Abstract
Background
Hepatocyte transplantation is a potential therapy for certain diseases of the liver, including hepatic failure. However, there is a limited supply of human livers as a source of cells and, after isolation, human hepatocytes can be difficult to expand in culture, limiting the number available for transplantation. Hepatocytes from other species, e.g., the pig, have therefore emerged as a potential alternative source. We searched the literature through the end of 2014 to assess the current status of experimental research into hepatocyte xenotransplantation.
Literature search and results
The literature search identified 51 reports of in vivo cross-species transplantation of hepatocytes in a variety of experimental models. Most studies investigated the transplantation of human (n=23) or pig (n=19) hepatocytes. No studies explored hepatocytes from genetically-engineered pigs. The spleen was the most common site of transplantation (n=23), followed by the liver (through the portal vein [n=6]) and peritoneal cavity (n=19). In 47 studies (92%), there was evidence of hepatocyte engraftment and function across a species barrier.
Conclusions
The data provided by this literature search strengthen the hypothesis that xenotransplantation of hepatocytes is feasible and potentially successful as a clinical therapy for certain liver diseases, including hepatic failure. By excluding vascular structures, hepatocytes isolated from genetically-engineered pig livers may address some of the immunological problems of xenotransplantation.
Keywords: Hepatocytes, Pig, Xenotransplantation
Introduction
Orthotopic liver allotransplantation is currently the treatment of choice for patients with end-stage liver disease. However, it is limited by a shortage of deceased human donors, which may result in a suitable allograft not being available when needed for a patient .with acute liver failure (ALF) or acute decompensation of chronic liver disease. Hepatocyte transplantation (Tx) is a potential alternative to whole liver Tx in the treatment of ALF or some liver-based metabolic disorders (1–23). Scaled-up isolation methods are available to isolate almost the entire hepatocyte population from human and pig livers (24–26). However, most healthy livers from deceased donors are prioritized for liver Tx, and so human hepatocytes that are healthy and functional are even more difficult to obtain than whole livers (11). Therefore, other species as sources of hepatocytes are being investigated, as discussed previously by others (27,28).
Pig hepatocyte xenoTx has several potential advantages (27,28), though evidence for some of these is limited :- (i) There could be an unlimited cell supply. (ii) Pigs have some metabolic similarities to humans (29–31). (iii) As the vascular endothelium of the liver is not transplanted, this may possibly reduce the risks of acute vascular rejection (32), though this is by no means certain. Our own preliminary studies indicate that there is less Gal expression and less human antibody binding to pig hepatocytes than to vascular endothelium (Ezzelarab M, et al, unpublished). There is also some evidence that hepatocytes show resistance to complement-mediated injury (33). (iv) Genetically-modified pigs should provide hepatocytes that to some extent are protected from the human humoral and cellular immune responses (34–36). (v) Conventional immunosuppressive therapy may possibly be sufficient to control rejection (37–39). (vi) Pig hepatocytes may be resistant to human viruses, such as the hepatitis and human immunodeficiency (HIV) viruses (40,41).
Before clinical trials of hepatocyte xenoTx are undertaken, evidence needs to be provided from animal studies that hepatocyte Tx across species barriers is likely to be successful. How long do hepatocytes from one species survive and function in another? Can adverse effects be anticipated?
We have reviewed the available literature on experimental hepatocyte xenoTx. We were unable to identify any report on clinical hepatocyte xenoTx. We did not review the literature on hepatocyte alloTx, which has been reviewed by others (5,6,42).
Literature Search
In the 35 years from January 1980 to December 2014, we identified 51 reports of in vivo hepatocyte Tx across species barriers (Table 1). We were unable to identify any studies before 1980. There was peak experimental activity in the period 2007–2014 (Figure 1).
Table 1.
Experimental hepatocyte xenotransplantation, 1994–2014: hepatocyte source species, recipient species, and number of published studies
| Source Species | Recipient Species | Number of Studies |
|---|---|---|
|
| ||
| Human | Mouse | 15 |
| Rat | 5 | |
| Pig | 3 | |
|
| ||
| Monkey | Mouse | 1 |
|
| ||
| Pig | Rat | 13 |
| Mouse | 4 | |
| Monkey | 1 | |
| Rabbit | 1 | |
|
| ||
| Rat | Mouse | 4 |
|
| ||
| Rabbit | Rat | 4 |
|
| ||
| TOTAL | 51 | |
Figure 1.
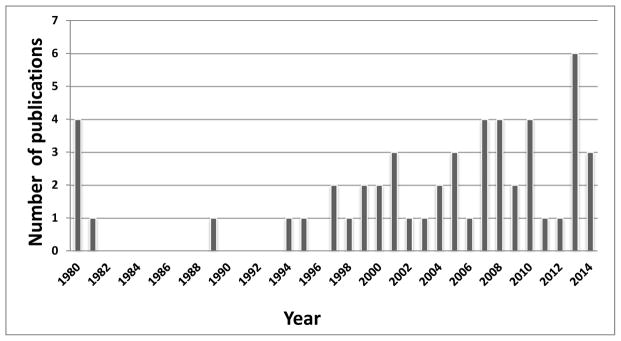
Number of publications on hepatocyte xenotransplantation, 1980–2014.
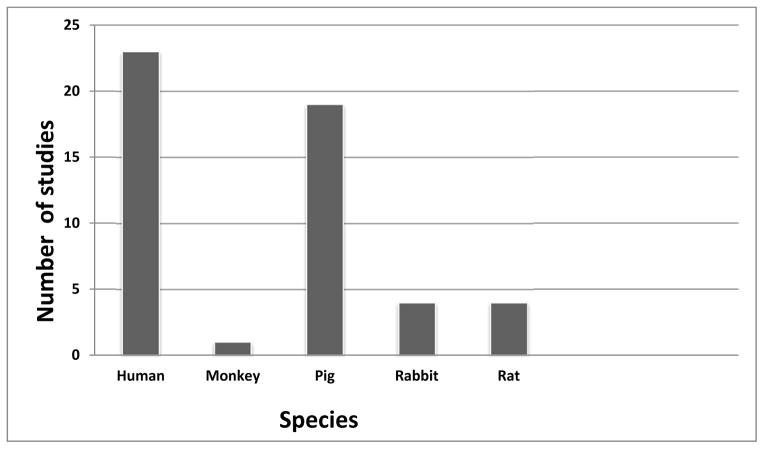
The majority of studies involved human (n=23) or pig (n=19) hepatocytes transplanted into a variety of other species (Figure 2). Rat (n=4), rabbit (n=4), and monkey (n=1) hepatocytes have also been transplanted. The most common recipient species were the mouse (n=24) and rat (n=22) (Table 1). Most commonly, the spleen (n=23) was the site of Tx, with the liver (via the portal vein, n=6) and peritoneal cavity (n=19) also being used. Details of all 51 experimental studies are provided in Tables 2–6.
Figure 2.
Source species of hepatocytes in studies of xenotransplantation, 1980–2014
Table 2.
