Abstract
Objective
Congenital diaphragmatic hernia (CDH) results in morbidity and mortality due to lung hypoplasia and persistent pulmonary hypertension (PH). We sought to define the relationship between fetal ultrasound markers of severity in CDH and the time to resolution of neonatal PH.
Study design
We conducted a retrospective study of fetuses with an antenatal ultrasound and left-sided CDH cared for at the University of California San Francisco (2002–12). Fetal liver position was classified on ultrasound as abdominal (entire liver within the abdomen) or thoracic (any portion of the liver within the thorax). Fetal stomach position was classified from least to most aberrant: abdominal, anterior left chest, mid-posterior left chest, or retrocardiac (right chest). Lung-to-head ratio (LHR) was determined from available scans at 20–29 weeks gestational age (GA). Routine neonatal echocardiograms were performed weekly for up to 6 weeks or until PH resolved, or until discharge. PH was assessed by echocardiogram using a hierarchy of ductus arteriosus level shunt, interventricular septal position, and tricuspid regurgitant jet velocity. Days to PH-free survival was defined as the age at which pulmonary artery pressure was estimated to be <2/3 systemic blood pressure. Cox proportional hazards models adjusted for GA at birth, era of birth, fetal surgery, and GA at ultrasound (LHR model only), with censoring at 100 days.
Results
Of 118 patients, fetal markers were available as follows: LHR (n=53), liver position (n=112), and stomach position (n=80). Fewer infants resolved PH if they had LHR<1 (p=0.006), thoracic liver position (p=0.001), or more aberrant stomach position (p<0.001). There was also a decreased rate of resolution of PH in infants with LHR <1 (hazard ratio 0.30, p=0.007), thoracic liver position (hazard ratio 0.38, p<0.001), and more aberrant stomach position (hazard ratios 0.28, p=0.002; 0.1, p<0.001; 0.07 p<0.001).
Conclusion
Fetal ultrasound markers of CDH severity are predictive not just of mortality but also of significant morbidity. LHR<1, thoracic liver, and aberrant stomach position are associated with delayed time to resolution of PH in infants with CDH and may be used to identify fetuses at high risk of persistent PH.
Keywords: Pulmonary vascular resistance, ultrasound, lung-to-head ratio
INTRODUCTION
Congenital diaphragmatic hernia (CDH) occurs in 1:4,000–5000 live births and is associated with significant morbidity and mortality.1,2 Contemporary care strategies have dramatically improved the survival of these infants, particularly at referral centers.3,4 Though this improvement is substantial, many of these infants are surviving with significant morbidity related to their pulmonary hypoplasia and pulmonary hypertension (PH) leading to prolonged need for assisted ventilation, home oxygen use, re-hospitalization, developmental delay, and complicated medication use.5–9
Despite the high rates of morbidity, research in this population has thus far focused heavily on only mortality and need for extracorporeal membrane oxygenation (ECMO). As such, antenatal sonographic markers of severity in fetuses with CDH have primarily been used to predict mortality and the need for ECMO, as well as to select candidates for fetal intervention. The lung-to-head ratio (LHR), particularly LHR<1, 10–18 and thoracic liver position (as compared to abdominal position) have been shown to be predictors of both death and need for ECMO use.11,14–16,18,19 More recently it has been demonstrated that increasingly abnormal stomach position is a strong predictor of not only death and ECMO, but also need for prolonged neonatal respiratory support.20 This is likely due to the relationship of stomach position to liver position.21,22
We and others have shown that persistence of PH beyond 2 to 3 weeks of life is associated with poor neonatal outcomes and thus there may be benefits to early identification of these infants at high risk of morbidity for antenatal counseling, selection for fetal intervention, and targeting future early neonatal therapies.8,23,24 This makes the prediction of persistent PH in infants with CDH an important and useful determination during fetal life. This study is the first to assess the ability of fetal LHR, liver position, and stomach position to predict both resolution of PH and the time required to achieve its resolution.
MATERIALS AND METHODS
Patient Data
We performed a retrospective cohort study of fetuses with antenatal ultrasound and left-sided Bochdalek-type congenital diaphragmatic hernia at our center, who were subsequently cared for after birth at the University of California San Francisco Benioff Children’s Hospital (2002–12). Fetuses were included if they had ≥ 1 neonatal echocardiogram and excluded if they had multiple congenital anomalies or a known or suspected syndrome. Clinical data were collected by chart review. Echocardiograms were re-reviewed by a reader blinded to clinical data. The outcome of interest was days to resolution of pulmonary hypertension, defined as the chronological age at which the echocardiogram estimated <2/3 systemic systolic pulmonary arterial pressure. Chronic lung disease was defined as requirement for any respiratory support at 56d of age or home oxygen if discharged earlier than 56d.25 Patients were managed using gentle ventilation and lung-sparing strategies as previously described.23,24 The Institutional Review Board of the University of California San Francisco approved this study.
