), in particular, have been hypothesised as neural mediators of reward learning deficits – and with the ACC also associated with anticipatory and motivational reward deficits (Der-Avakian et al., 2012). Studies examining differing facets of hedonic behaviour and their associations with specific brain regions, associated neural networks and neurotransmitter function may assist in differentiating melancholic and non-melancholic depression.
Turning to self-report findings in the Sydney sample, the depressive sub-types did not differ on self-reported measures of anhedonia, with comparable scores on consummatory and anticipatory hedonic capacity sub-scales of the TEPS. While these results suggest that consummatory and anticipatory hedonic capacity is similar in depressive sub-types, these were based solely on self-report measures that may be subject to recall bias and other limitations. Self-report measures lack psychometric features to define subtle differences in hedonic capacity (Liu et al., 2011), which may partially explain the lack of observed differences. Along these lines,Shankman et al. (2011) described differences between melancholic and non-melancholic patients in EEG asymmetries irrespective of current depression severity. Collectively, prior and current findings suggest that behavioural or physiological assessments probing more circumscribed aspects of anhedonic behaviour might be more sensitive to differentiate depressive sub-types. Further studies employing objective measures of consummatory and anticipatory hedonic capacity in depressive sub-types are warranted.
Contrary to our hypothesis, no significant associations were found between response bias (or reward learning) and self-reported anhedonia (TEPS and SHAPS) in either melancholic or non-melancholic participants, nor across the total sample. Evidence supporting the association between response bias and self-reported anhedonia in depressed patients is mixed. Liu and colleagues (2011) reported a positive correlation between SHAPS scores and response bias learning over time, and negative correlations between the anticipatory sub-scale of the TEPS and response bias learning over time in a clinically depressed sample. However, these correlations were only observed under a stressful condition (negative feedback was given irrespective of the participant’s performance on the PRT) – and with no associations observed in a non-stressful condition (and thus similar to that adopted in the current study). In an earlier study of depressed participants,Pizzagalli et al. (2008a) found no association between self-reported anhedonic symptoms (assessed by the anhedonic depression sub-scale of the Mood and Anxiety Symptom Questionnaire or MASQ; Watson et al., 1995) and response bias, but found that anhedonic symptoms correlated with the probability of missing the rich stimulus after a non-rewarded correct identification of rich stimuli.
While we did not find an association between self-reported anhedonia and response bias, positive correlations were observed between specific BIS/BAS sub-scales and response bias in the non-melancholic group. These findings are considered in turn. First, and consistent with our hypothesis, higher reward responsiveness scores (BAS-RR) were associated with an increased response bias. As noted earlier, BAS-RR scores reflect positive responses to the occurrence or anticipation of rewarding events. BAS-RR scores have previously been correlated with TEPS-anticipatory scores (Gard et al., 2006), and thus may be considered as representing anticipatory hedonic capacity. If conceptualised this way, our results suggest that increased anticipatory hedonic capacity in non-melancholic participants is associated with a tendency to engage in behaviours associated with potential reward. Anticipatory pleasure has previously been considered to be closely related to the engagement of motivational processes (Depue and Collins, 1999), supporting our results. Second, higher BIS scores were associated with an increased response bias in non-melancholic participants – indicating that high sensitivity to non-reward was related to an increased tendency to alter behaviour in favour of potential reward. In a previous study of healthy individuals, increased sensitivity to punishment (i.e., higher BIS scores) was observed in anhedonic relative to non-anhedonic individuals (Padrao et al., 2013). The authors hypothesised that anhedonic individuals have a tendency to choose non-risky bets in gambling tasks, reflecting (i) behavioural conditioning at a trait level to avoid punishment or non-reward, and (ii) a tendency to create negative expectations regarding upcoming events, resulting in avoidance of risky decisions. If non-reward vs. reward in the task employed in the current study is conceptualised as representing a level of ‘risk’, the increased tendency to respond to the stimulus that was previously rewarded (as opposed to the stimulus that was not rewarded) may be considered to be a less risky decision. An alternative explanation is also possible. In the current task, participants did not always receive feedback concerning their performance. The absence of feedback (i.e., non-reward) may have been interpreted differently by the depressive sub-types – with increased sensitivity to non-reward in non-melancholic participants contributing to an increased motivation to change their behaviour in favour of potential reward. Others have suggested that in the absence of feedback, depressed participants behave as if they are expecting failure, in contrast to healthy controls who behave as if they are expecting success (Elliott et al., 1998; Smoski et al., 2008). While the current study assessed positive reinforcement learning, future research probing negative reinforcement learning may assist in identifying putative markers associated with depressive sub-types. For example, negative reinforcement learning may be of particular relevance to the non-melancholic sub-type, linked to motivational associations with the behavioural inhibition system (BIS). The melancholic group did not show the same associations as that observed in the non-melancholic group, suggesting that the motivational salience of rewarding stimuli may differ between depressive sub-types.