Studies of human hepatocyte transplantation in other species
| Recipient species | Route of administration | Number of hepatocytes transplanted | Conclusions | Reference |
|---|---|---|---|---|
| Rat | Intraperitoneal | 1.5×107 | Cryopreserved hepatocytes remain liver function | Moscioni 1989 (97) |
| Rat | Intraperitoneal | 2×107 | Encapsulated hepatocytes survived and produced albumin | Wen 1998 (98) |
| Rat | Intraperitoneal | 2×107 | A 2-week course of cyclosporine resulted in graft function for 60 days | Wen 2000 (99) |
| Mouse (SCID/uPA) | Portal vein | 5×105 | Engrafted and partially repopulated liver | Dandri 2001(100) |
| Pig (pancreatec tomized) | Portal vein | 3 ×107 | Insulin-producing hepatocyte line controlled diabetes | Okitsu 2004 (101) |
| Mouse (SCID/uPA) | Spleen | 5.0 to 7.5×106 | Hepatocytes functioned | Tateno 2004 (102) |
| Mouse (SCID/uPA) | Spleen | 1×106 | Hepatocytes repopulated the liver and preserved normal function | Meuleman 2005 (103) |
| Mouse ( ALF) | Intraperitoneal | 50×106 | Hepatocytes functioned | Mai 2005 (61) |
| Pig (ALF) | Portal vein | 1 × 106 | Immortalized hepatocytes significantly prolonged the survival of ALF pigs | Totsugawa 2007 (104) |
| Mouse (SCID/uPA) | Spleen | 7.5 or 10×105 | Growth hormone enhanced proliferation of hepatocytes in SCID/uPA mice | Masumoto 2007 (105) |
| Mouse (SCID/ALF) | Spleen | 1×106 | Immortalized hepatocytes improved survival of ALF mouse | Tsuruga 2008 (106) |
| Rat (F344 nude) | Portal vein | 5×106 | Created immunodeficient rats that are suitable for hepatocyte Tx | Igarashi 2008 (107) |
| Mouse (SCID/uPA) | Spleen | 1×106 | Mouse NK cells played a critical role in the rejection of hepatocytes | Kawahara 2010 (108) |
| Mouse (ALF) | Intraperitoneal | 40×106 | Encapsulated immortalized hepatocytes improved survival | Sgroi 2011 (62) |
| Mouse (ALF) | Intraperitoneal | 3×106–6×106 | Hepatocytes survived | Link 2012 (63) |
| Rat (F344 nude) | Portal vein | 1 × 106 | Hepatocytes engrafted | Tachibana 2013 (109) |
| Fetal pigs and piglets | In utero and percutaneous | 1×107 in utero (fetal pigs) and 5×107 percutaneous (piglets) | In utero Tx allowed for stable engraftment | Fisher 2013 (110) |
| Mouse (uPA/SCID) | Spleen | 2.5×105 | Hepatocytes functioned | Tateno 2013 (111) |
| Mouse (nude) | Subcutaneous | 2.5×106 | Overexpression of RhoC can enhance the tumorigenicity of HL7702 cells | Xie 2013 (112) |
| Mouse (uPA/NOG) | Intraperitoneal | 2×106 | Hepatocytes survived and expanded | Gutti 2013 (113) |
| Mouse (uPA/SCID) | Spleen | 1×106 | Hepatocytes functioned | Ohtsuki 2014 (114) |
| Mouse (TK-NOG ) | Intraperitoneal | 1×106 | Human albumin produced | Kim 2014 (115) |
| Mouse (nude/ALF) | Spleen | 1×106 | Hepatocytes increased survival | Vidal 2014 (116) |
Abbreviations:
SCID/uPA mouse: The severe combined immunodeficiency/albumin linked-urokinase type plasminogen activator (SCID/Alb-uPA) human liver chimeric mouse model
F344 nude rat: F344 nude rats devoid of T cells were irradiated with X-rays and injected with bone marrow cells from SCID mice
ALF: Acute liver failure
uPA/NOG mice: Severe combined immunodeficiency/IL-2Rgc null (NOG) mice carry two copies of the mouse albumin promoter-driven urokinase-type plasminogen activator transgene.
RhoC: Ras homologous C (RhoC)
HL7702 cell: The HL7702 cell line was stably transfected with a RhoC expression vector and then subjected to cell proliferation, differentiation, colony formation, migration and invasion assays
TK-NOG mice: herpes simplex virus type 1 thymidine kinase [TK] transgene expressed within the liver of a highly immunodeficient mouse strain [NOG
Table 6.
Studies of rat hepatocyte transplantation in other species
| Recipient species | Route of administration | Number of hepatocytes transplanted | Conclusions | Reference |
|---|---|---|---|---|
| Mouse | Spleen/ intravenous/subcutaneous | 1×106 | Intravenous or intrasplenic Tx was followed by a significantly lower DTH response than subcutaneous Tx | Tanabe 1994 (135) |
| Mouse (Alb- uPA) | Spleen | 1–2×105 | Livers were reconstituted with rat hepatocytes | Rhim 1995 (136) |
| Mouse (ALF) | Spleen | 0.5×106 | Hepatocyte survival was maintained by weekly Jo2 administration | Vidal 2008 (137) |
| Mouse (uPA/SCID) | Spleen | 5.0–7.5×106 | Hepatocytes maintained their viability | Hata 2013 (138) |
Alb-uPA mice: albumin-urokinase (Alb-uPA) transgenic mice
DTH: The delayed type hypersensitivity
Jo2: specific anti-mouse Fas monoclonal antibody
Results and Discussion
Hepatocyte alloTx has been carried out in an effort to correct an inborn error of metabolism (3,5,6,8–13,18–20,43–50) or to provide support in patients with hepatic failure (2,4,5,11,14,51,52). In view of the persistent shortage of hepatocytes from deceased human donors, if hepatocyte Tx is going to play a significant therapeutic role, an alternative source of hepatocytes will be required.
The pig could fulfill this need, but there are few data on whether pig hepatocytes will survive in humans and, if so, whether they will be able to carry out the functions of human hepatocytes. The latter question will be particularly important if pig hepatocytes are transplanted in an effort to correct a metabolic disease in which replacement of a specific enzyme or hormone is required, e.g., glycogen storage disease, Crigler-Najjar syndrome type 1 (Table 7) (5,43,44,48,50), rather than when only detoxifying functions are required.
Table 7.
Hepatic metabolic disorders that potentially could be treated by hepatocyte xenotransplantation
| α1-antitrypsin deficiency |
| Arginino-succinate lyase deficiency |
| Bile acid synthesis disorders |
| Crigler-Najjar syndrome |
| Galactosemia |
| Glycogen storage disease type I (Van Gierke’s disease) |
| Glycogen storage disease type IV (Debrancher enzyme deficiency) |
| Hemochromatosis |
| Hereditary fructose intolerance |
| Hereditary tyrosinemia type I |
| Inherited Factor VII deficiency |
| Ornithine transcarbamylase deficiency |
| Peroxisomal biogenesis disease |
| Progressive familial intrahepatic cholestasis types 1, 2, and 3 |
| Urea cycle disorders |
| Wilson’s disease |
In the study by Nagata et al (37), between 1–2 billion wild-type (genetically-unmodified) pig hepatocytes (in a 1% alginate matrix) were injected directly into the parenchyma of the spleens of three cynomolgus monkeys (weighing 5–9kg), who received relatively intensive, but clinically-applicable, conventional immunosuppressive therapy. Our own very preliminary data suggest that conventional immunosuppressive therapy (based on calcineurin inhibition), unless very intensive, may be insufficient to prevent an adaptive immune response against even genetically-engineered pig hepatocytes in nonhuman primates (Iwase H, et al. unpublished), and therefore immunosuppressive regimens (based on T cell costimulation blockade) proven to be successful in pig vascularized solid organ Tx (53–56) may be required.
In Nagata’s study, graft function was determined by the measurement of porcine albumin. A peak of porcine albumin was detected in the blood within the first month. Following a single injection, the pig hepatocytes functioned for between 25 days (limited by death of the monkey from a cytomegalovirus infection) and >80 days. Following reTx on two occasions in one of the monkeys, porcine albumin was detected for >253 days (died from complications associated with replacement of a central venous catheter). Of considerable interest and relevance to future clinical trials was the observation that, although hepatocyte Tx was associated with a slight increase in anti-galactose-α1,3-galactose (Gal) IgG (considered to be within the normal range), there was no detectable increase in anti-nonGal antibody levels, suggesting that the Tx of hepatocytes from pigs genetically modified to delete expression of Gal (α1,3-galactosyltransferase gene-knockout pigs) might induce a minimal humoral immune response.