Imaging and echocardiographic data
Fetal sonographic markers of interest included the lung-to-head ratio (LHR), liver position and stomach position. LHR measurements were gathered from ultrasound reports or, if absent from the report, were directly measured from stored images, when available, by a single radiologist blinded to clinical outcome. LHR was only included in the analysis if the measurement was taken from a sonogram performed at 20–29 weeks gestational age (GA). All lung dimensions were measured on a transaxial view of the fetal chest at the level of the four-chamber view of the heart. The technique consistently used and reported at UCSF uses the aorta and lateral rib as landmarks for the lateral measurement, with the orthogonal anterior-posterior (AP) diameter measured from the cardiac atria to the posterior rib, then dividing by the head circumference.15,26 All analyses were done first including scans from 22 through 27 weeks’ GA 10,11 and then expanding to 20 to 29 weeks’ GA. As results did not differ when using these two inclusion criteria, data from the broader range of ages are presented to increase the information provided in our analyses. Liver position was gathered from fetal ultrasound reports, and verified by operative report from CDH repair, if surgery was performed. Six infants, all with intrathoracic liver on fetal sonography, died prior to surgical repair and thus liver position could not be verified. Liver position was categorized as abdominal (defined as entire liver within the abdomen) or intrathoracic (defined as any portion of the liver herniated into the thoracic cavity).
A single radiologist blinded to neonatal outcomes reviewed the initial fetal sonogram performed at our institution to determine stomach position when adequate images were available. Stomach position was classified based on the degree of herniation into the thoracic cavity while viewing the fetal thorax in the true axial plane at the level of the four-chamber view of the heart. Positions included abdominal, anterior left chest (defined as a portion of the fetal stomach contacting the anterior chest wall), mid-to-posterior left chest (defined as not contacting the anterior left chest wall and either contacting or possibly contacting the posterior left chest wall), or retrocardiac (defined as at least a portion of the stomach located posterior to the left atrium of the heart within the right chest).
Echocardiogram Protocol
Beginning in 2002, all infants with CDH underwent echocardiograms (Acuson Sequoia C256 and C512 and SC2000, Mountain View CA), per routine clinical protocol, within the first 48 hours of life and then weekly for the first 6 weeks of life or until resolution of pulmonary hypertension, death, or discharge. Standard clinical views were obtained to estimate the degree of elevation of pulmonary arterial pressure relative to systemic systolic blood pressure. Echocardiograms were classified into one of two categories: less than 2/3 systemic systolic pressures (no/mild PH) and greater than or equal to 2/3 systemic systolic pressures (PH). Classifications were made using a hierarchy of measurements involving (1) direction and velocity of flow via the ductus arteriosus (2) interventricular septum position and (3) peak tricuspid regurgitant (TR) jet velocity, by the modified Bernoulli equation and assuming right atrial pressure as 0 mmHg, as previously described.23,24
Statistical Analysis
Between groups univariate comparisons were made using Chi squared, Student’s t-test, Mann Whitney rank-sum, Kruskal Wallis, and ANOVA tests where appropriate. Kaplan Meier curves were compared using Log-rank tests. Cox proportional hazards multivariate models were created to assess the relationship between fetal markers of CDH severity and time to resolution of PH. Infants were censored at 100 days; infants who died were treated as being alive and never having resolved their PH and were censored at 100d of age to avoid informative censoring. We adjusted for GA at birth, era of birth (2002–2005, 2006–2008, and 2009–2012), history of fetal tracheal occlusion, and, for the LHR model only, GA at sonography. Finally, a Harrell’s C statistic was generated for each model to assess the predictive accuracy of each fetal marker in predicting time to resolution of PH (Stata 12.0 software; College Station, TX).
RESULTS
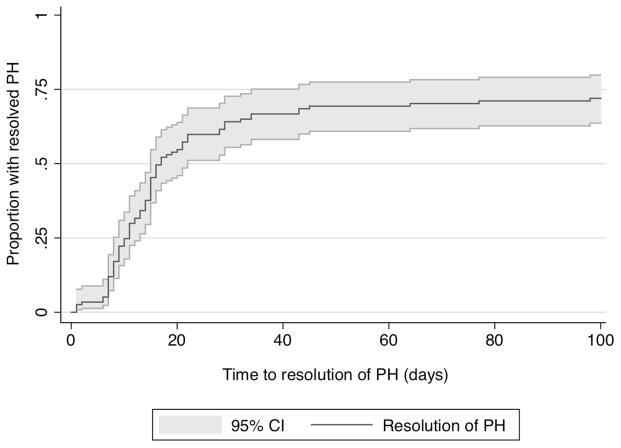
We identified 118 infants with left-sided CDH who had fetal sonography at UCSF. Cohort characteristics are described in Table 1. The cohort was predominantly term, with an overall mortality of 19% (23/118). Seventy-three percent (86/118) resolved their PH prior to death or discharge (Figure 1), with a median time to resolution, among those who resolved, of 14d (IQR 9, 20). Lung-to-head ratio was available in 89 (75%) patients, 53 of which were obtained from 20–29 weeks GA and thus included in the final analysis. There were 29 subjects in whom LHR was not noted in the fetal ultrasound report and original images were unavailable for primary review. Of the 118 subjects, 80 (68%) had adequate images available for determination of stomach position. Liver position was documented in 112 fetuses. The distribution of these measurements is shown in Table 2. There were no obvious differences in newborn characteristics between sub-cohorts, as determined by fetal measurement (Table 1). Additionally, there was no difference in proportion of subjects with available measurements by era across the 10-year study period for liver position, stomach position, or LHR and thus no suggestion of bias introduced by differential missing data by era.
