Introduction
The increasing stroke incidence and the long-term, severe disability of survivors requiring complex nursing care over extended time periods causes an enormous social and economic burden to ageing societies. Unfortunately, all clinical trials on neuroprotectants have failed thus far1. The only approved therapy is clot lysis (rTPa), which is restricted to only 4.5 hours post-stroke onset2.
Although rTPa application is statistically justified, it can result in severe adverse events3, complicating individual rTPa treatment decisions. Thus, the current strategies in stroke management are focused on prevention by identification of risk factors4 and intensive rehabilitation in the chronic phase5. However, stroke outcomes remain poor, causing a strong but unmet demand for alternative therapeutic approaches. The failure of the neuroprotective paradigm and limited eligibility for thrombolysis spawned an interest in stem cell-based neurorestoration6, which is characterized by a wide therapeutic window and is highly convergent with rehabilitation. On the other hand, stem cell-based tissue replacement may be aggravated by pathophysiological and anatomical features, while the beneficial effects of (stem) cells may not necessarily result from cellular restoration. Here, we review the state-of-the-art of cell-based stroke therapies and balance arguments supporting and challenging the concept of post-stroke tissue restoration. Moreover, we discuss the respective therapeutic mechanisms related to tissue restoration versus indirect means of regenerative support including practical issues such as transplantation time windows, routes of cell administration and potential detrimental effects.
Part I: the concept of cell replacement and arguments for its benefit
The replacement of lost brain tissue by transplanted cells has fired the imagination of researchers for decades. Studies on lesion-induced axonal sprouting of catecholamine neurons devised the fundamentals for brain regenerative strategies. Then, the functional connections of transplanted monoamine neurons were demonstrated while fetal nigral transplants were able to reverse parkinsonism in animal models and patients7. These early proof-of-principle studies supported the notion that replacement of lost brain cells by stem and progenitor cells may be viable option.
Neuronal replacement for stroke
The positive effects of dopaminergic neuron replacement in Parkinson’s disease are of great interest to stroke researchers. It was concurrently shown that fetal striatal grafts increased GABA release and reorganized GABAA receptors in the infarcted striato-pallidum, leading to improved spatial and conceptual learning. However, the integration of grafted cells in the host brain on the cellular level was not investigated8, while fetal tissue was considered insufficient to counter a high-prevalence disease like stroke. This resulted in a continuous search for unlimited sources of cells for replacement therapy.
Teratocarcinoma-derived neural progenitors are characterized by unrestricted expansion, and were found to differentiate to post-mitotic presumptive neurons (NT2N cells) in the presence of retinoic acid. The cells also showed molecular and structural polarity after transplantation into the rodent cerebrum, survived over one year in the nude mouse brain, and promoted behavioral recovery in ischemic rats9. These positive preclinical findings represented a translational cornerstone for the transfer of the neuronal replacement paradigm to the clinic. The first clinical stem cell trial for stroke (phase I, only patients in the chronic stroke phase10) proved the safety of the procedure. Detection of positive effects in a few patients missed statistical significance. However, serial [18F]fluorodeoxyglucose position emission tomography (PET) demonstrated a relationship between relative regional metabolic changes and the clinical performance of patients11. A neuropathological assessment of a patient from this trial revealed neurofilament-immunoreactive neurons at the graft location and the absence of neoplasms. Functional integration of transplanted cells with host neuronal circuitries was not investigated12. The subsequent phase II clinical trial resulted in improvements in some patients, but again no overall benefit was confirmed statistically13. However, the limited statistical power of early stage clinical trials must be considered when assessing results from such studies. Given the primary focus on safety aspects and the relatively small number of patients enrolled, any therapeutic effect must be of notable size to be detected at statistical significance. Although statistical prove of a therapeutic effect of realistic size cannot be expected in phase IIa/b studies, efficacy endpoints are often included in the study design and are considered crucial when deciding on the continuation with a respective therapy, particularly in the industrial environment. Although understandable from an economic perspective, this practice clearly bears the risk for false-negative results and premature abandonment of otherwise promising experimental therapies.
Since the outcome of clinical studies using post-mitotic, tumor-derived neurons has not been completely convincing, the strategy of using primary fetal-derived neural stem cells has been revived. Moreover, it is now speculated that true tissue restoration strategies will have to consider all major components of cerebral tissue, i.e. neurons, astro- and oligodendroglia, which may come with different challenges (Fig. 1A). One study convincingly showed neuronal differentiation, but integration of the graft was not studied15. Another study revealed not only neural differentiation of grafted embryonic medial ganglionic eminence (MGE) precursor cells, but also robust integration in host tissue, including synaptic connectivity and reorganized neuronal networks in the infarcted area, as evaluated by electron microscopy and electrophysiology on brain slices (Fig. 1B). This translated into sustained behavioral improvement14. In fact, the presence of vital cells exhibiting neur(on)al phenotypes that emerged from the graft and their meaningful functional integration into host brain tissue should be shown (Fig. 1C) when claiming true tissue restoration by a given approach (see Table for examples).
Figure 1. Post-stroke tissue restoration in theory and practice.
(A) In theory, tissue restoration strategies will have to target all three major cerebral cell populations: neurons, astro- and oligodendrocytes. Replacement of each individual component may come with different challenges. (B) In a recent study, MGE-grafted cells enhanced synaptophysin expression, which was quantified in the contralateral and ipsilateral sides (insets in brain slice scheme). Data are expressed as mean ± SEM. *p<0.05 versus vehicle (C) Representative current clamp trace of an action potential from an MGE neuron, implanted into the dorsal striatum of a stroked rat, elicited in response to a 400 pA current injection (left diagram). Sample trace of spontaneous excitatory postsynaptic currents (sEPSCs) in the MGE neuron held at −70 mV (native resting membrane potential for this cell was −56 mV; right diagram). (B) and (C) modified from by Daadi et al. (Cell Transplant. 2009;18:815–826. doi: 10.3727/096368909X470829; courtesy of Cognizant Communication Corporation, Putnam Valley, NY 10579, USA)14
Table.