The considerable experience of encapsulated pig islet xenoTx, which includes several small clinical trials (57), suggests that encapsulation is not yet fully successful in protecting islets from the primate immune response, and therefore is unlikely to be fully successful in protecting pig hepatocytes. However, several groups have demonstrated some protection by hepatocyte encapsulation (58–64).
Currently, there appears to be no experience of the Tx of hepatocytes from genetically-engineered pigs into other species, though data from other models of xenoTx strongly suggest that hepatocytes from these pigs will provide a greater likelihood of success compared with those from wild-type pigs [reviewed in (65,66). We would suggest that, for Tx into humans, hepatocytes from genetically-engineered pigs in which both Gal and N-glycolylneuraminic acid (NeuGc) expression is absent (67), and which express at least one human complement-regulatory protein, will be advantageous to graft survival. If pig-to-nonhuman primate hepatocyte Tx is observed to be identified with a thrombotic reaction, then possibly the additional expression of a human coagulation-regulatory protein might be valuable. If hepatocytes are found to phagocytose human red blood cells and/or platelets (which we believe is unlikely), then steps may need to be taken to genetically engineer the pig to prevent this (discussed in 68).
Whether pig hepatocytes will carry out all of the functions required to maintain homeostasis in humans remains uncertain (31, 69–72). In a review of physiologic compatibilities between human and pig organ systems, Ibrahim et al drew attention to the 65% structural similarity between human and pig albumin (69). Porcine clotting factors (II, V, VII, X, XII) have been studied (73,74) and have been shown to trigger the human coagulation system (75–77). Other metabolic aspects, including the elimination of drugs by porcine hepatocytes, were discussed by Ibrahim et al (69).
There is a little evidence from orthotopic pig liver Tx in nonhuman primates that pig hepatocyte function will at least provide some factors required by primates, but this evidence is very limited (30,65,78,79). Ekser and his colleagues demonstrated that in baboon recipients of livers from genetically-engineered pigs, although follow-up was for less than one week, many parameters of hepatic function, including coagulation, remained in the near-normal range. Western blot demonstrated that pig proteins (albumin, fibrinogen, haptoglobin, and plasminogen) were produced by the pig liver, and production of several coagulation factors was also confirmed. Apart from the experience of Nagata et al (37), there is no evidence in the pig-to-nonhuman model of hepatocyte Tx.
Extracorporeal pig liver perfusion with human blood has generally been for such short periods of time (hours) that few conclusions can be drawn. However, decreased levels of vitamin K-dependent clotting factors (VII and X) were documented to be produced by the pig liver (80–82). There is also some evidence that pig hepatocytes can remove bilirubin from human blood (81,83). Although unlikely, there is also a risk that pig hepatocytes will phagocytose human erythrocytes (84–88) and/or platelets (86,89–93); it is unlikely this will occur in the absence of vascular endothelial cells and Kupffer cells.
It is therefore difficult to come to any realistic conclusion on the efficacy of pig hepatocytes to provide the necessary metabolic functions that will be required after their Tx into humans.
A recent study by Komori et al (94), however, provides some encouragement. This group reported that adult pig hepatocytes yielded a 100-fold higher serum albumin level in immunodeficient mice than adult human hepatocytes (which in turn yielded a 1,000-fold higher level than fetal human hepatocytes). However, these findings differ from previous reports which showed either no significant difference in albumin production between human and pig (95) or lower levels of albumin in pigs than in humans (although total serum protein levels were equivalent). Function of pig hepatocytes transplanted into humans might also be affected by such factors as whether the recipient has hepatitis (96).
Clinical hepatocyte alloTx has demonstrated modestly encouraging results in the treatment of various inherited metabolic diseases, e.g., glycogen storage disease (44,48,50), Crigler-Najjar syndrome (43,50), ornithine transcarbamylase deficiency (50), and tyrosinemia type 1 (50).
The small survey reported here illustrates that there are data indicating that the Tx of hepatocytes can result in successful engraftment in widely-disparate species. For example, the Tx of human hepatocytes into the spleens of SCID or ALF mice, or into the portal vein of pigs with ALF, can provide life-supporting hepatic function and/or improvement in recipient survival (Table 2). Pig hepatocytes have been demonstrated to proliferate after implantation into extrahepatic sites in SCID mice or ALF rats (Table 4), and have also functioned for >8 months in a monkey receiving only conventional immunosuppressive therapy (37) (Table 3). Pig hepatocytes demonstrate some metabolic similarities to human hepatocytes (29–31). Rabbit hepatocytes have functioned in rats with ALF, and rat hepatocytes have engrafted and survived in ALF mice, allowing transient or definitive improvement of liver failure (Table 6).
Table 4.
Studies of pig hepatocyte transplantation in other species
| Recipient species | Route of administration | Number of hepatocytes transplanted | Conclusions | Reference |
|---|---|---|---|---|
| Rat (ALF) | Intraperitoneal | 4×107 | Hepatocytes prolonged survival | Makowka 1980a (118) |
| Rat (ALF) | Intraperitoneal | 4×107 | Presensitization did not affect survival | Makowka 1980b (119) |
| Rat (ALF) | Intraperitoneal | 4×107 | Hepatocytes prolonged survival | Makowka 1981 (120) |
| Rat (ALF) | Spleen | 1×108 | Cryopreserved hepatocytes supported hepatic function | Papalois 1997 (121) |
| Rabbit (Watanabe) | Portal vein | 1–2×108 | Lowered serum cholesterol by 30–60% for >100 days | Gunsalus 1997 (122) |
| Rat | Subcutaneous | 1×107 | Hepatocytes survived | Elçin 1999 (123)78) |
| Rat (cirrhotic) | Spleen | 1×107 | Hepatocytes functioned | Stefan 1999 (124) |
| Rat (ALF) | Intraperitoneal | 6×106 | After 95% hepatectomy, cryopreserved encapsulated hepatocytes improved survival | Sarkis 2000 (125) |
| Rat | Intraperitoneal | 45×106/capsul e×3 capsules | Encapsulated hepatocytes remained functional for >15 days | Benoist 2001 (58) |
| Rat (ALF) | Intraperitoneal | 6×107 | Macroencapsulated hepatocytes prevented death | Sarkis 2001 (126) |
| Mouse (Balb/c) | Spleen | 2×106 | Hepatocytes synthesized albumin | Nishitai 2002 (127) |
| Rat (cirrhotic) | Spleen | 5×106 | Hepatocytes prolonged survival | Nagata 2003 (128) |
| Mouse (SCID) | Spleen | 1×106 | Freshly isolated hepatocytes had better viability than cultured, preserved or cryopreserved hepatocytes | Nishitai 2005 (129) |
| Cynomolgus monkey | Spleen | 2×106 | With conventional immunosuppressive therapy, hepatocytes functioned for 253 days | Nagata 2007 (37) |
| Rat | Intraperitoneal | 30–40×106 cells/mL ×1.8ml | Cryopreserved hepatocytes retained biological activity without immunosuppressive therapy | Baldini 2008 (59) |
| Mouse (ALF) | Intraperitoneal | 1×107cells/ml | Encapsulated hepatocytes sustained hepatic function | Mei 2009 (60) |
| Rat (DPPIV- deficient) | Intraperitoneal | 1×107 | Hepatic irradiation advantageous | Yamanouchi 2009 (130) |
| Mouse (SCID) | Spleen | 1×106 | Hepatocytes cultured in SAPNFs corrected ALF and prolonged survival | Yamamoto 2010 (131) |
| Rat (ALF) | Spleen | 3×107 | Immortalized hepatocytes improved hepatic function and prolonged survival | Pan 2010 (132) |
SAPNF: self-assembling peptide nanofiber (SAPNF) to provide a provisional three-dimensional (3-D) support to interact with cells to control their function in vivo.