Table 1.
Characteristics of 118 Infants with CDH and Fetal Imaging
| Neonatal characteristics | All infants N= 118 | LHR available* N=53 | Liver position available N=112 | Stomach position available N=80 |
|---|---|---|---|---|
| Gestational age (weeks) | 38 (±2.3) | 37 (±2.6) | 38 (±2.3) | 38 (±2.2) |
| Male sex | 58% (68) | 53% (28) | 59% (66) | 55% (44) |
| Fetal tracheal occlusion surgery | 8% (9) | 15% (8) | 8% (9) | 10% (8) |
| Year of birth | ||||
| 2002 – 2005 | 30% (35) | 26% (14) | 29% (33) | 29% (23) |
| 2006 – 2008 | 25% (29) | 23% (12) | 25% (28) | 25% (20) |
| 2009 – 2012 | 46% (54) | 51% (27) | 46% (51) | 46% (37) |
| Time to resolution of PH (median days, IQR)† | 14 (9, 20) | 15 (10, 21) | 14 (9, 18) | 15 (9, 21) |
| Death | 19% (23) | 32% (17) | 21% (23) | 25% (20) |
| ECMO | 13% (15) | 15% (8) | 13% (15) | 15% (12) |
| Chronic lung disease | 23% (22) | 28% (10) | 22% (20) | 28% (17) |
Data reported as mean (± SD) or %(N) unless otherwise stated
Patients only included if fetal ultrasound was performed between 20 and 29 weeks gestational age. Three infants excluded for scan under 20 weeks; 33 excluded for scan 29 weeks and over.
Time to estimated pulmonary arterial pressures <2/3 systemic systolic pressures among those who resolved prior to death/discharge
Figure 1. Time to resolution of pulmonary hypertension.
Kaplan Meier curve of time to resolution of pulmonary hypertension in all patients, with 95% confidence interval.
Table 2.
Fetal Ultrasound Characteristics
| Ultrasound Parameter | |
|---|---|
| Lung to head ratio (LHR) (n=53) | 1.2 (±0.5) |
| Gestational age (weeks) at US§ | 24 (±2.4) |
| LHR <1 | 36% (19) |
| Liver position (n=112) | |
| Abdominal | 46% (52) |
| Intrathoracic | 54% (60) |
| Stomach position (n=80) | |
| Abdominal | 16% (13) |
| Anterior left chest | 23% (18) |
| Mid-to-posterior left chest | 43% (34) |
| Retrocardiac (right chest) | 19% (15) |
Data reported as mean (± SD) or %(N) unless otherwise stated
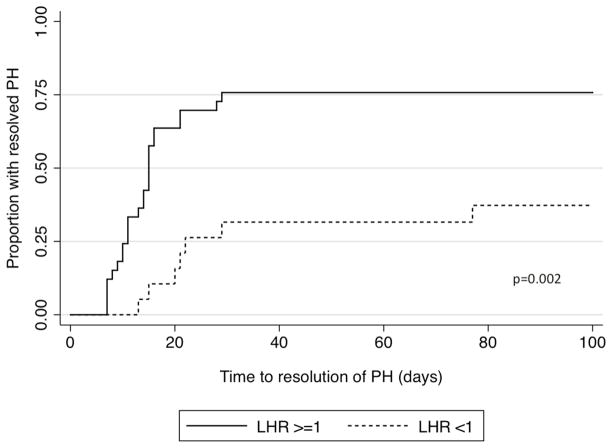
Infants with an LHR<1 had increased death prior to discharge (58% versus 18%, p=0.001), increased need for ECMO (32% versus 6%, p=0.012), and increased incidence of chronic lung disease (75% versus 14%, p=0.001) compared to infants with an LHR≥1. Additionally, a lower proportion of infants with LHR<1 had resolution of their PH prior to death or discharge (42% versus 76%, p=0.012, Figure 2) as well as a longer time to resolution of PH among those who ultimately resolved (median 22d versus 14d, p=0.003). After adjusting for GA at birth and GA at the time of fetal ultrasound, fetal tracheal occlusion, and era of birth, infants with LHR<1 continue to have a prolonged time to resolution (Table 3). However, LHR is only a moderate predictor of rate of resolution of PH with a C statistic of 0.68.
Figure 2. Time to resolution of pulmonary hypertension by lung-to-head ratio.
Kaplan Meier curve of time to resolution of pulmonary hypertension by LHR. (Black solid) LHR ≥1 and (black dash) LHR <1. Curves differ significantly by logrank test, p=0.002.
Table 3.