Cell therapies for stroke and supposed mechanisms
| Cell type | Cell sources | Therapeutic time window | Cellular replacement | Indirect repair mechanisms | Clinical trials |
|---|---|---|---|---|---|
| ESCs | blastocyst | up to months | yes | occasionally described, but not prevalent | no |
| NSCs | ESC-derived fetal tissue surgical specimen (adult tissue) |
hours to months | yes | stimulation of endogenous repair/plasticity angiogenesis neuroprotection (early transplantation) |
yes |
| GPs | ESC-derived fetal tissue surgical specimen (adult tissue) |
up to months | yes (oligodendrocytes only) | not described so far | yes (not for stroke) |
| MSCs | bone marrow cord blood placenta adipose tissue |
several days to one month | no, but differentiation capabilities occasionally reported | neuroprotection/anti-apoptosis immunomodulation/anti-inflammation glial scar modulation angiogenesis stimulation of endogenous repair/plasticity |
yes |
| HSCs | bone marrow cord blood |
hours to days | no, but differentiation capabilities occasionally reported | neuroprotection immunomodulation/anti-inflammation glial scar modulation angiogenesis stimulation of endogenous repair/plasticity |
yes |
| MNCs | bone marrow cord blood peripheral blood |
hours to days | no | neuroprotection/anti-apoptosis immunomodulation/anti-inflammation glial scar modulation angiogenesis stimulation of endogenous repair/plasticity |
yes |
| iPSCs | various, mainly fibroblasts | days to months | yes | neuroprotection/anti-apoptosis immunomodulation/anti-inflammation stimulation of endogenous repair/plasticity |
no |
The expression of neuronal markers has also been observed after transplantation of a human clonal stem cell line derived from the fetal neuroepithelium (ReNeuron Ltd.), although the cells did not show mature neuronal morphology16. Clinical trials were initiated17 with the PISCES (Pilot Investigation of Stem Cells in Stroke) study currently in long-term follow-up (NCT01151124). Transplantation of the NSI-566RSC line (Neuralstem Inc.) into a rat stroke model revealed that most cells remained at the striatal transplantation site, but a small amount of hNSE+ fibers extending dorsally and ventrally from the graft were identified. The functional relevance of this sprouting remains unclear18.
It was recently shown that human, induced pluripotent stem cell (iPSC)-derived, long-term neuroepithelial-like stem (hiPSC-lt-NES) cells can differentiate into neuroblasts and mature GABAergic neurons. iPSCs cells can be used in autologous scenarios, but a detailed knowledge of their integration is lacking. Inhibition of microglia/macrophage activation and mitigation of neuronal loss have been reported, and potentially indicate indirect mechanisms of action19.
Supplementation of glia
Supplementation of glia has not attracted major interest thus far. This may be related to the so-called glial scarring following stroke, primarily considered a plasticity-impairing phenomenon. While astroglia is present in abundance, there is scarce data on the fate of oligodendrocytes. We observed the extensive demyelination of still-viable axons after small striatal infarcts (unpublished data), which might cause clinical deficits. This stroke sequel could potentially be addressed by transplantation of glial/oligodendrocyte progenitors. The glial/oligodendrocyte precursors (GPs) exhibit an excellent differentiation potential and could become an attractive supplement for stroke therapy (Fig. 2)20. Indeed, remyelination and axon regeneration after olfactory-ensheathing glia transplantation has been observed in the rat, but the origin of this myelin (host or graft) remains unknown21.
Figure 2. Structural support of stem cell grafts.
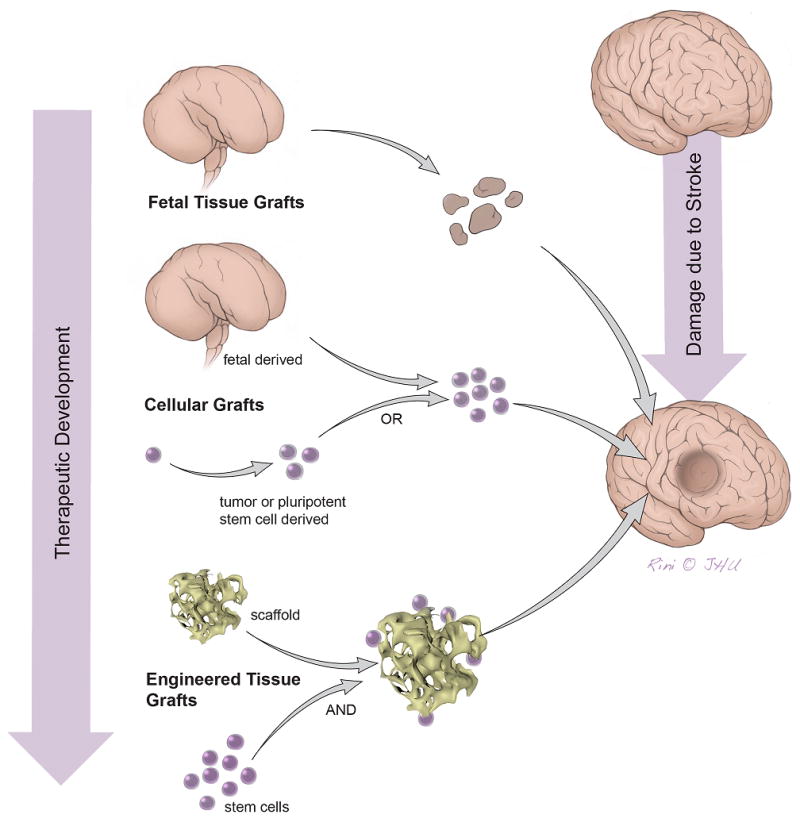
Overview on different cerebral tissue restoration strategies using pluripotent stem cell populations with or without structural support. Future, “true” restorative strategies will most likely have to rely on scaffold support.
