Abstract
Background
Reproductive hormones are known to impact innate mucosal immune function of the lower genital tract. Our objectives were to determine the effect of hormonal status on intrinsic anti-viral (HSV-1, HSV-2 and HIV-1) activity of cervicovaginal lavage (CVL).
Methods
CVL was collected from165 asymptomatic women which included post-menopausal women (n=29), women not on contraception in the days 1-14 (n=26) or days 15-28 (n=27) of the menstrual cycle, and women using the levonogerestrol intrauterine device (n=28), depomedroxyprogesterone acetate (n=28) or combined oral contraceptives (n=27). The anti-HSV-1/-2 and the anti-HIV-1 activity of the CVL were measured using plaque assays and the Jurkat-Tat-CCR5 assay, respectively.
Results
CVL from all of the groups had modest anti-viral activity. Anti-HIV-1 activity was decreased in CVL from postmenopausal women when compared to premenopausal women (11% vs. 34%, p=0.002). However there was no difference in anti-HIV-1 activity among premenopausal women regardless of phase of menstrual cycle or contraceptive use. Anti-HIV-1 activity was associated with the protein content of the CVL (r=0.44, p<0.001). There was no difference in anti-HSV-1 or -2 activity by hormonal group.
Conclusions
Menopause is associated with decreased innate HIV-1 activity in the lower genital tract, suggesting that factors in the vaginal fluid could play a role in increased susceptibility of HIV-1 infection in postmenopausal women. Hormonal contraceptive use, menopause and phase of menstrual cycle did not have a measurable impact on the intrinsic anti-HSV-1 or -2 activity.
Keywords: Menopause, Contraception, Human Immunodeficiency Virus, Herpes Simplex Virus, Innate Mucosal Immunity
Introduction
The prevalence of human immunodeficiency virus-1 (HIV-1) infection among older adults is increasing due to improved survival of HIV-infected patients on antiretroviral therapy as well as new primary HIV-1 infections in older patients [1]. In 2009, an estimated 12% of new HIV-1 infections in United States women occurred among those greater than 50 years of age [2]. People in this age group are less likely to be knowledgeable about HIV-1 [3], to be screened for HIV-1 or HIV-1-risk factors by their care providers [4], and to use condoms [3]. Additionally, biochemical, cellular and structural changes that occur at menopause may increase risk of HIV-1-acquisition in women [5]. Thus, postmenopausal women may be at greater risk for HIV-1-acquisition due to social, behavioral, and biological factors.
Cervicovaginal fluid is composed of cervical mucus, secretions from the upper genital tract, and transudate from the vaginal epithelium and provides an important first-line defense against pathogens, such as HIV-1 and herpes simplex virus-1 and 2 (HSV-1 and HSV-2, respectively). Previous studies have shown that cervicovaginal lavage (CVL) has both anti-HIV-1 [6-9] and anti-HSV-2 activities [10]. Components of the vaginal fluid such as antimicrobial peptides, cytokines, chemokines and bacterial products contribute to the innate immunity of the female reproductive tract.
The mucosal immune defense of the lower female genital tract has evolved to be responsive to reproductive hormones over the menstrual cycle to balance immune defense to infection with procreation, which is dependent on sperm penetration of the upper genital tract [11]. Keller and colleagues reported that the concentrations of secretory leukocyte protease inhibitor (SLPI), human beta-defensin-2, alpha defensins 1–3, lysozyme, and lactoferrin were lowest at mid-cycle when compared to both the proliferative and secretory stages of the menstrual cycle in CVL samples [12]. However, other studies have shown no effect of menstrual cycle stage on antimicrobial and cytokine levels within FRT secretions [10, 13, 14]. These differences could be due to different methods of CVL collection (such as the amount or type of lavage fluid used or the duration of lavage time) or significant intra- and inter-individual variation.