Resolution of pulmonary hypertension by fetal ultrasound parameter
| Survival to DC | Resolved PH prior to death or DC | Time to resolution of PH (days) * | Adjusted† Hazard Ratio | 95% CI | P value | |
|---|---|---|---|---|---|---|
|
| ||||||
| N (%) | N (%) | Median (IQR) | ||||
| Lung-to-head ratio (n=53)
| ||||||
| >=1 (n=34) | 28 (82%) | 26 (76%) | 14 (9, 15) | Ref | Ref | n/a |
| <1 (n=19) | 8 (42%) | 8 (42%) | 22 (18, 30) | 0.3 | (0.13, 0.72) | 0.007 |
|
| ||||||
| Liver position (n=112)
| ||||||
| Abdominal (n=52) | 51 (98%) | 46 (88%) | 11 (8, 15) | Ref | Ref | n/a |
| Intrathoracic (n=60) | 38 (63%) | 36 (60%) | 17 (12, 28) | 0.38 | (0.24, 0.61) | <0.001 |
|
| ||||||
| Stomach position (n=80)
| ||||||
| Abdominal (n=13) | 13 (100%) | 13 (100%) | 9 (6, 12) | Ref | Ref | n/a |
| Anterior left chest (n=18) | 17 (94%) | 17 (94%) | 15 (14, 16) | 0.28§ | (0.13, 0.62) | 0.002 |
| Mid-to-posterior left chest (n=34) | 24 (71%) | 21 (62%) | 17 (9, 22) | 0.1§ | (0.04, 0.23) | <0.001 |
| Retrocardiac (n=15) | 6 (40%) | 7 (47%) | 22 (11, 34) | 0.07§ | (0.02, 0.20) | <0.001 |
Abbreviations: PH - pulmonary hypertension; DC - discharge; IQR - interquartile range
Lower hazard ratios represent a decreased chance of resolution of pulmonary hypertension
Time to estimated pulmonary arterial pressures <2/3 systemic systolic pressures among those who resolved prior to death/discharge
After adjusting for gestational age, history of fetal surgery, birth era, and (for LHR model) gestational age at fetal ultrasound using Cox proportional hazards models
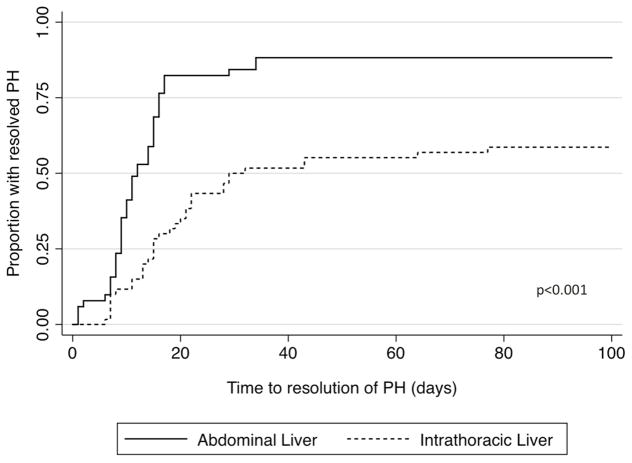
Infants with an intrathoracic liver had increased death prior to discharge (37% versus 2%, p<0.001), increased need for ECMO (25% versus 0%, p<0.001), and increased incidence of chronic lung disease (40% versus 10%, p<0.001) as compared to those with an abdominal liver position. A lower proportion of those with an intrathoracic liver had resolution of their PH (60% versus 88%, p=0.001, Figure 3) as well as a longer time to resolution (median 17d versus 11d, p=0.001), among those who ultimately resolved. After adjusting for GA at birth, fetal tracheal occlusion, and era of birth, infants with intrathoracic liver position continued to have a prolonged time to resolution (Table 3). Liver position is also only a moderate predictor of rate of resolution of PH with a C statistic of 0.66.
Figure 3. Time to resolution of pulmonary hypertension by liver position.
Kaplan Meier curve of time to resolution of pulmonary hypertension by liver position. (Black solid) Abdominal liver position and (black dash) intrathoracic liver position. Curves differ significantly by logrank test, p<0.001.