DPPIV: dipeptidyl peptidase IV.
Table 3.
Studies of monkey hepatocyte transplantation in other species
| Recipient species | Route of administration | Number of hepatocytes transplanted | Conclusions | Reference |
|---|---|---|---|---|
| Mouse (SCID) | Spleen | 1×106 | V5-treated hepatocytes improved survival | Tanaka 2006 (117) |
V5: anti-apoptotic pentapeptide, composed of Val-Pro-Met-Leu-Lys, has been demonstrated to suppress apoptosis in several types of human cells.
However, in view of differences in metabolic function and immune responses between the various species combinations that have formed the experimental models, it is hard to draw conclusions relating to clinical pig-to-human hepatocyte Tx from most of the studies. Indeed, there has been only one study in the clinically-relevant pig-to-nonhuman model (37).
The minimum number of hepatocytes required to provide meaningful improvement in hepatic function in another species remains uncertain. The fewest human hepatocytes required to improve ALF in a xenoTx model has to date been reported to be 5×105, and the fewest pig and rat hepatocytes has been 2×106 and 1–2×105, respectively. The study by Nagata et al in the pig-to-monkey model suggested that the injection of approximately 1.5–2.5×108/kg hepatocytes might be sufficient to have a clinical impact (37).
In summary, experimental experience to date provides optimism that pig hepatocytes transplanted into rat, mouse, and monkey are likely to engraft and function without excessive immunosuppressive therapy being required. However, any conclusion about the success of clinical pig hepatocyte Tx is clearly premature with respect to both metabolic function and immune response. Besides excluding vascular structures from the transplant product, which should reduce the possibility of antibody-mediated xenogeneic rejection, hepatocytes isolated from genetically-engineered pigs may address other immunological concerns. The number of hepatocytes that will be necessary for the outcome to be of clinical relevance, e.g., correction of hepatic failure or correction of an inborn error of metabolism, remains uncertain.
Table 5.
Studies of rabbit hepatocyte transplantation in other species
| Recipient species | Route of administration | Number of hepatocytes transplanted | Conclusions | Reference |
|---|---|---|---|---|
| Rat (ALF) | Intraperitoneal | 4×107 | Hepatocytes prolonged survival | Makowka 1980a (118) |
| Rat (ALF) | Intraperitoneal | 4×107 | Presensitization did not affect survival | Makowka 1980b (119) |
| Rat (ALF) | Spleen | 1×108 | Hepatocyte graft survival was improved by daclizumab | Papagoras 2007 (133) |
| Rat (ALF) | Spleen | 1×108 | Rapamycin offered no survival advantage | Lytras 2010 (134) |
Acknowledgments
Work on xenoTx in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959-01, #U01 AI068642, and # R21 A1074844, and # 1PO1 HL107152 by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA.
Abbreviations
- Alb-uPA
albumin linked-urokinase type plasminogen activator
- ALF
acute liver failure
- Gal
galactose-α1,3-galactose
- SCID
severe combined immunodeficient
- Tx
transplantation
Footnotes
Disclosure of conflict of interest
None of the authors reports a conflict of interest.
References
- 1.Mito M, Kusano M. Hepatocyte transplantation in man. Cell Transplant. 1993;2:65–74. [PubMed] [Google Scholar]
- 2.Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951–952. doi: 10.1097/00007890-199410270-00016. [DOI] [PubMed] [Google Scholar]
- 3.Reyes J, Rubenstein WS, Micles L. The use of cultured hepatocyte infusion via the portal vein for the treatment of ornithine transcarbamoylase deficiency by transplantation of enzymatically competent ABO/Rh-matched cells. Hepatology. 1996;24(Suppl):308A. Abstract. [Google Scholar]
- 4.Soriano HE, Wood RP, Kang DC, Ozaki CF, Finegold MJ, Bischoff FC. Hepatocellular transplantation in children with fulminant liver failure. Hepatology. 1997;26:443. [Google Scholar]
- 5.Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 6.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 1999;19:39–48. doi: 10.1055/s-2007-1007096. [DOI] [PubMed] [Google Scholar]
- 7.Allen KJ, Soriano HE. Liver cell transplantation: the road to clinical application. J Lab Clin Med. 2001;138:298–312. doi: 10.1067/mlc.2001.119148. [DOI] [PubMed] [Google Scholar]
- 8.Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, et al. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262–1267. doi: 10.1542/peds.111.6.1262. [DOI] [PubMed] [Google Scholar]
- 9.Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735–738. doi: 10.1097/01.TP.0000077420.81365.53. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78:1812–1814. doi: 10.1097/01.tp.0000146386.77076.47. [DOI] [PubMed] [Google Scholar]
- 11.Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant. 2004;4 (Suppl 6):7–13. doi: 10.1111/j.1600-6135.2004.0340.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitry RR, Dhawan A, Hughes RD, Bansal S, Lehec S, Terry C, et al. One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation. 2004;77:1614–1616. doi: 10.1097/01.tp.0000122224.98318.19. [DOI] [PubMed] [Google Scholar]
- 13.Hughes RD, Mitry RR, Dhawan A. Hepatocyte transplantation for metabolic liver disease: UK experience. J R Soc Med. 2005;98:341–345. doi: 10.1258/jrsm.98.8.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvao FH, de Andrade JR, DR, de Andrade DR, Martins BC, Marson AG, Bernard CV, et al. Hepatocyte transplantation: State of the art. Hepatol Res. 2006;36:237–247. doi: 10.1016/j.hepres.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Dhawan A, Mitry RR, Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. J Inherit Metab Dis. 2006;29:431–435. doi: 10.1007/s10545-006-0245-8. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 17.Najimi M, Smets F, Sokal E. Hepatocyte transplantation: Current and future developments. Curr Opin Organ Transplant. 2007;12:503–508. [Google Scholar]
- 18.Lysy PA, Najimi M, Stephenne X, Bourgois A, Smets F, Sokal EM. Liver cell transplantation for Crigler-Najjar syndrome type I: update and perspectives. World J Gastroenterol. 2008;14:3464–3470. doi: 10.3748/wjg.14.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puppi J, Tan N, Mitry RR, Hughes RD, Lehec S, Mieli-Vergani G, et al. Hepatocyte transplantation followed by auxiliary liver transplantation--a novel treatment for ornithine transcarbamylase deficiency. Am J Transplant. 2008;8:452–457. doi: 10.1111/j.1600-6143.2007.02058.x. [DOI] [PubMed] [Google Scholar]
- 20.Quaglia A, Lehec SC, Hughes RD, Mitry RR, Knisely AS, Devereaux S, et al. Liver after hepatocyte transplantation for liver-based metabolic disorders in children. Cell Transplant. 2008;17:1403–1414. doi: 10.3727/096368908787648083. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick E, Mitry RR, Dhawan A. Human hepatocyte transplantation: state of the art. J Intern Med. 2009;266:339–357. doi: 10.1111/j.1365-2796.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 22.Pietrosi G, Vizzini GB, Gruttadauria S, Gridelli B. Clinical applications of hepatocyte transplantation. World J Gastroenterol. 2009;15:2074–2077. doi: 10.3748/wjg.15.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhawan A, Strom SC, Sokal E, Fox IJ. Human hepatocyte transplantation. Methods Mol Biol. 2010;640:525–534. doi: 10.1007/978-1-60761-688-7_29. [DOI] [PubMed] [Google Scholar]