Finally, previous research has suggested that anhedonia impacts on motivational aspects of approach behaviour (appetitive) rather than on the consummatory process (Padrao et al., 2013). While melancholic and non-melancholic participants in this study did not differ on self-reported appetitive or consummatory hedonic capacity, further work is required to determine whether motivational aspects of approach behaviour may differ in depressive sub-types using objective measures of anhedonia.
Study limitations should be emphasized. First, although all MDD participants at the Boston site were unmedicated, the majority of Sydney-based MDD participants were medicated. However, the lack of effects involving Site indicates that systematic medication effects were unlikely. Second, sample size at each site was relatively small, which might have limited statistical power (but this was circumvented by aggregating data across sites). Third, the potential for fatigue effects among Sydney participants cannot be excluded, being initially involved in a 2-hour clinical assessment prior to completing research components. Fourth, the effects of anxiety were not controlled for - an important feature to examine in light of previous reports of associations between stress, anhedonic behaviour and reduced reward response bias (Bogdan & Pizzagalli, 2006). Finally, average depression severity scores indicated that the sample was at the lower end of the severity range, and participants were excluded from study participation on the basis of significant cognitive impairment. These aspects may have excluded those participants who were more likely to be suffering from severe melancholic depression, suggesting that the current effects might underestimate differences between depressive sub-types.
In summary, our results suggest that melancholic depression is characterized by a reduced ability to modulate behaviour as a function of reinforcement history. Moreover, initial evidence suggests that PRT performance may be related more to trait motivational processes associated with reward-based learning, rather than self-reported hedonic capacity. While studies to date employing this task have provided strong evidence for an insensitivity to reward-relevant information in depression, they have been unable to clarify whether reinforcement deficits are driven by reduced hedonic capacity, diminished motivation or a combination of the two (Treadway and Zald, 2011). Our results suggest reinforcement deficits measured in this way are linked with approach and avoidance motivational systems. Furthermore, self-reported motivational processes were linked with reward-based learning in non-melancholic participants, but showed no such associations in those with melancholia. Results support the view that anhedonia represents a complex system requiring multi-faceted assessment (Der-Avakian et al., 2012). Clinically, reward system dysfunction can lead to diminished engagement in pleasurable activities, contributing to maintenance and exacerbation of depressive symptoms (Pizzagalli et al., 2005), negatively impacting on quality of life. Identification of differential reward system deficits in depressive sub-types may ultimately improve the phenotypic definition of depression, whilst assisting in the development of tailored behavioural and biological treatment interventions to improve illness trajectories.
Supplementary Material
Acknowledgement
The authors would like to thank Bianca Blanch, Matthew Hyett, Rebecca Graham, Stacey McCraw and Georgia McClure for assistance with data collection and data entry.
Role of Funding Source
Funding for the Sydney site component of this study was provided by NHMRC Program Grant (1037196). The NHMRC had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the manuscript for publication. Dr. Pizzagalli was partially supported by NIMH grant R01 MH068376 and MH101521.