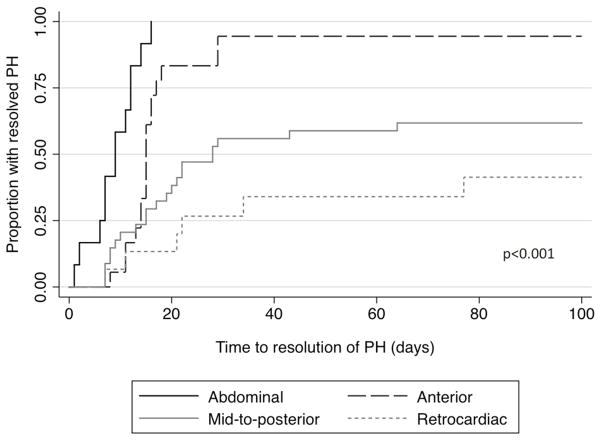
Increasing herniation of stomach into the thoracic cavity (abdominal versus anterior left chest versus mid-to-posterior left chest versus retrocardiac/right chest) was also associated with increasing risk of death (0%, 6%, 29%, and 60%, respectively, p<0.001), increasing need for ECMO (0%, 0%, 15%, and 47%, p=0.001), and increasing incidence of chronic lung disease (8%, 18%, 38%, and 67%, p=0.03). Furthermore, the proportion of those who ultimately resolved their PH decreased with progressive malposition of the stomach (100%, 94%, 62%, and 47%, p<0.001, Figure 4) while the time to resolution increased (median 9d, 15d, 17d, and 22d, p=0.002). After adjusting for GA at birth, fetal tracheal occlusion, and era of birth, infants with progressively abnormal stomach position continued to have a prolonged time to resolution (Table 3). Stomach position is a somewhat better predictor of rate of resolution of PH with a C statistic of 0.73.
Figure 4. Time to resolution of pulmonary hypertension by stomach position.
Kaplan Meier curve of time to resolution of pulmonary hypertension by stomach position. (Black solid) abdominal, (black dash) anterior, (grey solid) mid-posterior, and (grey dash) retrocardiac stomach position. Curves differ significantly by logrank test, p<0.001.
In a sensitivity analysis, evaluations of the effect of fetal markers on time to resolution of PH were repeated with censoring at the time of death for those infants who expired at < 100d of age. There were no substantial effects on the estimated Hazard Ratios (data not shown).
COMMENT
Despite recent improvements in care strategies, congenital diaphragmatic hernia remains a challenging problem for providers both pre- and postnatally. Not only are providers faced with the challenge of predicting mortality, but as survival improves, we must begin to consider the significant morbidities faced by this population. Persistent pulmonary hypertension is a commonly seen in this population and, when persistent, it is well known to be a major cause of mortality and morbidity among infants with CDH. Despite this, fetal sonographic markers that are routinely used in evaluation of CDH have not been used to predict persistent PH in these infants. In this study, we demonstrate that fetal ultrasound markers previously found to be associated with neonatal survival, including LHR ≥1, abdominal liver position and less abnormal stomach position, are also strongly associated with a decreased time to resolution of PH.
As treatment strategies evolve, the survival rate for CDH continues to improve, from 50% in previous eras, to 75–90% in single center reports.3,4,27 However, among survivors, pulmonary hypertension remains a major cause of morbidity 5,6,8,23,28. We have recently confirmed, in a large cohort, that the persistence of PH, as defined in the current study, was itself a biomarker of severe disease in infants with CDH, predicting prolonged ventilation, prolonged respiratory support of any level, need for supplemental home oxygen, and death.24 The current study extends these findings to quantitative antenatal measurements, showing that resolution of PH, as well as the timing of resolution, can be predicted from fetal ultrasound markers already being widely employed. Progressively abnormal fetal measurements are associated with a delay in the usual neonatal fall in pulmonary vascular resistance. Healthy term newborns achieve an estimated pulmonary arterial pressure < 2/3 systemic pressure by 24 hours of life,29 yet infants with CDH with even the best outcomes do not achieve this milestone until 1–3 weeks of age.24 Infants with CDH with estimated pulmonary arterial pressures that remain elevated beyond this time are those at highest risk for poor outcomes and thus may benefit from early identification. As fetal intervention and treatment strategies continue to develop, fetuses and infants may be specifically targeted for antenatal or early neonatal intervention based on unfavorable fetal markers, making these even more valuable for patient and provider decision-making over time.
Our results support recent studies concluding that lower LHR, o/e LHR, o/e total fetal lung volume, and intrathoracic liver position were predictive of an increased incidence of PH.30–32 The current study utilizes our validated approach to the definition and the timing of the assessment of PH in newborns with CDH, 23,24 and thus it expands the findings from other groups by evaluating PH as an outcome over time, in relationship to fetal measurements. A major strength of our study is the use of routine weekly echocardiograms for all infants with CDH. With this clinical protocol, we have not only added the ability to assess resolution of PH over time, but we have also removed the potential selection bias in studies using data from echocardiograms obtained only for clinical indications.8,28,32 Thus, we have determined not only the ultimate resolution of PH, but also an unbiased quantification of the time to its resolution.