It is well established that the function of the innate immune system declines with aging [15]; and several studies have demonstrated immune senescence of the lower genital tract mucosal defense [5]. The loss of estrogen after menopause results not only in the thinning of the vaginal epithelium, but also the decline of antimicrobial concentrations in vaginal secretions and protective components of the vaginal microbiome including lactobacilli [16]. With menopause, there is a decrease of cervical mucus production [17] and a significant decrease in vaginal fluid viscosity as measured in CVL [18]. In addition to mediators present in cervicovaginal fluid, changes in the numbers of CD4+ T-cells in the genital tract have also been reported in postmenopausal women. Meditz, et al, compared 22 healthy premenopausal women to 24 postmenopausal women and reported that the percentage of CD4+ T cells expressing the CCR5 receptor in both the cervix and the blood were increased in the postmenopausal women. These authors postulated that postmenopausal women could have an increased risk of HIV-1 [19].
Exogenous reproductive hormones used for contraception impact the concentrations of immune factors in the vaginal fluid, secreted from the epithelial cells lining both the upper and lower genital tract. Secretions from polarized uterine epithelial cells can inhibit growth or kill pathogens, including HIV-1 [20]. Incubation of polarized uterine epithelial cells with estradiol increased the capacity of the secretions to inhibit Staphylococcus aureus growth, indicating that estradiol modulates expression of immune factors [21]. Endometrial biopsies obtained after exposure to depot medroxyprogesterone acetate (DMPA) showed decreased expression of SLPI compared with biopsies obtained at prior to DMPA treatment [22]. The dose, route of administration and type of progestin may have different effects on the lower genital tract defense. Combined oral contraceptive pills (COCs) as well as the levonorgestrel intrauterine device (LNG-IUD) have been associated with decreased expression of human beta-defensin-1 and -2, but not SLPI [23]. The primary aim of this study was to characterize the impact of endogenous and exogenous reproductive hormones on the innate anti-HSV-1, anti-HSV-2 and anti-HIV-1 activities of vaginal fluid collected by CVL.
Materials and Methods
Following Institutional Review Board approval by the University of Pittsburgh, informed consent was obtained from healthy, asymptomatic, HIV-negative women who were either between 18-46 years of age or over the age of 50. The study population and enrollment procedures were previously described in detail [18]. Briefly, we enrolled women into the study who fell into the six following categories: 1) premenopausal women not using exogenous hormones in days 1-14, 2) days 15-28 of the menstrual cycle, 3) combined-oral contraceptive (COCs), 4) medroxyprogesterone acetate (DMPA), 5) levonorgestrel IUD (LNG-IUD), and 6) postmenopausal based on age >50 years with amenorrhea for at least 1 year. Postmenopausal women receiving hormone replacement therapy were excluded. None of the postmenopausal women reported taking supplements containing phytoestrogens. A vaginal swab for pH, wet mount microscopy and Gram stain was collected. For collection of the CVL, 10 mL of sterile normal saline was placed into the vagina; a lavage was performed for 1 minute and placed into 15 mL conical vial with 100 μL of protease inhibitor (Sigma-Aldrich), similar to the CVL technique done in previous studies of anti-HIV activity [9]. Participant samples were given a unique identifier upon arrival to the laboratory, thus all of the investigators preforming the laboratory assays were masked to the hormonal status of the woman from whom the sample was obtained. All CVL samples were stored at -70°C until they were thawed for immediate testing. The protein content of the CVL samples was determined by the Lowry method.
