Abstract
Substance use disorders, including cannabis use disorders and associated negative consequences, are best considered chronic and in need of continuing care. In contrast, most treatment efficacy studies evaluate a fixed number of intervention sessions at a single point in time. The present study evaluated the efficacy of posttreatment Maintenance Check-Ups (MCUs) in maintaining and improving outcomes following nine sessions of motivational enhancement treatment/cognitive behavioral treatment (MET/CBT). Adults dependent on cannabis (n = 74) were randomly assigned to the MCU or a No Check-Up (NCU) condition and followed up at 3-and 9-months. MCU sessions occurred 1 and 4 months following the completion of the base treatment. Additional MET/CBT sessions were available to participants throughout the follow-up period. The MCUs specifically encouraged treatment re-entry for those showing ongoing signs of disorder. Participants in the MCU condition reported significantly greater abstinent rates at both follow-ups and were using on fewer days at the 3-month but not the 9-month follow-up. Contrary to hypotheses, MCU participants did not attend more additional treatment and differences in rates of cannabis use emerged prior to the first MCU session. Future research with longer follow-up periods and longer monitoring of outcomes is needed to fully evaluate the utility of MCUs or other forms of continuing care.
Keywords: Motivational Enhancement Therapy, marijuana treatment, aftercare, continuing care, cannabis
1. Introduction
The risk of developing cannabis dependence for those who have ever used the substance is estimated at 9% (Anthony, Warner, & Kessler, 1994) and may be as high as 50% for daily users (van der Pol et al., 2013). Heavy long-term cannabis use has been linked to impairment in cognitive functioning, health, employment, and psychiatric functioning (Hall, 2014; Stephens & Banes, 2013). A similar profile of cannabis-related consequences is seen in samples of adults seeking treatment for cannabis dependence (Budney, Radonovich, Higgins, & Wong, 1998; Stephens, Roffman, & Curtin, 2000; Stephens, Roffman, & Simpson, 1994).
The short-term effectiveness of motivational enhancement therapy (MET) and cognitive behavioral treatments (CBT) for cannabis use disorders has been demonstrated in multiple studies (e.g., Budney, Higgens, Radonovich, & Novy, 2000; Copeland, Swift, Roffman, & Stephens, 2001; Stephens et al., 2000). The largest randomized trial for cannabis dependent adults conducted at multiple sites identified a 9-session MET/CBT intervention to be more effective than a 2-session MET intervention (Marijuana Treatment Project Research Group, 2004) and resulted in a published treatment manual on brief treatment of cannabis dependence (Steinberg et al., 2005). However, there is wide individual variability in treatment success. Point abstinence rates immediately following treatment are typically in the range of 20–40%. There is evidence of significant improvement in another subset of participants who do not become abstinent. However, outcomes for those who initially abstain or reduce use are not stable. Most participants do not achieve or maintain clear improvement from baseline functioning over the course of long term follow-up (Lozano, Stephens, & Roffman, 2006). There is a need for the development of interventions that assist in extending gains made during an initial treatment episode and in reducing the rate of relapse.
Reviews of the continuing care literature (McKay, 2005, 2006) emphasize that post-acute care monitoring of substance use status ought to be the standard of care for substance use disorder interventions. Following the completion of acute care, as symptoms wax and wane over time, the focus and intensity of both ongoing monitoring and treatment re-engagement options should be flexible and personalized to each client’s specific needs (e.g., Brown, Seraganian, Tremblay, & Annis, 2002; Stout, Rubin, Zwick, Zywiak, & Bellino, 1999). Lowering the burden of continuing care for the client (e.g., time, energy, expense), particularly during periods in which the client is being successful with abstinence/non-problematic use, is likely to prevent attrition from ongoing monitoring and promote continuing care participation (McKay et al., 2010). When treatment re-engagement is necessary, tailoring its focus, intensity, and duration to the individual client’s needs is likely to promote greater compliance. For example, Recovery Management Checkups (RMC) have been used to encourage early reintervention of postdischarge relapsers from outpatient and inpatient treatment (Dennis, Scott, & Funk, 2003). RMCs involve quarterly assessments of participant functioning following treatment and use feedback and motivational interviewing to encourage a return to treatment when indicated. The probability of transitioning to treatment reentry and recovery was increased with RMC exposure (Scott, Dennis, & Foss, 2005). Those who received RMC were judged less likely to be in need of treatment after 24 months.