Currently, fetal assessment of diaphragmatic hernia is done through measurement of various fetal parameters by both magnetic resonance imaging (MRI) and ultrasonography. While MRI assessment of fetal lung volume has utility, it may be limited due to cost, availability, and expertise required for interpretation. Sonographic measurements that are frequently used include the lung-to-head ratio (with or without use of the observed-to-expected LHR), liver position, and more recently, stomach position. Our current data confirm the results of previous studies showing increased mortality and use of ECMO in patients with LHR <1, liver herniated into the thorax and more aberrant stomach position.10–18,32 This is despite the previously noted limitations of these measurements, which include challenges in accurate determination of liver position due to difficulties in distinguishing liver from lung by fetal ultrasound,11,14–16,18,19,22 the difficult learning curve to achieve competency,33 moderate inter-observer agreement and change in LHR with GA (which may or may not be improved by the use of observed-to-expected LHR),16,18,32 and the variability in classification of stomach position, from current proposals in the literature.20,21,34,35
Our study was limited in its ability to accurately measure pulmonary arterial pressures. Cardiac catheterization is the gold standard for the assessment of pulmonary arterial pressures, though this procedure is invasive so not repeatedly used in this population. Previous work in infants with congenital heart disease has shown Doppler echocardiography to produce accurate estimates of pulmonary arterial pressures.36 In infants with chronic lung disease (including infants with CDH), Mourani and colleagues found TR jet alone did not accurately predict PH by cardiac catheterization; however, multiple echocardiographic parameters together did predict PH.37 Similarly, our previous work shows that utilizing the hierarchy of echocardiographic measurements employed in this study, echocardiographic findings are related to important clinical outcomes, despite the lack of confirmation by cardiac catheterization.24
Another limitation of our study concerns the accuracy and availability of the LHR measurement as it is dependent on GA. Since just over half of our study population had available LHR measurements within our defined GA window, there could be bias in our analysis related to LHR. However, we found no difference in the demographics or clinical outcomes of infants based on available sonographic measurements (Table 1). Further, we have shown that there is no difference in the proportion of subjects with missing data across the study period. It is known that LHR increases in a non-linear fashion as GA increases38 and as such, a variety of ranges for GA have been analyzed to assess ability to predict outcome. Yang et al. found that LHR was more accurate when measured at 24–34 weeks’ GA, and less so at 20–24 weeks’ GA, while Jani et al. showed that LHR at 22–28 weeks’ GA in fetuses with intrathoracic liver position was strongly predictive of neonatal outcome. Other studies have suggested using an observed-to-expected (o/e) LHR since it may be independent of GA,38,39 though recent evidence by Quintero et al. call this into question.40 Their results demonstrate that o/e LHR is not independent of GA and should also be interpreted with caution. In this study, we have focused on LHR rather than o/e LHR and included only measurements from fetuses at 20–29 weeks’ GA. Of note, the majority of included measurements lie within previously accepted ranges, with a median gestational age of 24.2 weeks (IQR 22, 26). As noted, our results did not change with this wider inclusion period in comparison to measurements obtained from 22–27 weeks’ GA. Further, despite this potential limitation, we found that an LHR <1 is associated with a decreased survival and an increased use of ECMO, as previously shown by others.
Conclusions
In conclusion, we have shown that fetal ultrasound markers of survival in CDH are also strongly associated with time to resolution of pulmonary hypertension. LHR ≥1, abdominal liver position, and less abnormal stomach position are favorable antenatal prognostic signs for short-term morbidity. Incorporation of these findings can be useful during prenatal counseling and may identify individual high-risk fetuses who might benefit from intrauterine or early neonatal interventions, as PH is both a major cause of morbidity and a marker of illness severity in CDH. Given the close relationships of various fetal markers, future studies with a larger sample size might define an antenatal decision algorithm to identify combinations of findings yielding prediction of the fetuses at highest risk for neonatal morbidity or mortality.
Acknowledgments
FUNDING SOURCES: Author LA Lusk was supported by a grant from the National Institute of Child Health and Human Development (T32 HD-07162). Author AM Basta was supported by a grant from the National Institute of Biomedical Imaging and Bioengineering (T32 EB001631).
We would like to thank Dr. Mary Norton (University of California San Francisco) for her critical review and assistance with the development of this manuscript.
Footnotes
CONFLICTS OF INTEREST: The authors report no conflicts of interest.