- 24.Gerlach J, Kloppel K, Schon MR, Brombacher J, Courtney JM, Unger J, et al. Comparison of pig hepatocyte isolation using intraoperative perfusion without warm ischemia and isolation of cells from abattoir organs after warm ischemia. Artif Organs. 1993;17:950–953. doi: 10.1111/j.1525-1594.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 25.Gerlach JC, Brombacher J, Courtney JM, Neuhaus P. Nonenzymatic versus enzymatic hepatocyte isolation from pig livers for larger scale investigations of liver cell perfusion systems. Int J Artif Organs. 1993;16:677–681. [PubMed] [Google Scholar]
- 26.Gerlach JC, Brombacher J, Kloppel K, Schnoy N, Neuhaus P. Comparison of four methods for mass hepatocyte isolation from pig and human livers. Transplantation. 1994;57:1318–1322. doi: 10.1097/00007890-199405150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Bonavita AG, Quaresma K, Cotta-de-Almeida V, Pinto MA, Saraiva RM, Alves LA. Hepatocyte xenotransplantation for treating liver disease. Xenotransplantation. 2010;17:181–187. doi: 10.1111/j.1399-3089.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- 28.Lima-Quaresma KR, Bonavita AG, Cytrangulo MK, Pinto MA, Alves LA. Hepatocyte xenotransplantation. Methods Mol Biol. 2012;885:245–249. doi: 10.1007/978-1-61779-845-0_15. [DOI] [PubMed] [Google Scholar]
- 29.Soucek P, Zuber R, Anzenbacherova E, Anzenbacher P, Guengerich FP. Minipig cytochrome P450 3A, 2A and 2C enzymes have similar properties to human analogs. BMC Pharmacol. 2001;1:11. doi: 10.1186/1471-2210-1-11. Epub 2001. Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekser B, Echeverri GJ, Hassett AC, Yazer MH, Long C, Meyer M, et al. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90:483–493. doi: 10.1097/TP.0b013e3181e98d51. (2010a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekser B, Bianchi J, Ball S, Iwase H, Walters A, Ezzelarab M, et al. Comparison of hematologic, biochemical, and coagulation parameters in alpha1,3-galactosyltransferase gene-knockout pigs, wild-type pigs, and four primate species. Xenotransplantation. 2012;19:342–354. doi: 10.1111/xen.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cattan P, Zhang B, Braet F, Atia N, Conti F, Conjeaud H, et al. Comparison between aortic and sinusoidal liver endothelial cells as targets of hyperacute xenogeneic rejection in the pig to human combination. Transplantation. 1996;62:803–810. doi: 10.1097/00007890-199609270-00018. [DOI] [PubMed] [Google Scholar]
- 33.Koch CA, Kanazawa A, Nishitai R, Knudsen BE, Ogata K, Plummer TB, et al. Intrinsic resistance of hepatocytes to complement-mediated injury. J immunol (Baltimore, Md : 1950) 2005;174:7302–7309. doi: 10.4049/jimmunol.174.11.7302. [DOI] [PubMed] [Google Scholar]
- 34.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 35.Cooper DK, Ekser B, Burlak C, Ezzelarab M, Hara H, Paris L, et al. Clinical lung xenotransplantation--what donor genetic modifications may be necessary? Xenotransplantation. 2012;19:144–158. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper DK, Hara H, Ezzelarab M, Bottino R, Trucco M, Phelps C, et al. The potential of genetically-engineered pigs in providing an alternative source of organs and cells for transplantation. J Biomed Res. 2013;27:249–253. doi: 10.7555/JBR.27.20130063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagata H, Nishitai R, Shirota C, Zhang JL, Koch CA, Cai J, et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132:321–329. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Hara H, Campanile N, Tai HC, Long C, Ekser B, Yeh P, et al. An in vitro model of pig liver xenotransplantation--pig complement is associated with reduced lysis of wild-type and genetically modified pig cells. Xenotransplantation. 2010;17:370–378. doi: 10.1111/j.1399-3089.2010.00602.x. [DOI] [PubMed] [Google Scholar]
- 39.Satyananda V, Hara H, Ezzelarab MB, Phelps C, Ayares D, Cooper DK. New concepts of immune modulation in xenotransplantation. Transplantation. 2013;96:937–945. doi: 10.1097/TP.0b013e31829bbcb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanazawa A, Platt JL. Prospects for xenotransplantation of the liver. Semin Liver Dis. 2000;20:511–522. doi: 10.1055/s-2000-13159. [DOI] [PubMed] [Google Scholar]
- 41.Onions D, Cooper DK, Alexander TJ, Brown C, Claassen E, Foweraker JE, et al. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation. 2000;7:143–155. doi: 10.1034/j.1399-3089.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342–347. doi: 10.1097/TP.0b013e31823b72d6. [DOI] [PubMed] [Google Scholar]
- 43.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 44.Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 45.Ambrosino G, Varotto S, Strom SC, Guariso G, Franchin E, Miotto D, et al. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant. 2005;14:151–157. doi: 10.3727/000000005783983250. [DOI] [PubMed] [Google Scholar]
- 46.Stephenne X, Najimi M, Smets F, Reding R, de Ville de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058–2061. doi: 10.1111/j.1600-6143.2005.00935.x. [DOI] [PubMed] [Google Scholar]
- 47.Stephenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–1323. doi: 10.1053/j.gastro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Lee KW, Lee JH, Shin SW, Kim SJ, Joh JW, Lee DH, et al. Hepatocyte transplantation for glycogen storage disease type Ib. Cell Transplant. 2007;16:629–637. doi: 10.3727/000000007783465019. [DOI] [PubMed] [Google Scholar]
- 49.Meyburg J, Das AM, Hoerster F, Lindner M, Kriegbaum H, Engelmann G, et al. One liver for four children: first clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation. 2009;87:636–641. doi: 10.1097/TP.0b013e318199936a. [DOI] [PubMed] [Google Scholar]
- 50.Ribes-Koninckx C, Ibars EP, Calzado Agrasot MA, Bonora-Centelles A, Miquel BP, Vila Carbo JJ, et al. Clinical outcome of hepatocyte transplantation in four pediatric patients with inherited metabolic diseases. Cell Transplant. 2012;21:2267–2282. doi: 10.3727/096368912X637505. [DOI] [PubMed] [Google Scholar]
- 51.Bilir B, Durham J, Krysl J, Karrer F, Kumpe D, Ostrowska A, et al. Transjugular intra-portal transplantation of cryopreserved human hepatocytes in a patient with acute liver failure. Hepatology. 1996;24(Suppl):308A. (Abstract) [Google Scholar]
- 52.Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, et al. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000;6:32–40. doi: 10.1002/lt.500060113. [DOI] [PubMed] [Google Scholar]