Brain tissue engineering for stroke
While the above-mentioned approaches were focused on the delivery of cell suspensions to the living brain tissue, there have been attempts to repopulate the post-stroke tissue cavity in the chronic phase. Any physiological guidance structure such as radial glia or white matter bundles is likely to be absent in a lesion cavity, but may be crucially required for ensuring graft differentiation at the desired location. Moreover, when repopulating larger brain lesions, the neuroanatomical structure of the repopulated brain elements must be resembled while larger transplants will have to provide vascular-like elements for adequate blood supply and a structural support of the tissue matrix. These aspects call for scaffolds to support the graft (Fig. 2). It was demonstrated that injectable scaffolds, such as polyglycolic acid (PLGA) particles, can serve as a structural support for neural stem cells when delivered directly to the stroke cavity22. Moreover, the efficient integration of such neuro-scaffolds within host tissue has been observed. The various scaffolding approaches for brain tissue engineering have been extensively reviewed23. Matrigel scaffolds were shown to provide a permissive environment for neuronal differentiation, with some transplanted cells revealing spontaneous action potentials or excitatory postsynaptic currents by post mortem whole-cell patch-clamp records24. This coincided with behavioral improvements, but again, the existence of direct connections between the transplanted neurons and host circuitries were not investigated. Polymer fibrous scaffolds have been shown to excellently support neural stem cells, and differentiated neurons probably reconstituted some anatomical connections, including long-distance projections25. Some authors even propose channel-like, large matrices seeded with cells in vitro before neurosurgical implantation into the infarcted hemisphere (Fig. 2)26.
Part II: recovery without replacement and the prevalence of indirect mechanisms
Functional recovery was originally interpreted as directly related to graft survival and integration. Thus, ensuring these aspects was thought important for therapeutic success, but eventually became a substantial challenge. In their pioneering study, Bühnemann and colleagues reported initial engraftment and impressive subsequent expansion of murine ESC-derived neural stem cells (NSCs), leading to newly formed tissue four weeks after transplantation in the rat brain27. Differentiation into all neural lineages, projections into the host tissue, and even appropriate electrophysiological activities of graft-derived neurons and astroglia were observed. However, most of the grafts disappeared between four and 12 weeks after transplantation and those that survived were subject to a significant decline in graft size of 90% or more. Despite thorough immunosuppression, enhanced microglial activity, rather than apoptosis and/or inappropriate metabolic graft support, were reportedly responsible for this loss.
On the other hand, neuronal replacement does not necessarily ameliorate functional impairments28, while allogeneic transplantation leading to differentiation and structural integration may be successful in the healthy brain, but not necessarily after stroke29. Experimental transplantation of various adult stem and progenitor cell-containing populations is frequently performed systemically to facilitate future clinical translation30, 31, and has been repeatedly reported to improve neuronal function after stroke without neuronal replacement or prolonged graft survival, usually in a dose-dependent fashion32. Mesenchymal stem cells (MSCs) are a prominent example. Although a differentiation capability into neuronal cells has been proposed33, MSCs are thought to exert their therapeutic potential primarily by secretion of numerous paracrine factors, so called “bystander effects”34, 35. Since MSCs were shown to robustly improve multiple outcome measures after experimental stroke36, early stage clinical studies using MSCs are currently underway to invest the approach in the clinical environment. The uncomplicated MSCs derivation and the possibility for autologous use make them even more attractive. Although optimal conditions of application remain to be defined for experimental therapies employing adult cell populations37, such therapies were also reported to have much wider time windows than rTPa lysis and can address several degenerative or pro-regenerative mechanisms after stroke38 apart from cell replacement (Fig. 3, Table). The following paragraphs will discuss challenges for tissue replacement approaches and alternative strategies utilizing “bystander effects” exerted by NSCs and selected non-neuronal, adult stem cell populations.
Figure 3. Pathophysiological aspects following ischemic stroke.
A plethora of stroke sequelae contribute to immediate and delayed cell loss and impaired regeneration. Transplanted cells have been shown to affect relevant pathophysiological mechanisms, but successful treatment seems to be highly dependent on the time point of transplantation. When administered in the acute phase of stroke, cells can attenuate primary neural cell death, detrimental neuroinflammation and promote vascular repair and neovascularization. These early effects were frequently observed together with low or absent cell survival and integration, or even rely on cell scavenging. By contrast, the modulation of factors determining stroke outcome in the chronic phase seems to require prolonged presence of transplanted cells. Therapeutically relevant brain repair and attenuation of chronic inflammation demands the secretion of sufficient amounts of trophic factors and continuous manipulation of perivascular spaces.
Considerable challenges for tissue replacement therapies in the adult lesioned brain
Although replacement of lost cerebral tissue theoretically seems to be the most attractive strategy to achieve sustained restoration of neuronal function in the chronic phases after stroke26, related therapeutic concepts may be hampered by practical difficulties even if graft survival was ultimately ensured. Stem cell differentiation must be perfectly synchronized with respect to functional, spatial, and temporal dimensions in order to ensure a permanent and sustained therapeutic effect. Functional aspects comprise the frequency and type of graft-derived neuronal, astro- and oligodendroglial cells, as well as the exact numerical ratio between those in a particular brain area to be repopulated. Adequate access to cerebral blood supply and the neuroimmunological integrity of the graft must be ensured. Newborn cells further need to integrate at the correct place and at the correct time to interact meaningfully with each other, and, most importantly, with the remaining host brain tissue. Moreover, a plethora of pathophysiological responses take place after ischemic stroke, with some occurring simultaneously and most being detrimental39, and all of which must be taken into consideration.