Anti-HIV-1 Assay
The innate anti-HIV-1 activity of CVL was tested using the Jurkat-Tat-CCR5 assay. Jurkat-Tat-CCR5 cells (kindly provided by Quentin J. Sattentau, University of Oxford) were maintained in RPMI-1640 (cRPMI) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin along with 250 μg/mL Hygromycin B (for tat selection) and 500 μg/mL Geneticin (for CCR5 selection). After washing, 5×104 cells were added to each well of a 96-well plate; each treatment was performed in triplicate. Assays to evaluate the effect of CVL on Jurkat cell viability were performed in parallel with the anti-HIV activity assay. CVL was diluted to 1:16 and added to the appropriate wells and an equal volume of RPMI only (for viability assays) or RPMI containing 3000 TCID50 of HIV-1BaL with 2 μg of DEAE (for efficacy assays) was added to a final volume of 200 μL resulting in a 1:32 final dilution of the CVL. Control wells for viability assays consisted of medium only and for efficacy assays consisted of medium with HIV-1. The 96 well plates were incubated in a humidified chamber at 37°C/5% CO2. On day 4, half the volume of culture medium was removed and fresh medium was replenished. On day 7, cell viability and anti-HIV activity was determined. For cell viability, half the medium was removed from the toxicity plate and an equal volume of CellTiter-Glo (Promega, Madison WI) was added. Luminescence was measured, averaged, and cell viability was determined as deviations from the medium only wells. For anti-HIV-1 activity, half of the medium was collected and HIV-1 p24gag antigen was quantified by ELISA (Alliance; Perkin-Elmer, Waltham, MA) and averaged. HIV-1 suppression was defined as the one minus the quotient of HIV-1 p24gag antigen with the CVL and the control multiplied by 100. Triplicates rarely differed from each other. When it occurred, the outlier value was removed only if it exceeded 10-fold difference from the other two values. Assays were repeated only when the controls did not fall into the range of expected values.
Anti-HSV Assays
HSV-1 or -2 (10 μl with 105 -106 TCID50) was mixed with 90 μl of CVL and incubated at 37°C for 1 hour. Two 40 μL aliquots were taken from each sample, diluted to 400 μl with RPMI 1640 medium (Fisher) with 1% fetal calf serum (Atlanta Biologicals) and added to Vero cell monolayers in two separate wells on 12 well tissue culture plates (Falcon) for 1 hour. The wells were then washed and overlaid with 0.4% agarose in RPMI 1640 medium containing 2% fetal calf serum. The plates were incubated for 5 days at 37°C following which 100 μL of dye (MTT 5mg/mL in PBS) (Sigma) was added to each well and after 3 hours the number of plaques was counted. Control samples contained only virus and medium and the difference between the plaque number of the control samples and the CVL-virus mixtures was used as a measure of antiviral activity. The duplicate anti-HSV activities from each CVL were averaged and there were no significant variation between duplicates. HSV suppression was defined as one minus this percentage. The CVL samples were assess for HSV-1 and -2 immunoglobulin G (IgG) using an enzyme-linked immunosorbent assay (GenWay, San Diego).
Statistical Analysis
One-way analysis of variance and Spearman rank correlation coefficient were used to evaluate the associations of anti-viral activity with hormonal group and CVL protein content, using IBM SPSS Statistical software version 22 (IBM Corp, Armonk, NY, USA).
Results
Demographic Characteristics
The demographic characteristics of the study population are shown in Table 1 and were previously published [18]. Women in the postmenopausal group had a mean age of 56 years as compared to the women in the premenopausal groups who had a mean age of 29. There were no statistically significant differences in the premenopausal groups, except that women using DMPA were more likely to identify as black (p<0.001) and report using tobacco (p=0.046). Additionally, there was no statistically significant difference in the number of sexual partners (p=0.06). Women using hormonal contraception and postmenopausal women were less likely to report using condoms [18]. Motile sperm suggesting very recent sexual exposure were not noted to be present in any of the wet mount fluids evaluated by microscopy at the time of enrollment. By Gram stain, sperm were noted in only 6 (3.6%) of the women, only one of whom was post-menopausal.