PAPER PRESENTATION: This paper was presented as a platform presentation at the Western Society for Pediatric Research (Jan 23, 2014, Carmel, CA) as well as the Annual Meeting of the Pediatric Academic Society (May 3–6, 2014, Vancouver, British Columbia, Canada).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million California births, 1989–1997. Birth defects research. Part A, Clinical and molecular teratology. 2006 Mar;76(3):170–174. doi: 10.1002/bdra.20230. [DOI] [PubMed] [Google Scholar]
- 2.Balayla J, Abenhaim HA. Incidence, predictors and outcomes of congenital diaphragmatic hernia: a population-based study of 32 million births in the United States. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013 Nov 29; doi: 10.3109/14767058.2013.858691. [DOI] [PubMed] [Google Scholar]
- 3.Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002 Mar;37(3):357–366. doi: 10.1053/jpsu.2002.30834. [DOI] [PubMed] [Google Scholar]
- 4.Bagolan P, Casaccia G, Crescenzi F, Nahom A, Trucchi A, Giorlandino C. Impact of a current treatment protocol on outcome of high-risk congenital diaphragmatic hernia. J Pediatr Surg. 2004 Mar;39(3):313–318. doi: 10.1016/j.jpedsurg.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Bos A, Tibboel D, Koot V, Hazebroek F, Molenaar J. Persistent Pulmonary Hypertension in High-Risk Congenital Diaphragmatic Hernia: Incidence and Vasodilator Therapy. J Pediatr Surg. 1993;28:1463–1465. doi: 10.1016/0022-3468(93)90431-j. [DOI] [PubMed] [Google Scholar]
- 6.Iocono JA, Cilley RE, Mauger DT, Krummel TM, Dillon PW. Postnatal pulmonary hypertension after repair of congenital diaphragmatic hernia: predicting risk and outcome. J Pediatr Surg. 1999 Feb;34(2):349–353. doi: 10.1016/s0022-3468(99)90207-5. [DOI] [PubMed] [Google Scholar]
- 7.Thibeault DW, Haney B. Lung volume, pulmonary vasculature, and factors affecting survival in congenital diaphragmatic hernia. Pediatrics. 1998;101(2):289–295. doi: 10.1542/peds.101.2.289. [DOI] [PubMed] [Google Scholar]
- 8.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;39(3):307–312. doi: 10.1016/j.jpedsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Kinsella JP, Truog WE, Walsh WF, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe persistent pulmonary hypertension of the newborn. The Journal of pediatrics. 1997;131:55–62. doi: 10.1016/s0022-3476(97)70124-0. [DOI] [PubMed] [Google Scholar]
- 10.Aspelund G, Fisher JC, Simpson LL, Stolar CJ. Prenatal lung-head ratio: threshold to predict outcome for congenital diaphragmatic hernia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012 Jul;25(7):1011–1016. doi: 10.3109/14767058.2011.608442. [DOI] [PubMed] [Google Scholar]
- 11.Jani J, Keller RL, Benachi A, et al. Prenatal prediction of survival in isolated left-sided diaphragmatic hernia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2006 Jan;27(1):18–22. doi: 10.1002/uog.2688. [DOI] [PubMed] [Google Scholar]
- 12.Keller RL, Glidden DV, Paek BW, et al. The lung-to-head ratio and fetoscopic temporary tracheal occlusion: prediction of survival in severe left congenital diaphragmatic hernia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2003 Mar;21(3):244–249. doi: 10.1002/uog.44. [DOI] [PubMed] [Google Scholar]
- 13.Laudy JA, Van Gucht M, Van Dooren MF, Wladimiroff JW, Tibboel D. Congenital diaphragmatic hernia: an evaluation of the prognostic value of the lung-to-head ratio and other prenatal parameters. Prenatal diagnosis. 2003 Aug;23(8):634–639. doi: 10.1002/pd.654. [DOI] [PubMed] [Google Scholar]
- 14.Lipshutz GS, Albanese CT, Feldstein VA, et al. Prospective analysis of lung-to-head ratio predicts survival for patients with prenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg. 1997 Nov;32(11):1634–1636. doi: 10.1016/s0022-3468(97)90471-1. [DOI] [PubMed] [Google Scholar]
- 15.Metkus AP, Filly RA, Stringer MD, Harrison MR, Adzick NS. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg. 1996 Jan;31(1):148–151. doi: 10.1016/s0022-3468(96)90338-3. discussion 151–142. [DOI] [PubMed] [Google Scholar]
- 16.Odibo AO, Najaf T, Vachharajani A, Warner B, Mathur A, Warner BW. Predictors of the need for extracorporeal membrane oxygenation and survival in congenital diaphragmatic hernia: a center's 10-year experience. Prenatal diagnosis. 2010 Jun;30(6):518–521. doi: 10.1002/pd.2508. [DOI] [PubMed] [Google Scholar]
- 17.Knox E, Lissauer D, Khan K, Kilby M. Prenatal detection of pulmonary hypoplasia in fetuses with congenital diaphragmatic hernia: a systematic review and meta-analysis of diagnostic studies. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010 Jul;23(7):579–588. doi: 10.3109/14767050903551400. [DOI] [PubMed] [Google Scholar]
- 18.Hedrick HL, Danzer E, Merchant A, et al. Liver position and lung-to-head ratio for prediction of extracorporeal membrane oxygenation and survival in isolated left congenital diaphragmatic hernia. American journal of obstetrics and gynecology. 2007 Oct;197(4):422.e421–424. doi: 10.1016/j.ajog.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Mullassery D, Ba'ath ME, Jesudason EC, Losty PD. Value of liver herniation in prediction of outcome in fetal congenital diaphragmatic hernia: a systematic review and meta-analysis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010 May;35(5):609–614. doi: 10.1002/uog.7586. [DOI] [PubMed] [Google Scholar]