A perfect interaction of stem cell-borne neural populations is observed during embryo- and fetogenesis under physiological conditions, resulting in the human brain as one of the most complex organs and its neocortex as a masterpiece of evolution. Despite important advances in the understanding of brain and cortical development40, 41, our knowledge regarding the cellular, biochemical, and electrophysiological processes directing this interplay still remains fragmentary42. On the other hand, even minor disturbances during this susceptible and delicate process can have significant consequences43, 44, and there is evidence for a strict regulation and temporal limitation of structured postnatal neurogenesis45. This may, at least partly, account for the observation of teratomas46 or teratocarcinomas47 after transplantation of pluripotent stem cells into the adult mammalian brain. These tumors resemble rather primitive tissue formations, such as the neural tube, and typically emerge when precise regulatory processes of cell growth and differentiation are absent or have ceased48. Thus, the assumption that the highly complex and incompletely understood pre- and early postnatal cerebral development can be locally recapitulated in the lesioned and adult brain only by placing pluripotent cells at a desired location may be simplistic. The risk of uncontrolled differentiation may be controlled effectively by ensuring a limited expansion potential of the grafted cells by previous differentiation49, selection50, or the induction of suicide genes into transplanted cells. But, as yet, we can present neither a convincing concept of how to precisely orchestrate and control stem cell differentiation and integration in the adult and lesioned brain, nor can we be confident that this regulation occurs both spontaneously and autonomously.
However, promising opportunities to beneficially influence the course of lesion development and maturation with stem cell-based therapies, which do not necessarily rely on cellular differentiation and integration, and might therefore not require a detailed understanding of the underlying processes, could be utilized alternatively. This becomes especially relevant since our understanding of stroke as a focal event embedded in a systemic pathophysiological context is currently completing. Moreover, this approach can be considered advantageous from a pragmatic point of view: it may be easier to realize than complete deciphering of fundamental cerebral developmental biology.
Extracerebral causes of neuronal damage and the impact of stem cell transplantation
Recent data suggest that stroke, formerly recognized as a brain-specific disease, is accompanied by a number of pathophysiological processes throughout the entire organism. In particular, the interaction between the immune and the central nervous system (CNS) was highlighted when immigrating splenic monocytes were identified as major contributors to delayed brain damage51. A beneficial influence of adult stem cell-containing populations, such us human umbilical cord blood cells, was found countering these processes52, and an interruption of the splenic response by intravenously injected NSCs has been described after hemorrhagic stroke53. Systemically administered hematopoietic stem cells have been reported to accumulate in the spleen, down-regulate inflammatory genes and to attenuate deleterious brain inflammation after stroke54. It was moreover reported in an animal sepsis model that intravenously injected MSCs could reprogram pulmonary macrophages to reduce inflammatory cytokine secretion55. From these observations, it is tempting to speculate that immunomodulatory effects of transplanted cells are independent from the cell type, but rather a result of an interaction with specific macrophage populations being responsible for the maintenance of daily immune tolerance56.
Neuroprotection, growth factors, and angiogenesis: the role of bystander effects
Interestingly, there is no study that convincingly shows that neuronal differentiation is a prerequisite for functional improvement following stroke, which is consequently discussed as strongly related to trophic effects rather than neuronal replacement57. If the repopulation of the lesioned brain is too challenging, a promising alternative approach is the prevention of neuronal cell death beyond the acute phase. In fact, a formidable amount of tissue damage has been reported to occur in the subacute phase of stroke39, providing excellent opportunities for therapeutic intervention.
It has been suggested that the neuroprotective properties of NSCs may be a fundamental characteristic of their biological constitution58. If the cells are a source of restorative processes, they must be more resistant against the detrimental influences that occur in the hostile micro-milieu after CNS damage. NSCs can modulate the local environment to prevent such influences59, thereby supporting the actions of neighboring neurons as a “bystander effect.” NSCs can, among others, also produce a broad spectrum of trophic factors, such as nerve growth factor NGF, brain-derived neurotrophic factor (BDNF), and glia-derived neurotrophic factor (GDNF)60, which play pivotal roles in neuroprotection, as they mitigate caspase-mediated apoptosis in the injured CNS61. GDNF has been reported to be neuroprotective62 by promoting cell survival, but also enhances axonal outgrowth and synaptogenesis. To augment the growth factor-mediated neuroprotective potential of NSCs, therapeutic approaches that employ the induced overexpression of factors such as BDNF63 for the treatment of ischemic stroke, or GDNF64 for hemorrhagic stroke, have been suggested. These strategies can even be combined with the use of cytoprotectants, making transplanted NSCs more resilient against oxidative stress and reperfusion injury65, 66. In all these cases, permanent survival of the graft is not a necessary condition to elicit the beneficial effect. Next to the growth-factor-exerted neuroprotection in the subacute stage following stroke, the support of angiogenesis may contribute to the beneficial effects mediated by NSCs. In particular, the normalization of cerebral blood flow (CBF) and blood-brain barrier integrity in peri-lesional areas is a relevant factor for functional restoration following stroke67. Indeed, NSCs were shown to restore blood-brain barrier integrity and enhance angiogenesis in the post-ischemic brain68. This is probably mediated by vascular endothelial growth factor (VEGF), which can also help to preserve the microvasculature after cerebral ischemia69. Thus, neural stem cells have the potential to preserve and restore an adequate CBF in the post-stroke brain. Importantly, “bystander effects” by secretion of immunomodulatory or (neuro)trophic paracrine factors as reviewed above are considered a very important if not universal therapeutic mechanism which is also exerted by adult stem cell populations such as MSCs35 and umbilical cord blood mononuclear cells (MNCs)70, 71.