Table 1. Demographic Characteristics.
| Days 1-14 (N=26) | Days 15-28 (N=27) | OCPs (N=27) | DMPA (N=28) | LNG-IUD (N=28) | Post-menopausal (N=29) | |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 29.8 ± 7.8 | 27.0 ± 6.5 | 28.6 ± 9.5 | 29.4 ± 6.1 | 29.0 ± 5.2 | 56.2 ± 7.0 |
| BMI (mean ± SD) | 30.8 ± 9.8 | 25.1 ± 6.5 | 25.4 ± 5.9 | 27.3 ± 6.4 | 27.6 ± 6.2 | 30.6 ± 7.0 |
| Race | ||||||
| White | 14 (53.8%) | 14 (51.9%) | 24 (88.9%) | 9 (32.1%) | 22 (78.6%) | 22 (75.9%) |
| Black | 10 (38.5%) | 7 (25.9%) | 2 (7.4%) | 19 (67.9%) | 5 (17.9%) | 7 (24.1%) |
| Asian | 0 | 3 (11.1%) | 1 (3.7%) | 0 | 1 (3.6%) | 0 |
| Other | 2 (7.7%) | 3 (11.1%) | 0 | 0 | 0 | 0 |
| Condom use (most or all of the time) | 10 (38.5%) | 13 (48.1%) | 3 (11.1%) | 6 (21.4%) | 4 (14.3%) | 0 |
| Current # partners | ||||||
| None | 12 (46.2%) | 5 (18.5%) | 7 (25.9%) | 7 (25.0%) | 4 (14.3%) | 14 (48.3%) |
| One | 12 (46.2%) | 21 (77.8%) | 19 (70.4%) | 20 (71.4%) | 22 (78.6%) | 15 (51.7%) |
| Two or more | 2 (7.7%) | 1 (3.7%) | 1 (3.7%) | 1 (3.6%) | 2 (7.1%) | 0 |
| Current smoker | 6 (23.1%) | 5 (18.5%) | 3 (11.1%) | 11 (39.3%) | 2 (7.1%) | 4 (13.8%) |
| Bacterial Vaginosis | 8 (30.7%) | 5 (18.5%) | 1 (3.7%) | 8 (28.6%) | 2 (2.1%) | N/A |
Anti-Viral Activities
Overall, CVL moderately suppressed both HIV and HSV growth (Table 2). All women of reproductive age had similar levels of endogenous anti-HIV-1 activity irrespective of time in cycle or contraceptive hormone use (Table 2). By contrast, post-menopausal women had significantly lower levels of anti-HIV-1 activity than all of the women of reproductive age. After combining all of the results from 136 premenopausal women and comparing these to the 28 postmenopausal, postmenopausal women had significantly less anti-HIV-1 activity than the CVL from premenopausal women (34% vs 11%, p=0.002, Figure 1). Postmenopausal women had significantly less protein in the CVL (Table 2); and protein content of the CVL was positively correlated with innate anti-HIV-1 activity (r=0.44, p<0.001). Although postmenopausal women had numerically lower endogenous anti-HSV activity than women of reproductive age, there was no statistically significant difference in anti-HSV-1 or -2 activity of the CVL samples among the groups characterized by hormonal status (Table 2). Anti-HSV IgG was detected in the CVL of 19 (11.5%) of the 165 women. We found no correlation between the presence of IgG in the CVL and innate anti HSV-1 or HSV-2 activity.
Table 2. The Association of Reproductive Hormones with Innate Antiviral Activity.
| Group | Number | HSV-1 Suppression vs. Control (%) | HSV-2 Suppression vs. Control (%) | HIV-1 Suppression vs. Control (%) | Ratio of CVL Protein Content (Premenopausal: Postmenopausal) |
|---|---|---|---|---|---|
| Postmenopausal | 29 | 23.22 ± 15.46 | 36.56 ± 29.36 | 10.96 ± 29.41* | 1 |
| Days 1-14 | 26 | 31.20 ± 15.91 | 40.23 ± 31.50 | 39.29 ± 40.57 | 2.2 |
| Days 15-28 | 27 | 30.21 ± 29.38 | 39.26 ± 36.85 | 26.49 ± 38.45 | 2.6 |
| OCP | 27 | 28.09 ± 21.18 | 52.86 ± 24.42 | 35.77 ± 31.72 | 2.7 |
| DMPA | 28 | 31.95 ± 22.03 | 38.25 ± 41.61 | 32.95 ± 35.00 | 1.8 |
| LNG-IUD | 28 | 25.09 ± 26.34 | 48.18 ± 30.55 | 37.88 ± 37.22 | 3.1 |
| P-Value** | 0.63 | 0.38 | 0.04*** | <0.001 |
One postmenopausal result outlier excluded.