- 53.Iwase H, Ekser B, Satyananda V, Bhama J, Hara H, Ezzelarab M, et al. Pig-to-baboon heart transplantation - initial experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015 doi: 10.1111/xen.12167. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwase H, Ekser B, Satyananda V, Zhou H, Hara H, Bajona P, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. 2015. Transplant Immunol. 2015 doi: 10.1016/j.trim.2015.02.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, Ayares D, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148:1106–1113. doi: 10.1016/j.jtcvs.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, Lewis BG, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–489. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wynyard S, Nathu D, Garkavenko O, Denner J, Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21:309–323. doi: 10.1111/xen.12102. [DOI] [PubMed] [Google Scholar]
- 58.Benoist S, Sarkis R, Barbu V, Honiger J, Baudrimont M, Lakehal F, et al. Survival and functions of encapsulated porcine hepatocytes after allotransplantation or xenotransplantation without immunosuppression. Surgery. 2001;129:606–616. doi: 10.1067/msy.2001.112961. [DOI] [PubMed] [Google Scholar]
- 59.Baldini E, Cursio R, De Sousa G, Margara A, Honiger J, Saint-Paul MC, et al. Peritoneal implantation of cryopreserved encapsulated porcine hepatocytes in rats without immunosuppression: viability and function. Transplant Proc. 2008;40:2049–2052. doi: 10.1016/j.transproceed.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 60.Mei J, Sgroi A, Mai G, Baertschiger R, Gonelle-Gispert C, Serre-Beinier V, et al. Improved survival of fulminant liver failure by transplantation of microencapsulated cryopreserved porcine hepatocytes in mice. Cell Transplant. 2009;18:101–110. doi: 10.3727/096368909788237168. [DOI] [PubMed] [Google Scholar]
- 61.Mai G, Nguyen TH, Morel P, Mei J, Andres A, Bosco D, et al. Treatment of fulminant liver failure by transplantation of microencapsulated primary or immortalized xenogeneic hepatocytes. Xenotransplantation. 2005;12:457–464. doi: 10.1111/j.1399-3089.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 62.Sgroi A, Mai G, Morel P, Baertschiger RM, Gonelle-Gispert C, Serre-Beinier V, et al. Transplantation of encapsulated hepatocytes during acute liver failure improves survival without stimulating native liver regeneration. Cell Transplant. 2011;20 :1791–1803. doi: 10.3727/096368911X564976. [DOI] [PubMed] [Google Scholar]
- 63.Link TW, Arifin DR, Long CM, Walczak P, Muja N, Arepally A, et al. Use of magnetocapsules for in vivo visualization and enhanced survival of xenogeneic HepG2 cell transplants. Cell Med. 2012;4:77–84. doi: 10.3727/215517912X653337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jitraruch S, Dhawan A, Hughes RD, Filippi C, Soong D, Philippeos C, et al. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS One. 2014;9:e113609. doi: 10.1371/journal.pone.0113609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 66.van der Windt DJ, Bottino R, Kumar G, Wijkstrom M, Hara H, Ezzelarab M, et al. Clinical islet xenotransplantation: how close are we? Diabetes. 2012;61:3046–3055. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 68.Cooper DK, Ekser B, Burlak C, Ezzelarab M, Hara H, Paris L, et al. Clinical lung xenotransplantation – what genetic modifications to the pig may be necessary? Xenotransplantation. 2012;19:144–158. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13:488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 70.Hammer C. Xenotransplantation for liver therapy or: Can porcine hepatocytes generate physiological functions sufficient for a human patient in ALF? Int J Artif Organs. 2002;25:1019–1028. doi: 10.1177/039139880202501018. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Qin S, Ding Y, Li S, Yang G, Zhang J, et al. Reference values of biochemical and hematological parameters for Guizhou minipigs. Exp Biol Med (Maywood) 2011;236:477–482. doi: 10.1258/ebm.2011.010283. [DOI] [PubMed] [Google Scholar]
- 72.Hammer C, Thein E. Physiological aspects of xenotransplantation. Xenotransplantation. 2002;9:303–305. doi: 10.1034/j.1399-3089.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Tan W, Qin S, Zhang J, Bu H, Li Y, et al. Cloning of the full-length cDNA of porcine antithrombin III and comparison with its human homolog. Comp Med. 2009;59:372–377. [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Qiao J, Tan W, Lu Y, Qin S, Zhang J, et al. Characterization of porcine factor VII, X and comparison with human factor VII, X. Blood Cells Mol Dis. 2009;43 :111–118. doi: 10.1016/j.bcmd.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Sun X, Cheng J, Wang L, Wei Q, Li S, et al. Study of hepatic function matching between Banna minipig inbred and humans. Transplant Proc. 2004;36:2492–2494. doi: 10.1016/j.transproceed.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L, Li Y, Liu J, Zeng Y, Zeng R, Cheng J. Activation of human coagulation system by liver-derived clotting factors of Banna minipig inbred line. Transplant Proc. 2004;36:2490–2491. doi: 10.1016/j.transproceed.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L, Li Y, Jiang H, Liu J, Zeng Y, Cheng J. Comparison of hepatic coagulant, fibrinolytic, and anticoagulant functions between Banna minipig inbred line and humans. Transplantation. 2005;79:1128–1131. doi: 10.1097/00007890-200505150-00031. [DOI] [PubMed] [Google Scholar]
- 78.Ekser B, Gridelli B, Cooper DK. Porcine alanine transaminase after liver allo- and xenotransplantation. Xenotransplantation. 2012;19:52–55. doi: 10.1111/j.1399-3089.2011.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramirez P, Chavez R, Majado M, Munitiz V, Muñoz A, Hernandez Q, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70:989–998. doi: 10.1097/00007890-200010150-00001. [DOI] [PubMed] [Google Scholar]
- 80.Adham M, Ducerf C, Vernet M, Rigal D, de la Roche E, Bizollon T, et al. Changes in serum proteins during isolated pig liver xenoperfusion. Transplant Proc. 1997;29:3015. doi: 10.1016/s0041-1345(97)00765-3. [DOI] [PubMed] [Google Scholar]
- 81.Adham M, Sab JM, Ducerf C, Tassaux D, Vianey-Saban C, Chevallier M, et al. Correction of acute liver cell failure disorders through liver xenoperfusion: experimental study. Transplant Proc. 1997;29:3013–3014. doi: 10.1016/s0041-1345(97)00764-1. [DOI] [PubMed] [Google Scholar]
- 82.Adham M, Vianey-Saban C, Ducerf C, Boyer S, de la Roche E, Taibi A, et al. Plasma amino acid study during discordant liver xenoperfusion. Transplant Proc. 1997;29:3016. doi: 10.1016/s0041-1345(97)00766-5. [DOI] [PubMed] [Google Scholar]
- 83.Luo Y, Levy G, Ding J, Qi J, Chakbrati S, Garcia BM, et al. HDAF transgenic pig livers are protected from hyperacute rejection during ex vivo perfusion with human blood. Xenotransplantation. 2002;9:36–44. doi: 10.1034/j.1399-3089.2002.0o140.x. [DOI] [PubMed] [Google Scholar]
- 84.Rees MA, Butler AJ, Chavez-Cartaya G, Wight DG, Casey ND, Alexander G, et al. Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood. Transplantation. 2002;73:1194–1202. doi: 10.1097/00007890-200204270-00003. [DOI] [PubMed] [Google Scholar]
- 85.Rees MA, Butler AJ, Negus MC, Davies HF, Friend PJ. Classical pathways complement destruction is not responsible for the loss of human erythrocytes during porcine liver perfusion. Transplantation. 2004;77:1416–1423. doi: 10.1097/01.tp.0000121135.24688.a3. [DOI] [PubMed] [Google Scholar]