The neurovascular niche and post-stroke brain plasticity
The preservation and recovery of the cerebral microvasculature is of importance beyond the maintenance of CBF because of a close physiological interplay between angiogenesis and neurogenesis. This interaction, demanding spatial proximity, has been characterized as occurring in the neurovascular unit or neurovascular niche72. It was further suggested that blood vessels represent directional structures for migrating endogenous neural stem and progenitor cells73. Hence, it comes as no surprise that the potent pro-angiogenic factor VEGF was not only shown to play a major role in the regulation of the neurovascular niche74, but also in the recruitment of neural stem cells into it75. The neurovascular niche has been discussed as relevant for post-stroke recovery76, and NSCs may support this role by contributing to the preservation of the cerebral microvasculature.
Functional improvement has often been observed within a relatively short time after NSCs transplantation77. De novo generated neurons, regardless of emerging from endogenous or exogenous stem cells, are very unlikely to have induced this recovery78. Recovery was therefore suggested to be caused by other processes than draft-derived neuronal differentiation and the impact of NSCs on brain plasticity was investigated. An enhanced density in the network of corticostriatal, -thalamic, and -spinal connections was observed in stem cell-treated rats, which was related to functional recovery. Interestingly, VEGF was again found to be a key mediator of these effects79. Moreover, increased dendritic branching80 and increased synaptic plasticity has been reported81, both related to swift functional recovery within three weeks after transplantation. This time is shorter than the time anticipated as being required for neuronal differentiation and integration, which indirectly supports the notion that functional recovery induced by NSCs transplantation is not dependent on neuronal replacement.
Neuroimmunological aspects
Ischemic cell death leads to damage associated molecular pattern (DAMP) signaling and sterile tissue inflammation that contributes to brain damage, but also orchestrates clearance and wound healing processes82. Moreover, a persistent pro-inflammatory environment reduces the regenerative potential of the brain83 and impairs brain function84. Immunomodulation by transplanted cells therefore represents a promising option to ameliorate acute and long-term stroke consequences. In fact, it was reported that intravenously transplanted NSC exhibit a pathotropism towards the inflamed brain and could arbitrate long-term immunomodulatory effects85. Both NSCs and MSCs hold a receptor and ligand machinery enabling them to follow chemogradients and transmigrate into the ischemic brain. Interestingly, the mechanisms of stem cell homing resemble in many aspects that of leukocytes that drive post-stroke inflammation86, 87. In experimental stroke models, transplanted NSCs were found in the ischemic lesion border where they cause a down-regulation of pro-inflammatory cytokines88. MSCs were shown to effectively inhibit the infiltration of detrimental leukocyte populations, thereby improving functional outcome89, 90. Systemically transplanted NSCs and MSCs could further migrate towards draining lymph nodes and suppress antigen-specific T cell responses91, 92, a process that may be highly relevant for long-term outcome of stroke82, 93.
Translationally relevant aspects of tissue-restoring and bystander cell therapies: time windows, administration routes and potential adverse effects
The predominant modes of action (see Table) exerted by a particular cell therapy will have a significant impact on its practical implementation. One of the clinically most important features is the time window in which a therapy is effective. Given the narrow time window of rTPA lysis94, any significant extension of the time window would provide a clear benefit. Indeed, time windows between 4 hours and 7 days have been described for MNCs from cord blood95 and bone marrow96, whereas time windows of up to a month are reported for MSCs97. However, “bystander effects” targeting post-stroke pathomechanisms are most likely restricted to a time window since those processes damp as lesion maturating proceeds (Fig. 3). In contrast, the time window of a therapy initiating a stem cell-based brain tissue restoration would be theoretically unlimited. Therapy induction in the chronic phase, when the hostile post-stroke environment82 has ceased, may even be beneficial.
Another clinically important aspect is the route of cell administration and related safety concerns. Cell therapies exerting tissue restoration or relying on intracerebral “bystander effects” will require local (i.e. intraparenchymal, intraventricular) or intraarterial cell delivery, respectively. While stereotaxic cell transplantation comes at a small, but considerable risk of secondary damage98, intraarterial administration of larger cells such as MSCs or the application excessive cell numbers may lead to secondary infarctions99, 100. While this risk is not apparent for smaller cell populations such as MNCs or GPs99, those may be in turn pass cerebral circulation in significant numbers without reaching their primary site of action. Intravenous cell administration is discussed as a clinically unproblematic approach, especially useful for cell populations exerting “bystander effects” that are thought to target also extra-cerebral causes of post-stroke neuronal damage. In this scenario, larger cells can be trapped within so called filter organs featuring an extensive capillary network such as the lungs or the spleen. This risk is particularly prominent for MSCs, but also for NSCs101. Potential consequences of the trapping phenomenon including splenic or pulmonary microinfarction are currently unclear and may hence require further investigation. Next to the induction of cerebral micro-infarction or secondary brain tissue damage, local and intraarterial cell administration may come at the risk of tumor-like neoplasms or tissue overgrowth, which is prevalent for cell populations exhibiting a strong proliferation potential41. These potentially detrimental side effects must be ruled out for a certain cell therapy approach before considering its clinical application.