P-value from one way analysis of variance
HIV-1 suppression was lower among postmenopausal women compared to reproductive-aged women (11% vs. 34%, respectively; P=0.002)
Figure 1. Innate anti-HIV-1 Activity of Vaginal Fluid Comparing 136 Reproductive Aged Women to 28 Postmenopausal Women (P=0.002).
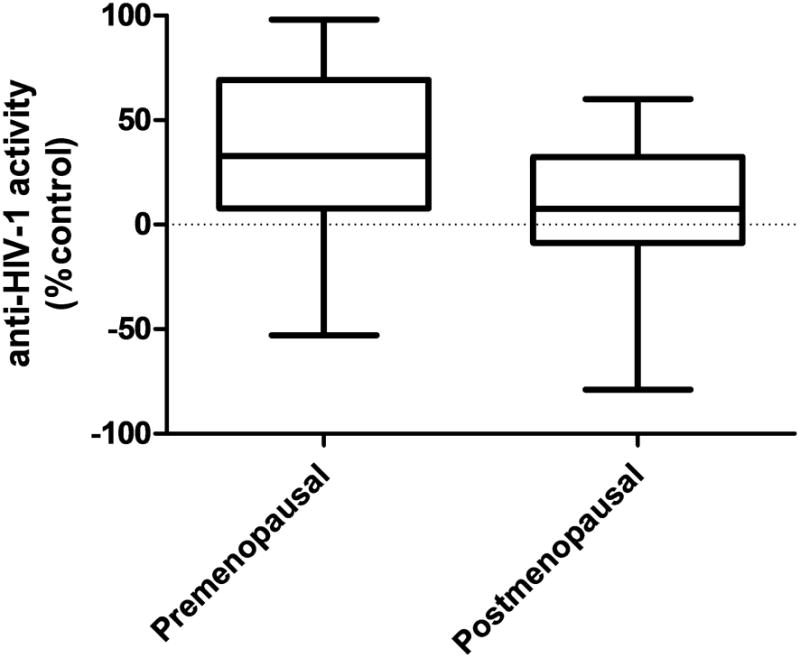
Figure 1 compares the median anti-HIV-1 activity for postmenopausal women to that of premenopausal women. Postmenopausal women had significantly less innate anti-HIV-1 activity than women of reproductive age (11% vs. 34%, respectively).
Comment
CVL samples from postmenopausal women had significantly less anti-HIV-1 activity than CVL samples from premenopausal women. These findings suggest that factors present in CVL contribute to the innate mucosal vaginal immunity against HIV-1 are modulated by reproductive hormones and have increased activity when estrogen is present. These data also support a potential increased vulnerability of postmenopausal women to HIV-1 infection. Postmenopausal women have decreased CVL protein content [18]; and CVL protein was positively associated with anti-HIV-1 activity. Therefore, the decreased genital anti-HIV-1 activity seen in postmenopausal women could partly be due to decreased CVL protein content. In the present study, phase of menstrual cycle and use of exogenous contraceptive hormones were not significantly associated with the innate anti-HIV-1 activity of vaginal fluid.