- 86.Burlak C, Twining LM, Rees MA. Terminal sialic acid residues on human glycophorin A are recognized by porcine Kupffer cells. Transplantation. 2005;80:344–352. doi: 10.1097/01.tp.0000162974.94890.9f. [DOI] [PubMed] [Google Scholar]
- 87.Rees MA, Butler AJ, Brons IG, Negus MC, Skepper JN, Friend PJ. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation. 2005;12:13–19. doi: 10.1111/j.1399-3089.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 88.Waldman JP, Vogel T, Burlak C, Coussios C, Dominguez J, Friend P, et al. Blocking porcine sialoadhesin improves extracorporeal porcine liver xenoperfusion with human blood. Xenotransplantation. 2013;20:239–251. doi: 10.1111/xen.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ekser B, Lin C, Long C, Echeverri GJ, Hara H, Ezzelarab M, et al. Potential factors influencing the development of thrombocytopenia and consumptive coagulopathy after genetically modified pig liver xenotransplantation. Transpl Int. 2012;25:882–896. doi: 10.1111/j.1432-2277.2012.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ekser B, Long C, Echeverri G, Hara H, Ezzelarab M, Lin C, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 91.Ezzelarab M, Ekser B, Gridelli B, Iwase H, Ayares D, Cooper DK. Thrombocytopenia after pig-to-baboon liver xenotransplantation: where do platelets go? Xenotransplantation. 2011;18:320–327. doi: 10.1111/j.1399-3089.2011.00679.x. [DOI] [PubMed] [Google Scholar]
- 92.Paris LL, Chihara RK, Sidner RA, Tector AJ, Burlak C. Differences in human and porcine platelet oligosaccharides may influence phagocytosis by liver sinusoidal cells in vitro. Xenotransplantation. 2012;19:31–39. doi: 10.1111/j.1399-3089.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- 93.Paris LL, Chihara RK, Reyes LM, Sidner RA, Estrada JL, Downey SM, et al. ASGR1 expressed by porcine enriched liver sinusoidal endothelial cells mediates human platelet phagocytosis in vitro. Xenotransplantation. 2011;18:245–251. doi: 10.1111/j.1399-3089.2011.00639.x. [DOI] [PubMed] [Google Scholar]
- 94.Komori J, DeWard AD, Gramignoli R, Strom SC, Fontes P, Lagasse E. Potential barriers to human hepatocyte transplantation in MUP-uPA(tg(+/+))Rag2(−)γC9−/−) mice. Cell Transplant. 2014;23:1537–1544. doi: 10.3727/096368913X672046. [DOI] [PubMed] [Google Scholar]
- 95.Langsch A, Giri S, Acikgöz A, Jasmund I, Frericks B, Bader A. Interspecies difference in liver-specific functions and biotransformation of testosterone of primary rat, porcine and human hepatocyte in an organotypical sandwich culture. Toxicol Lett. 2009;188:173–179. doi: 10.1016/j.toxlet.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 96.Cheng YB, Wang YJ, Zhang SC, Liu J, Chen Z, Li JJ, et al. Response of porcine hepatocytes in primary culture to plasma from severe viral hepatitis patients. World J Gastroenterol. 2005;11:7585–7590. doi: 10.3748/wjg.v11.i48.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moscioni AD, Roy-Chowdhury J, Barbour R, Brown LL, Roy-Chowdhury N, Competiello LS, et al. Human liver cell transplantation. Prolonged function in athymic-Gunn and athymic-analbuminemic hybrid rats. Gastroenterology. 1989;96:1546–1551. [PubMed] [Google Scholar]
- 98.Wen L, Calmus Y, Honiger J, Conti F, Capeau J, Weill B, et al. Encapsulated xenogeneic hepatocytes remain functional after peritoneal implantation despite immunization of the host. J Hepatol. 1998;29:960–968. doi: 10.1016/s0168-8278(98)80124-4. [DOI] [PubMed] [Google Scholar]
- 99.Wen L, Grude P, Conti F, Honiger J, Capeau J, Nordlinger B, et al. Suppression of humoral immunization against encapsulated xenogeneic hepatocytes and prolongation of their function by 2-week cyclosporine treatment in the rat. Surgery. 2000;127:301–308. doi: 10.1067/msy.2000.103882. [DOI] [PubMed] [Google Scholar]
- 100.Dandri M, Burda MR, Torok E, Pollok JM, Iwanska A, Sommer G, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- 101.Okitsu T, Kobayashi N, Jun HS, Shin S, Kim SJ, Han J, et al. Transplantation of reversibly immortalized insulin-secreting human hepatocytes controls diabetes in pancreatectomized pigs. Diabetes. 2004;53:105–112. doi: 10.2337/diabetes.53.1.105. [DOI] [PubMed] [Google Scholar]
- 102.Tateno C, Yoshizane Y, Saito N, Kataoka M, Utoh R, Yamasaki C, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol. 2004;165:901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, et al. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41:847–856. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- 104.Totsugawa T, Yong C, Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, et al. Survival of liver failure pigs by transplantation of reversibly immortalized human hepatocytes with Tamoxifen-mediated self-recombination. J Hepatol. 2007;47:74–82. doi: 10.1016/j.jhep.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 105.Masumoto N, Tateno C, Tachibana A, Utoh R, Morikawa Y, Shimada T, et al. GH enhances proliferation of human hepatocytes grafted into immunodeficient mice with damaged liver. J Endocrinol. 2007;194:529–537. doi: 10.1677/JOE-07-0126. [DOI] [PubMed] [Google Scholar]
- 106.Tsuruga Y, Kiyono T, Matsushita M, Takahashi T, Kasai H, Matsumoto S, et al. Establishment of immortalized human hepatocytes by introduction of HPV16 E6/E7 and hTERT as cell sources for liver cell-based therapy. Cell Transplant. 2008;17:1083–1094. [PubMed] [Google Scholar]
- 107.Igarashi Y, Tateno C, Tanaka Y, Tachibana A, Utoh R, Kataoka M, et al. Engraftment of human hepatocytes in the livers of rats bearing bone marrow reconstructed with immunodeficient mouse bone marrow cells. Xenotransplantation. 2008;15:235–245. doi: 10.1111/j.1399-3089.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- 108.Kawahara T, Douglas DN, Lewis J, Lund G, Addison W, Tyrrell DL, et al. Critical role of natural killer cells in the rejection of human hepatocytes after xenotransplantation into immunodeficient mice. Transpl Int. 2010;23:934–943. doi: 10.1111/j.1432-2277.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 109.Tachibana A, Tateno C, Yoshizato K. Repopulation of the immunosuppressed retrorsine-treated infant rat liver with human hepatocytes. Xenotransplantation. 2013;20:227–238. doi: 10.1111/xen.12037. [DOI] [PubMed] [Google Scholar]
- 110.Fisher JE, Lillegard JB, McKenzie TJ, Rodysill BR, Wettstein PJ, Nyberg SL. In utero transplanted human hepatocytes allow postnatal engraftment of human hepatocytes in pigs. Liver Transpl. 2013;19:328–335. doi: 10.1002/lt.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tateno C, Miya F, Wake K, Kataoka M, Ishida Y, Yamasaki C, et al. Morphological and microarray analyses of human hepatocytes from xenogeneic host livers. Lab Invest. 2013;93:54–71. doi: 10.1038/labinvest.2012.158. [DOI] [PubMed] [Google Scholar]