Summary
While the initial stem cell transplantation studies in stroke were aimed toward a cell replacement strategy, there is growing evidence that many of the beneficial effects are mediated by indirect mechanisms, such as trophic support and immunomodulation. This has been paralleled by an increasing understanding of the considerable difficulties that may challenge tissue replacement strategies in the adult lesioned brain. However, state-of-the-art, rapid technological advances, e.g. in the field of biomaterials, may support or enable “true” neurorestoration by cellular replacement or even de novo formation of structured and functional brain tissue. This would allow us to capitalize on both functional recovery by neurorestoration, as well as functional preservation and enhanced plasticity by indirect stem cell-mediated effects.
Supplementary Material
Acknowledgments
We thank Mary McAllister for editorial assistance and associate professor David Rini, Department of Art as Applied to Medicine, Johns Hopkins University for creating figure artwork.
Funding
NIH grant 1R21NS081544, Maryland Stem Cell Research foundation Exploratory grant 0178-00, National Centre for Research and Development, European research area network NEURON grant “MEMS-IRBI”.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CBF
cerebral blood flow
- ChAT
choline acetyltransferase
- CNS
central nervous system
- DAMP
danger associated molecular pattern
- ESC
embryonic stem cell
- GABA
gamma-aminobutyric acid
- GDNF
glia-derived neurotrophic factor
- GPs
glial/oligodendrocyte precursors
- hNSE
human neuron-specific enolase
- IL-1β
interleukin-1β
- iPSCs
induced pluripotent stem cells
- MCAO
middle cerebral artery occlusion
- MSCs
mesenchymal stem cells
- MNCs
mononuclear cells
- NGF
nerve growth factor
- NSCs
neural stem cells
- NT2N
Ntera2/D1 neuron-like
- PET
position emission tomography
- PLGA
poly(lactic-co-glycolic acid)
- rTPa
recombinant tissue plasminogen
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
None.
References
- 1.Auriel E, Bornstein NM. Neuroprotection in acute ischemic stroke--current status. J Cell Mol Med. 2010;14:2200–2202. doi: 10.1111/j.1582-4934.2010.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Balami JS, Sutherland BA, Buchan AM. Complications associated with recombinant tissue plasminogen activator therapy for acute ischaemic stroke. CNS Neurol Disord Drug Targets. 2013;12:155–169. doi: 10.2174/18715273112119990050. [DOI] [PubMed] [Google Scholar]
- 4.Starby H, Delavaran H, Andsberg G, Lovkvist H, Norrving B, Lindgren A. Multiplicity of risk factors in ischemic stroke patients: Relations to age, sex, and subtype - a study of 2,505 patients from the lund stroke register. Neuroepidemiology. 2014;42:161–168. doi: 10.1159/000357150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 6.Jakala P, Jolkkonen J. Time for a neurorestorative therapy in stroke. Expert Opin Biol Ther. 2012;12:267–270. doi: 10.1517/14712598.2012.656086. [DOI] [PubMed] [Google Scholar]
- 7.Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, et al. Grafts of fetal dopamine neurons survive and improve motor function in parkinson’s disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- 8.Aihara N, Mizukawa K, Koide K, Mabe H, Nishino H. Striatal grafts in infarct striatopallidum increase gaba release, reorganize gabaa receptor and improve water-maze learning in the rat. Brain Res Bull. 1994;33:483–488. doi: 10.1016/0361-9230(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 9.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (nt2n cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 10.Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 11.Meltzer CC, Kondziolka D, Villemagne VL, Wechsler L, Goldstein S, Thulborn KR, et al. Serial [18f] fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. Neurosurgery. 2001;49:586–591. doi: 10.1097/00006123-200109000-00011. discussion 591–582. [DOI] [PubMed] [Google Scholar]
- 12.Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, et al. Clonal human (hnt) neuron grafts for stroke therapy: Neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 14.Daadi MM, Lee SH, Arac A, Grueter BA, Bhatnagar R, Maag AL, et al. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant. 2009;18:815–826. doi: 10.3727/096368909X470829. [DOI] [PubMed] [Google Scholar]
- 15.Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci. 2007;26:605–614. doi: 10.1111/j.1460-9568.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- 16.Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Mack GS. Reneuron and stemcells get green light for neural stem cell trials. Nature biotechnology. 2011;29:95–97. doi: 10.1038/nbt0211-95. [DOI] [PubMed] [Google Scholar]
- 18.Tajiri N, Quach DM, Kaneko Y, Wu S, Lee D, Lam T, et al. Behavioral and histopathological assessment of adult ischemic rat brains after intracerebral transplantation of nsi-566rsc cell lines. PLoS One. 2014;9:e91408. doi: 10.1371/journal.pone.0091408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatarishvili J, Oki K, Monni E, Koch P, Memanishvili T, Buga AM, et al. Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor Neurol Neurosci. 2014;32:547–558. doi: 10.3233/RNN-140404. [DOI] [PubMed] [Google Scholar]
- 20.Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi X, Kang Y, Hu Q, Chen C, Yang L, Wang K, et al. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain Res. 2010;1317:257–267. doi: 10.1016/j.brainres.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 22.Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by plga particles. Biomaterials. 2009;30:2985–2994. [Google Scholar]
- 23.Delcroix GJ, Schiller PC, Benoit JP, Montero-Menei CN. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials. 2010;31:2105–2120. doi: 10.1016/j.biomaterials.2009.11.084. [DOI] [PubMed] [Google Scholar]
- 24.Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, et al. Transplantation of human neural precursor cells in matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood Flow Metab. 2010;30:534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nature biotechnology. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 26.Dihne M, Hartung HP, Seitz RJ. Restoring neuronal function after stroke by cell replacement: Anatomic and functional considerations. Stroke. 2011;42:2342–2350. doi: 10.1161/STROKEAHA.111.613422. [DOI] [PubMed] [Google Scholar]