This is a large study of innate anti-viral activity (both HSV-1, -2 and HIV-1) of CVL in women stratified by contraceptive use, phase of menstrual cycle and menopause. One limitation of the present study is that the classification of hormonal status was determined by participant self-report and was not verified by review of medical records or measurement of reproductive or contraceptive hormones in the plasma. However, misclassification of post-menopause was unlikely given the mean age of the women (56.2 years). Large standard deviation values indicate significant variation in the innate anti-viral activities of CVF between women even using the same methods of contraception or in the same phase of the menstrual cycle. This significant population level variation in anti-viral activity could have limited the detection differences between groups. Some of the variation in anti-HSV activity could have been due to previous exposure to HSV and an adaptive immune response. Serum was not collected from women to test for past exposure to HSV-1 and HSV-2. However, there was no association between the presence of IgG against HSV-1 and HSV-2 in the CVL sample and innate antiviral effect. Our results in premenopausal women differ from those previously published by Shust et al. reporting decreased anti-HSV-2 activity with oral contraceptive use versus women in the luteal phase not using exogenous hormones (p=0.09) [10]. The methods for detection of anti-HSV activity and study design (multiple samples were taken from the same study participant) were different from the present study, which may account for the difference in results.
Despite these limitations, we were able to show that postmenopausal women have decreased anti-HIV-1 activity in the CVL when compared to premenopausal women. Our findings confirm other studies that reported that antimicrobial activity of genital tract secretions of women is higher in the presence of estrogen [11]. We also showed that anti-HIV activity was related to protein content of CVL. Postmenopausal women have decreased levels of protein in their CVL compared to women of reproductive age[18], suggesting that cervicovaginal protein content may be mediated by the presence of estrogen. Use of DMPA has been associated with a reduction in serum estradiol levels [24], which could be related biologic mechanism of the increased risk of HIV infection in women using DMPA [25-29]. Further studies should identify which immune factors are hormonally regulated and may contribute to enhanced susceptibility of postmenopausal women or women using DMPA for contraception to HIV. Clinicians should also be aware of the possible increased risk of HIV in postmenopausal women so that sexually active postmenopausal women who are not in monogamous relationships or do not know the HIV serostatus of their partners can be counseled to use barrier methods of protection.
Acknowledgments
This study was supported by the US National Institutes of Health Grant Number AI 082639.
Footnotes
Disclosure: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gebo KA, Moore RD. Treatment of HIV infection in the older patient. Expert Rev Anti Infect Ther. 2004;2:733–743. doi: 10.1586/14789072.2.5.733. [DOI] [PubMed] [Google Scholar]
- 2.Prejean J, Song R, Hernandez A, Ziebell T, Green T, Walker F, Lin L, An Q, Mermin J, Lansky A, Hall HI. Estimated HIV incidence in the United States, 2006-2009. PLoS ONE. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prati G, Mazzoni D, Zani B. Psychosocial predictors and HIV-related behaviors of older adults verses middle-aged and younger adults. J Aging Health. 2014 Jun 19; doi: 10.1177/0898264314538664. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Levy JA, Ory MG, Crystal S. HIV/AIDS interventions for midlife and older adults: Current status and challenges. J Acquir Immune Defic Syndr. 2003;33:S59–S67. doi: 10.1097/00126334-200306012-00002. [DOI] [PubMed] [Google Scholar]