- 20.Kitano Y, Okuyama H, Saito M, et al. Re-evaluation of stomach position as a simple prognostic factor in fetal left congenital diaphragmatic hernia: a multicenter survey in Japan. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011 Mar;37(3):277–282. doi: 10.1002/uog.8892. [DOI] [PubMed] [Google Scholar]
- 21.Cordier AG, Cannie MM, Guilbaud L, et al. Stomach position versus liver-to-thoracic volume ratio in left-sided congenital diaphragmatic hernia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014 Apr 25; doi: 10.3109/14767058.2014.906576. [DOI] [PubMed] [Google Scholar]
- 22.Bootstaylor BS, Filly RA, Harrison MR, Adzick NS. Prenatal sonographic predictors of liver herniation in congenital diaphragmatic hernia. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1995 Jul;14(7):515–520. doi: 10.7863/jum.1995.14.7.515. [DOI] [PubMed] [Google Scholar]
- 23.Keller RL, Tacy TA, Hendricks-Munoz K, et al. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. American journal of respiratory and critical care medicine. 2010 Aug 15;182(4):555–561. doi: 10.1164/rccm.200907-1126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusk LA, Wai KC, Moon-Grady AJ, Steurer MA, Keller RL. Persistence of pulmonary hypertension by echocardiography predicts short-term outcomes in congenital diaphragmatic hernia. The Journal of pediatrics. 2015 Feb;166(2):251–256. e251. doi: 10.1016/j.jpeds.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American journal of respiratory and critical care medicine. 2001 Jun;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 26.Keller RL. Antenatal and postnatal lung and vascular anatomic and functional studies in congenital diaphragmatic hernia: implications for clinical management. American journal of medical genetics. Part C, Seminars in medical genetics. 2007 May 15;145C(2):184–200. doi: 10.1002/ajmg.c.30130. [DOI] [PubMed] [Google Scholar]
- 27.Downard CD, Jaksic T, Garza JJ, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003 May;38(5):729–732. doi: 10.1016/jpsu.2003.50194. [DOI] [PubMed] [Google Scholar]
- 28.Wynn J, Krishnan U, Aspelund G, et al. Outcomes of congenital diaphragmatic hernia in the modern era of management. The Journal of pediatrics. 2013 Jul;163(1):114–119. e111. doi: 10.1016/j.jpeds.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner JR, Boys RJ, Hunter S, Hey E. Non-invasive assessment of pulmonary arterial pressure in healthy neonates. Archives of disease in childhood. 1991;66:386–390. doi: 10.1136/adc.66.4_spec_no.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spaggiari E, Stirnemann JJ, Sonigo P, Khen-Dunlop N, De Saint Blanquat L, Ville Y. Prenatal Prediction Of Pulmonary Arterial Hypertension In Congenital Diaphragmatic Hernia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2014 Jun 27; doi: 10.1002/uog.13450. [DOI] [PubMed] [Google Scholar]
- 31.Ruano R, Takashi E, da Silva MM, Campos JA, Tannuri U, Zugaib M. Prediction and probability of neonatal outcome in isolated congenital diaphragmatic hernia using multiple ultrasound parameters. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2012 Jan;39(1):42–49. doi: 10.1002/uog.10095. [DOI] [PubMed] [Google Scholar]
- 32.Garcia AV, Fingeret AL, Thirumoorthi AS, et al. Lung to head ratio in infants with congenital diaphragmatic hernia does not predict long term pulmonary hypertension. J Pediatr Surg. 2013 Jan;48(1):154–157. doi: 10.1016/j.jpedsurg.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Cruz-Martinez R, Figueras F, Moreno-Alvarez O, et al. Learning curve for lung area to head circumference ratio measurement in fetuses with congenital diaphragmatic hernia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010 Jul;36(1):32–36. doi: 10.1002/uog.7577. [DOI] [PubMed] [Google Scholar]
- 34.Hatch EI, Jr, Kendall J, Blumhagen J. Stomach position as an in utero predictor of neonatal outcome in left-sided diaphragmatic hernia. J Pediatr Surg. 1992 Jun;27(6):778–779. doi: 10.1016/s0022-3468(05)80116-2. [DOI] [PubMed] [Google Scholar]
- 35.Mann PC, Morriss FH, Jr, Klein JM. Prediction of survival in infants with congenital diaphragmatic hernia based on stomach position, surgical timing, and oxygenation index. American journal of perinatology. 2012 May;29(5):383–390. doi: 10.1055/s-0032-1304817. [DOI] [PubMed] [Google Scholar]
- 36.Skinner JR, Stuart AG, O'Sullivan J, Heads A, Boys RJ, Hunter S. Right heart pressure determination by Doppler in infants with tricuspid regurgitation. Archives of disease in childhood. 1993 Aug;69(2):216–220. doi: 10.1136/adc.69.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008 Feb;121(2):317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jani J, Nicolaides KH, Keller RL, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007 Jul;30(1):67–71. doi: 10.1002/uog.4052. [DOI] [PubMed] [Google Scholar]
- 39.Jani JC, Benachi A, Nicolaides KH, et al. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2009 Jan;33(1):64–69. doi: 10.1002/uog.6141. [DOI] [PubMed] [Google Scholar]
- 40.Quintero RA, Kontopoulos EV, Quintero LF, Landy DC, Gonzalez R, Chmait RH. The observed vs. expected lung-to-head ratio does not correct for the effect of gestational age on the lung-to-head ratio. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013 Apr;26(6):552–557. doi: 10.3109/14767058.2012.736000. [DOI] [PubMed] [Google Scholar]