- 27.Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, et al. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- 28.Bliss TM, Kelly S, Shah AK, Foo WC, Kohli P, Stokes C, et al. Transplantation of hnt neurons into the ischemic cortex: Cell survival and effect on sensorimotor behavior. J Neurosci Res. 2006;83:1004–1014. doi: 10.1002/jnr.20800. [DOI] [PubMed] [Google Scholar]
- 29.Dubois-Dauphin M, Julien S. Stem cell-derived neurons grafted in the striatum are expelled out of the brain after chronic cortical stroke. Stroke. 2010;41:1807–1814. doi: 10.1161/STROKEAHA.110.578427. [DOI] [PubMed] [Google Scholar]
- 30.Boltze J, Reich DM, Hau S, Reymann KG, Strassburger M, Lobsien D, et al. Assessment of neuroprotective effects of human umbilical cord blood mononuclear cell subpopulations in vitro and in vivo. Cell Transplant. 2012;21:723–737. doi: 10.3727/096368911X586783. [DOI] [PubMed] [Google Scholar]
- 31.Kranz A, Wagner DC, Kamprad M, Scholz M, Schmidt UR, Nitzsche F, et al. Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res. 2010;1315:128–136. doi: 10.1016/j.brainres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Janowski M, Date I. Systemic neurotransplantation--a problem-oriented systematic review. Reviews in the neurosciences. 2009;20:39–60. doi: 10.1515/revneuro.2009.20.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Taran R, Mamidi MK, Singh G, Dutta S, Parhar IS, John JP, et al. In vitro and in vivo neurogenic potential of mesenchymal stem cells isolated from different sources. Journal of biosciences. 2014;39:157–169. doi: 10.1007/s12038-013-9409-5. [DOI] [PubMed] [Google Scholar]
- 34.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 35.Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, et al. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271–2285. doi: 10.1016/j.biochi.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weise G, Lorenz M, Posel C, Maria Riegelsberger U, Storbeck V, Kamprad M, et al. Transplantation of cryopreserved human umbilical cord blood mononuclear cells does not induce sustained recovery after experimental stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2014;34:e1–9. doi: 10.1038/jcbfm.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckert MA, Vu Q, Xie K, Yu J, Liao W, Cramer SC, et al. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1322–1334. doi: 10.1038/jcbfm.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 40.Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 42.Breunig JJ, Haydar TF, Rakic P. Neural stem cells: Historical perspective and future prospects. Neuron. 2011;70:614–625. doi: 10.1016/j.neuron.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selemon LD, Ceritoglu C, Ratnanather JT, Wang L, Harms MP, Aldridge K, et al. Distinct abnormalities of the primate prefrontal cortex caused by ionizing radiation in early or midgestation. J Comp Neurol. 2013;521:1040–1053. doi: 10.1002/cne.23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- 45.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seminatore C, Polentes J, Ellman D, Kozubenko N, Itier V, Tine S, et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke. 2010;41:153–159. doi: 10.1161/STROKEAHA.109.563015. [DOI] [PubMed] [Google Scholar]
- 47.Erdo F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 48.Lawinger P, Venugopal R, Guo ZS, Immaneni A, Sengupta D, Lu W, et al. The neuronal repressor rest/nrsf is an essential regulator in medulloblastoma cells. Nat Med. 2000;6:826–831. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- 49.Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of parkinson’s disease: Effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- 50.Chung S, Shin BS, Hedlund E, Pruszak J, Ferree A, Kang UJ, et al. Genetic selection of sox1gfp-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J Neurochem. 2006;97:1467–1480. doi: 10.1111/j.1471-4159.2006.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory t cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 52.Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 54.Schwarting S, Litwak S, Hao W, Bahr M, Weise J, Neumann H. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke. 2008;39:2867–2875. doi: 10.1161/STROKEAHA.108.513978. [DOI] [PubMed] [Google Scholar]
- 55.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin e(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nature reviews Immunology. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guzman R. Cellular stroke therapy: From cell replacement to trophic support. Expert Rev Cardiovasc Ther. 2009;7:1187–1190. doi: 10.1586/erc.09.121. [DOI] [PubMed] [Google Scholar]
- 58.Imitola J, Park KI, Teng YD, Nisim S, Lachyankar M, Ourednik J, et al. Stem cells: Cross-talk and developmental programs. Philos Trans R Soc Lond B Biol Sci. 2004;359:823–837. doi: 10.1098/rstb.2004.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carletti B, Piemonte F, Rossi F. Neuroprotection: The emerging concept of restorative neural stem cell biology for the treatment of neurodegenerative diseases. Curr Neuropharmacol. 2011;9:313–317. doi: 10.2174/157015911795596603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen N, Lee SB, Lee YS, Lee KH, Ahn JY. Neuroprotection by ngf and bdnf against neurotoxin-exerted apoptotic death in neural stem cells are mediated through trk receptors, activating pi3-kinase and mapk pathways. Neurochem Res. 2009;34:942–951. doi: 10.1007/s11064-008-9848-9. [DOI] [PubMed] [Google Scholar]
- 62.Du Y, Zhang X, Tao Q, Chen S, Le W. Adeno-associated virus type 2 vector-mediated glial cell line-derived neurotrophic factor gene transfer induces neuroprotection and neuroregeneration in a ubiquitin-proteasome system impairment animal model of parkinson’s disease. Neurodegener Dis. 2013;11:113–128. doi: 10.1159/000334527. [DOI] [PubMed] [Google Scholar]
- 63.Chang DJ, Lee N, Choi C, Jeon I, Oh SH, Shin DA, et al. Therapeutic effect of bdnf-overexpressing human neural stem cells (hb1.F3.Bdnf) in a rodent model of middle cerebral artery occlusion. Cell Transplant. 2013;22:1441–1452. doi: 10.3727/096368912X657323. [DOI] [PubMed] [Google Scholar]