Footnotes
Conflict of Interest
Professor Parker has received honoraria from Aspen, Lundbeck, Janssen and Servier pharmacetucial companies in recent years for activities unrelated to this project. In the last three years Dr. Iosifescu has been a consultant for Avanir, CNS Response, INSYS Therapeutics, Lundbeck, Otsuka, Servier, Sunovion and has received research support through the Icahn School of Medicine at Mount Sinai from Alkermes, Astra Zeneca, Brainsway, Euthymics, Neosync, Roche, Shire. In the past three years, Dr. Pizzagalli has received honoraria/consulting fees from Advanced Neuro Technology North America, Otsuka America Pharmaceutical, Pfizer, and Servier for activities unrelated to this project. For a comprehensive list of lifetime disclosures of Dr. Fava, see http://mghcme.org/faculty/faculty-detail/maurizio_fava.
Contributors
Author Fletcher designed the study, wrote the protocol and managed the literature searches. Parker contributed to conceptual design. Pizzagalli, Fletcher and Iosifescu undertook the data processing and statistical analyses. Fletcher wrote the first draft of the manuscript. All authors edited the manuscript. All authors contributed to and have approved the final manuscript.
References
- Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Curr. Opin. Psychol. doi: 10.1016/j.copsyc.2014.12.011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- AhnAllen CG, Liverant GL, Gregor KL, Kamholz BW, Levitt JJ, Gulliver SB, Pizzagalli DA, Koneru VK, Kaplan GB. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Res. 2012;196:9–14. doi: 10.1016/j.psychres.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders, fourth ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders, fifth ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories –IA and –II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol. Beh. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol. Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J. Pers. Soc. Psychol. 1994;67:319–333. [Google Scholar]
- Carver CS. Affect and the functional bases of behavior: On the dimensional structure of affective experience. Pers. Soc. Psychol. Rev. 2001;5:345–356. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav. Brain Sci. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of instrumental action. Curr. Dir. Psychol. Sci. 1995;4:162–167. [Google Scholar]
- Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol. Med. 1998;28:559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients: the pleasure scale. Arch. Gen. Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J. Res. Personal. 2006;40:1086–1102. [Google Scholar]
- Gray JA. A critique of Eyesenck’s theory of personality. In: Eyesenck HJ, editor. A Model for Personality. Berlin: Springer-Verlag; 1981. pp. 246–276. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P, Jouvent R, Lancrenon S, Roumengous V, Feline A. The Pleasure-Displeasure Scale: use in the evaluation of depressive illness. Encephale. 1986;12:149–154. [PubMed] [Google Scholar]
- Hyett MP, Parker GB, Proudfoot J, Fletcher K. Examining age effects on prototypic melancholic symptoms as a strategy for refining definition of melancholia. J. Affect. Disord. 2008;109:193–197. doi: 10.1016/j.jad.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Klein D. Endogenomorphic depression. A conceptual and terminological revision. Arch. Gen. Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Klein D. Depression and anhedonia. In: Clark DC, Fawcett J, editors. Anhedonia and Affect Deficit States. New York: PMA publishing; 1987. pp. 1–14. [Google Scholar]
- Lemke MR, Puhl P, Koethe N, Winkler T. Psychomotor retardation and anhedonia in depression. Acta Psychiatrica Scand. 1999;99:252–256. doi: 10.1111/j.1600-0447.1999.tb07221.x. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J. Clin. Psychol. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Liu W-H, Chan RCK, Wang L-Z, Huang J, Cheung EFC, Gong Q-Y, Gollan JK. Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Prog. Neuro. Psychopharmacol. Biol. Psychiatry. 2011;35:1045–1052. doi: 10.1016/j.pnpbp.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Loas G. Vulnerability to depression: A model centered on anhedonia. J. Affect. Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- Loas G, Boyer P. Anhedonia in endogenomorphic depression. Psychiatry Res. 1996;60:57–65. [Google Scholar]
- Macmillan NA, Creelman DC. Detection Theory: A User’s Guide. New York: Cambridge University Press; 1991. [Google Scholar]