- 112.Xie S, Zhu M, Lv G, Geng Y, Chen G, Ma J, et al. Overexpression of Ras homologous C (RhoC) induces malignant transformation of hepatocytes in vitro and in nude mouse xenografts. PloS one. 2013;8:e54493. doi: 10.1371/journal.pone.0054493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gutti TL, Knibbe JS, Makarov E, Zhang J, Yannam GR, Gorantla S, et al. Human hepatocytes and hematolymphoid dual reconstitution in treosulfan-conditioned uPA-NOG mice. Am J Pathol. 2014;184:101–109. doi: 10.1016/j.ajpath.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ohtsuki S, Kawakami H, Inoue T, Nakamura K, Tateno C, Katsukura Y, et al. Validation of uPA/SCID mouse with humanized liver as a human liver model: protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases by LC-MS/MS. Drug Metab Dispos. 2014;42:1039–1043. doi: 10.1124/dmd.114.057646. [DOI] [PubMed] [Google Scholar]
- 115.Kim MK, Choi HJ, Kwon I, Pierson RN, Cooper DK, Soulillou JP, et al. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of xenocorneal transplantation. Xenotransplantation. 2014;21:420–430. doi: 10.1111/xen.12129. [DOI] [PubMed] [Google Scholar]
- 116.Vidal I, Blanchard N, Chenard-Neu MP, Bachellier P, Heyd B, Staedtler F, et al. Increased survival despite failure of transplanted human hepatocyte implantation into liver parenchyma of nude mice with repeated lethal jo2-induced liver deficiency. Cell Transplant. 2014;23:1557–1572. doi: 10.3727/096368913X667501. [DOI] [PubMed] [Google Scholar]
- 117.Tanaka K, Kobayashi N, Gutierrez AS, Rivas-Carrillo JD, Navarro-Alvarez N, Chen Y, et al. Prolonged survival of mice with acute liver failure with transplantation of monkey hepatocytes cultured with an antiapoptotic pentapeptide V5. Transplantation. 2006;81:427–437. doi: 10.1097/01.tp.0000188693.48882.18. [DOI] [PubMed] [Google Scholar]
- 118.Makowka L, Rotstein LE, Falk RE, Falk JA, Nossal NA, Langer B, et al. Allogeneic and xenogeneic hepatocyte transplantation in experimental hepatic failure. Transplantation. 1980;30:429–435. doi: 10.1097/00007890-198012000-00009. [DOI] [PubMed] [Google Scholar]
- 119.Makowka L, Rotstein LE, Falk RE, Falk JA, Langer B, Nossal NA, et al. Reversal of toxic and anoxic induced hepatic failure by syngeneic, allogeneic, and xenogeneic hepatocyte transplantation. Surgery. 1980;88:244–253. [PubMed] [Google Scholar]
- 120.Makowka L, Rotstein LE, Falk RE, Falk JA, Zuk R, Langer B, et al. Studies into the mechanism of reversal of experimental acute hepatic failure by hepatocyte transplantation. 1. Can J Surg. 1981;24:39–44. [PubMed] [Google Scholar]
- 121.Papalois A, Arkadopoulos N, Kostopanagiotou G, Theodorakis K, Peveretos P, Golematis B, et al. Experimental xenotransplantation of fresh isolated and cryopreserved pig hepatocytes: a biochemical and morphological study. Transplant Proc. 1997;29:2096–2098. doi: 10.1016/s0041-1345(97)00249-2. [DOI] [PubMed] [Google Scholar]
- 122.Gunsalus JR, Brady DA, Coulter SM, Gray BM, Edge AS. Reduction of serum cholesterol in Watanabe rabbits by xenogeneic hepatocellular transplantation. Nat Med. 1997;3:48–53. doi: 10.1038/nm0197-48. [DOI] [PubMed] [Google Scholar]
- 123.Elcin YM, Dixit V, Lewin K, Gitnick G. Xenotransplantation of fetal porcine hepatocytes in rats using a tissue engineering approach. Artif Organs. 1999;23:146–152. doi: 10.1046/j.1525-1594.1999.06222.x. [DOI] [PubMed] [Google Scholar]
- 124.Stefan AM, Coulter S, Gray B, LaMorte W, Nikelaeson S, Edge AS, et al. Xenogeneic transplantation of porcine hepatocytes into the CCl4 cirrhotic rat model. Cell Transplant. 1999;8:649–659. doi: 10.1177/096368979900800611. [DOI] [PubMed] [Google Scholar]
- 125.Sarkis R, Benoist S, Honiger J, Baudrimont M, Delelo R, Balladur P, et al. Transplanted cryopreserved encapsulated porcine hepatocytes are as effective as fresh hepatocytes in preventing death from acute liver failure in rats. Transplantation. 2000;70:58–64. [PubMed] [Google Scholar]
- 126.Sarkis R, Honiger J, Chafai N, Baudrimont M, Sarkis K, Delelo R, et al. Semiautomatic macroencapsulation of fresh or cryopreserved porcine hepatocytes maintain their ability for treatment of acute liver failure. Cell Transplant. 2001;10:601–607. [PubMed] [Google Scholar]
- 127.Nishitai R, Plummer TB, Platt JL. Detection of albumin synthesis in transplanted porcine hepatocytes in mice. Liver Transpl. 2002;8:972–974. doi: 10.1053/jlts.2002.35172. [DOI] [PubMed] [Google Scholar]
- 128.Nagata H, Ito M, Cai J, Edge AS, Platt JL, Fox IJ. Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology. 2003;124:422–431. doi: 10.1053/gast.2003.50065. [DOI] [PubMed] [Google Scholar]
- 129.Nishitai R, Koch CA, Ogata K, Knudsen BE, Plummer TB, Butters KA, et al. Toward the survival and function of xenogeneic hepatocyte grafts. Liver Transpl. 2005;11:39–50. doi: 10.1002/lt.20305. [DOI] [PubMed] [Google Scholar]
- 130.Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258–267. doi: 10.1002/hep.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamamoto T, Navarro-Alvarez N, Soto-Gutierrez A, Yuasa T, Iwamuro M, Kubota Y, et al. Treatment of acute liver failure in mice by hepatocyte xenotransplantation. Cell Transplant. 2010;19:799–806. doi: 10.3727/096368910X508915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pan X, Du W, Yu X, Sheng G, Cao H, Yu C, et al. Establishment and characterization of immortalized porcine hepatocytes for the study of hepatocyte xenotransplantation. Transplant Proc. 2010;42:1899–1906. doi: 10.1016/j.transproceed.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 133.Papagoras D, Papalois A, Tsaroucha A, Lytras D, Kyriazanos J, Giannakou N, et al. Beneficial effect of an antibody against interleukin-2 receptor (daclizumab) in an experimental model of hepatocyte xenotransplantation. World J Gastroenterol. 2007;13:1435–1437. doi: 10.3748/wjg.v13.i9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lytras D, Papalois A, Tsaroucha AK, Papagoras D, Kyriazanos J, Lambropoulou M, et al. Xenotransplantation of hepatocytes in rats with acute liver failure using sirolimus for immunosuppression. J Int Med Res. 2010;38:546–557. doi: 10.1177/147323001003800217. [DOI] [PubMed] [Google Scholar]
- 135.Tanabe S, Taura Y, Furusawa S, Hirota Y, Tanaka M, Nakaichi M, et al. Delayed type hypersensitivity responses in mice transplanted with rat hepatocytes. J Vet Med Sci. 1994;56:1143–1148. doi: 10.1292/jvms.56.1143. [DOI] [PubMed] [Google Scholar]
- 136.Rhim JA, Sandgren EP, Palmiter RD, Brinster RL. Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc Natl Acad Sci U S A. 1995;92:4942–4946. doi: 10.1073/pnas.92.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vidal I, Blanchard N, Alexandre E, Gandillet A, Chenard-Neu MP, Staedtler F, et al. Improved xenogenic hepatocyte implantation into nude mouse liver parenchyma with acute liver failure when followed by repeated anti-Fas antibody (Jo2) treatment. Cell Transplant. 2008;17:507–524. doi: 10.3727/096368908785096051. [DOI] [PubMed] [Google Scholar]
- 138.Hata T, Uemoto S, Fujimoto Y, Murakami T, Tateno C, Yoshizato K, et al. Transplantation of engineered chimeric liver with autologous hepatocytes and xenobiotic scaffold. Ann Surg. 2013;257:542–547. doi: 10.1097/SLA.0b013e31825c5349. [DOI] [PubMed] [Google Scholar]