- 5.Wira C, Patel M, Ghosh M, Mukura L, Fahey J. Innate immunity in the human female reproductive tract: Endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS ONE. 2010;5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahey T, Ghosh M, Fahey JV, Shen Z, Mukura LR, Song Y, Cu-Uvin S, Mayer KH, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. Selective impact of HIV disease progression on the innate immune system in the human female reproductive tract. PLoS ONE. 2012;7:e38100. doi: 10.1371/journal.pone.0038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson BL, Ghosh M, Raker C, Fahey J, Song Y, Rouse DJ, Wira CR, Cu-Uvin S. In vitro anti-HIV-1 activity in cervicovaginal secretions from pregnant and nonpregnant women. Am J Obstet Gynecol. 2012;207:65.e1–65.e10. doi: 10.1016/j.ajog.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dezzutti CS, Hendrix CW, Marrazzo JM, Pan Z, Wang L, Louissaint N, Kalyoussef S, Torres NM, Hladik F, Parikh U, Mellors J, Hillier SL, Herold BC. Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators. PLoS ONE. 2011;6:e23136. doi: 10.1371/journal.pone.0023136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shust GF, Cho S, Kim M, Madan RP, Guzman EM, Pollack M, Epstein J, Cohen HW, Keller MJ, Herold BC. Female genital tract secretions inhibit herpes simplex virus infection: correlation with soluble mucosal immune mediators and impact of hormonal contraception. Am J Reprod Immunol. 2010;63:110–119. doi: 10.1111/j.1600-0897.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wira CR, Fahey JV, Rodriguez-Garcia M, Shen Z, Patel MV. Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. Am J Reprod Immunol. 2014;72:236–258. doi: 10.1111/aji.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller MJ, Guzman E, Hazrati E, Kasowitz A, Cheshenko N, Wallenstein S, Cole AL, Cole AM, Profy AT, Wira CR, Hogarty K, Herold BC. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 13.Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol. 2002;187:561–568. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 14.Patel MV, Ghosh M, Fahey JV, Ochsenbauer C, Rossoll RM, Wira CR. Innate immunity in the vagina (Part II): anti-HIV activity and antiviral content of human vaginal secretions. Am J Reprod Immunol. 2014;72:22–33. doi: 10.1111/aji.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogra PL. Aging and its possible impact on mucosal immune responses. Aging Res Rev. 2010;9:101–106. doi: 10.1016/j.arr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Goetz EJ, Huang MC, Don J, Patel K, Schwartz JB, Fast K, Ferrucci L, Madara K, Taub DD, Longo DL. Gender specificity of altered human immune cytokine profiles in aging. FASEB J. 2010;24:3580–3589. doi: 10.1096/fj.10-160911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw JL, Petraki C, Watson C, Bocking A, Diamandis EP. Role of tissue kallikrein-related peptidases in cervical mucus remodeling and host defense. Biol Chem. 2008;389:1513–1522. doi: 10.1515/BC.2008.171. [DOI] [PubMed] [Google Scholar]
- 18.Chappell CA, Rohan LC, Moncla BJ, Wang L, Meyn LA, Bunge K, Hillier SL. The effects of reproductive hormones on the physical properties of cervicovaginal fluid. Am J Obstet Gynecol. 2014 Mar 21; doi: 10.1016/j.ajog.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meditz A, Moreau K, MaWhinney S, Gozansky W, Melander K, Kohrt W, Wierman M, Connick E. CCR5 Expression is Elevated in Endocervical CD4+ T-Cells in Healthy Postmenopausal Women. J Acquir Innune Defic Syndr. 2012 Mar 1;59(3):221–228. doi: 10.1097/QAI.0b013e31823fd215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, Hill ET, Fahey JV. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 2011;4:335–342. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li A, Felix JC, Yang W, Jain JK. Effect of mifepristone on the expression of endometrial secretory leukocyte protease inhibitor in new medroxyprogesterone acetate users. Fertil Steril. 2008;90:872–875. doi: 10.1016/j.fertnstert.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Fleming DC, King AE, Williams AR, Critchley HO, Kelly RW. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril. 2003;79:856–863. doi: 10.1016/s0015-0282(02)04930-0. [DOI] [PubMed] [Google Scholar]
- 24.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000 Sep;96(3):431–9. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 25.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. The Lancet infectious diseases. 2013 Jul 18; doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 26.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet infectious diseases. 2012 Jan;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007 Aug 20;21(13):1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 28.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010 Jul 17;24(11):1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison CS, Chen PL, Kwok C, et al. Hormonal Contraception and the Risk of HIV Acquisition: An Individual Participant Data Meta-analysis. PLoS Med. 2015 Jan 22;12(1):e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]