- McCarthy D, Davison M. Signal probability, reinforcement, and signal detection. J. Exp. Anal. Behav. 1979;32:373–382. doi: 10.1901/jeab.1979.32-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: some conjectures. Bull. Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- Nakonezny PA, Carmody TJ, Morris DW, Kurian BT, Trivedi MH. Psychometric evaluation of the Snaith–Hamilton pleasure scale in adult outpatients with major depressive disorder. Int. Clin. Psychopharmacol. 2010;25:328–333. doi: 10.1097/YIC.0b013e32833eb5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Padrao G, Mallorqui A, Cucurell D, Marco-Pallares J, Rodriguez-Fornells A. Neurophysiological differences in reward processing in anhedonics. Cogn. Affect. Behav. Neurosci. 2013;13:102–115. doi: 10.3758/s13415-012-0119-5. [DOI] [PubMed] [Google Scholar]
- Parker G, Hadzi-Pavlovic D. Melancholia: A Disorder of Movement and Mood. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- Parker G, Roy K, Hadzi-Pavlovic D, Wilhelm K, Mitchell P. The differential impact of age on the phenomenology of melancholia. Psychol. Med. 2001;31:1231–1236. doi: 10.1017/s0033291701004603. [DOI] [PubMed] [Google Scholar]
- Parker G, Fletcher K, Barrett M, Synnott H, Breakspear M, Rees AM, Hadzi-Pavlovic D. Inching toward Bethlehem: mapping melancholia. J. Affect. Disord. 2010a;123:291–298. doi: 10.1016/j.jad.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Parker G, Fink M, Shorter E, Taylor MA, Akiskal H, Berrios G, Bolwig T, Brown WA, Carroll B, Healy D, Klein DF, Koukopoulos A, Michels R, Paris J, Rubin RT, Spitzer R, Swartz C. Issues for DSM-5: whither melancholia? The case for its classification as a distinct mood disorder. Am. J. Psychiatry. 2010b;167:745–747. doi: 10.1176/appi.ajp.2010.09101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Hyett MP, Friend P, Hadzi-Pavlovic D. Does age impact on rating melancholic and non-melancholic depressive symptoms? J. Affect. Disord. 2013a;147:318–324. doi: 10.1016/j.jad.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Parker G, McCraw S, Blanch B, Hadzi-Pavlovic D, Synnott H, Rees AM. Discriminating melancholic and non-melancholic depression by prototypic clinical features. J. Affect. Disord. 2013b;144:199–207. doi: 10.1016/j.jad.2012.06.042. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J. Psychiatric Res. 2013;47:1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PJ. Pharmacotherapy for major depression with melancholic features: relative efficacy of tricyclic versus selective serotonin reuptake inhibitor antidepressants. J. Affect. Disord. 1996;39:1–6. doi: 10.1016/0165-0327(96)00014-6. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O-Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal detection approach. Biol. Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Bogdan R, Ratner KG, Jahn AL. Increased perceived stress is associated with blunted hedonic capacity: Potential implications for depression research. Beh. Res. Ther. 2007;45:2742–2753. doi: 10.1016/j.brat.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in Major Depressive Disorder: evidence from a probabilistic reward task. J Psychiatry Res. 2008a;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacol. 2008b;196:221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with Major Depressive Disorder. Am. J. Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Ann. Rev. Clin. Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Sarapas C, Klein DN. The effect of pre- vs. post-reward attainment on EEG asymmetry in melancholic depression. Int. J. Psychophysiol. 2011;79:287–295. doi: 10.1016/j.ijpsycho.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Smoski MJ, Lynch TR, Rosenthal Z, Cheavens JS, Chapman AL, Krishnan RR. Decision-making and risk aversion among depressive adults. J. Beh. Ther. Exp. Psychiatry. 2008;39:567–576. doi: 10.1016/j.jbtep.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP. Anhedonia: a neglected symptom of psychopathology. Psych. Med. 1993;23:957–966. doi: 10.1017/s0033291700026428. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone: the Snaith-Hamilton Pleasure Scale. Br. J. Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Taylor MA, Fink M. Restoring melancholia in the classification of mood disorders. J. Affect. Disord. 2008;105:1–14. doi: 10.1016/j.jad.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J. Clin. Child. Psychol. 1999;28:366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, Claes S. Reduced reward learning predicts outcome in Major Depressive Disorder. Biol. Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J. Abnorm. Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.