- 64.Lee HJ, Park IH, Kim HJ, Kim SU. Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage. Gene Ther. 2009;16:1066–1076. doi: 10.1038/gt.2009.51. [DOI] [PubMed] [Google Scholar]
- 65.Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, et al. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sakata H, Niizuma K, Wakai T, Narasimhan P, Maier CM, Chan PH. Neural stem cells genetically modified to overexpress cu/zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke. 2012;43:2423–2429. doi: 10.1161/STROKEAHA.112.656900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mostany R, Chowdhury TG, Johnston DG, Portonovo SA, Carmichael ST, Portera-Cailliau C. Local hemodynamics dictate long-term dendritic plasticity in peri-infarct cortex. J Neurosci. 2010;30:14116–14126. doi: 10.1523/JNEUROSCI.3908-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, et al. Intracarotid injection of fluorescence activated cell-sorted cd49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- 69.Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via hif-1alpha-regulated vegf signaling. J Cereb Blood Flow Metab. 2008;28:1530–1542. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hau S, Reich DM, Scholz M, Naumann W, Emmrich F, Kamprad M, et al. Evidence for neuroprotective properties of human umbilical cord blood cells after neuronal hypoxia in vitro. BMC Neurosci. 2008;9:30. doi: 10.1186/1471-2202-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reich DM, Hau S, Stahl T, Scholz M, Naumann W, Emmrich F, et al. Neuronal hypoxia in vitro: Investigation of therapeutic principles of hucb-mnc and cd133+ stem cells. BMC Neurosci. 2008;9:91. doi: 10.1186/1471-2202-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev. 2010;6:238–244. doi: 10.2174/157340310791658802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: Vegf- and bdnf-mediated cross-talk between neural stem cells and endothelial cells: An in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt NO, Koeder D, Messing M, Mueller FJ, Aboody KS, Kim SU, et al. Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res. 2009;1268:24–37. doi: 10.1016/j.brainres.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 76.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu W, Chen X, Hu C, Li J, Yu Z, Cai W. Transplantation of neural stem cells expressing hypoxia-inducible factor-1alpha (hif-1alpha) improves behavioral recovery in a rat stroke model. J Clin Neurosci. 2010;17:92–95. doi: 10.1016/j.jocn.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 78.Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 79.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minnerup J, Kim JB, Schmidt A, Diederich K, Bauer H, Schilling M, et al. Effects of neural progenitor cells on sensorimotor recovery and endogenous repair mechanisms after photothrombotic stroke. Stroke. 2011;42:1757–1763. doi: 10.1161/STROKEAHA.110.599282. [DOI] [PubMed] [Google Scholar]
- 81.Patkar S, Tate R, Modo M, Plevin R, Carswell HV. Conditionally immortalised neural stem cells promote functional recovery and brain plasticity after transient focal cerebral ischaemia in mice. Stem Cell Res. 2012;8:14–25. doi: 10.1016/j.scr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131:2564–2578. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 86.Andres RH, Choi R, Pendharkar AV, Gaeta X, Wang N, Nathan JK, et al. The ccr2/ccl2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke. 2011;42:2923–2931. doi: 10.1161/STROKEAHA.110.606368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pluchino S, Cossetti C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia. 2013;61:1379–1401. doi: 10.1002/glia.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 89.Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY, et al. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by tgf-beta. Neurobiology of disease. 2013;58:249–257. doi: 10.1016/j.nbd.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Wang LQ, Lin ZZ, Zhang HX, Shao B, Xiao L, Jiang HG, et al. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS neuroscience & therapeutics. 2014;20:317–326. doi: 10.1111/cns.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 2007;61:209–218. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- 92.Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, Slavin S, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Archives of neurology. 2008;65:753–761. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- 93.Urra X, Miro F, Chamorro A, Planas AM. Antigen-specific immune reactions to ischemic stroke. Frontiers in cellular neuroscience. 2014;8:278. doi: 10.3389/fncel.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hacke W, Lichy C. Thrombolysis for acute stroke under antiplatelet therapy: Safe enough to be beneficial? Nat Clin Pract Neurol. 2008;4:474–475. doi: 10.1038/ncpneuro0867. [DOI] [PubMed] [Google Scholar]
- 95.Boltze J, Schmidt UR, Reich DM, Kranz A, Reymann KG, Strassburger M, et al. Determination of the therapeutic time window for human umbilical cord blood mononuclear cell transplantation following experimental stroke in rats. Cell Transplant. 2012;21:1199–1211. doi: 10.3727/096368911X589609. [DOI] [PubMed] [Google Scholar]
- 96.de Vasconcelos Dos Santos A, da Costa Reis J, Diaz Paredes B, Moraes L, Jasmin, Giraldi-Guimaraes A, et al. Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res. 2010;1306:149–158. doi: 10.1016/j.brainres.2009.09.094. [DOI] [PubMed] [Google Scholar]
- 97.Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 98.Potts MB, Silvestrini MT, Lim DA. Devices for cell transplantation into the central nervous system: Design considerations and emerging technologies. Surg Neurol Int. 2013;4:S22–30. doi: 10.4103/2152-7806.109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW, et al. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab. 2013;33:921–927. doi: 10.1038/jcbfm.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui L, Kerkela E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6:11. doi: 10.1186/scrt544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