In the present study, we adapted RMCs for the treatment of cannabis dependence. All participants received up to 9 MET/CBT sessions within 12 weeks; the ‘required’ 9 session phase of treatment ended after the 9th session or 12 weeks, whichever happened first. For half of the participants, Maintenance Check-Ups (MCUs) were scheduled 1 and 3 months after the completion of the initial treatment period. The intention of check-ups was to bolster success via affirmation of reduced cannabis use, identification of the need for additional treatment via repeated assessment, and encouragement to return to treatment when needed. In contrast to the RMC protocol in which only the clients who were identified as “in need” of treatment received an RMC session, all participants in the MCU condition in the present study received the maintenance sessions. In order to reduce barriers to treatment re-entry that might occur if a participant had to initiate treatment at a new facility or with a new counselor, all participants were given the option of returning to MET/CBT treatment with their original therapist anytime during the 6-month follow-up period. We hypothesized that:
Both intervention conditions, with and without MCUs, would lead to reduced cannabis use and associated problems following the initial nine sessions of MET/CBT treatment, thus replicating previous findings;
The MCUs would lead to greater reductions in cannabis use and related negative consequences at a follow-up assessment occurring after the check-up sessions;
Greater reductions in cannabis use and problems at later follow-ups would be mediated in part by increased attendance at additional therapy for those who continued to experience problems related to their cannabis use.
2. Method
2.1 Design
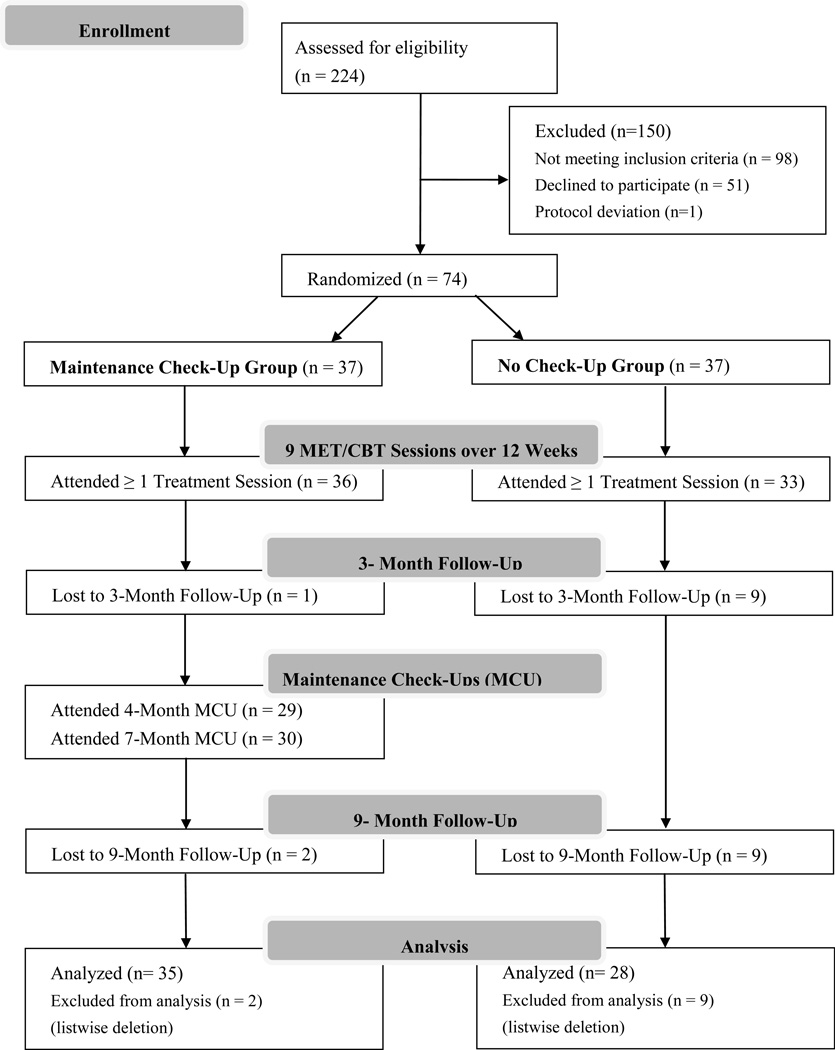
Participants (N=74) were randomly assigned to either a Maintenance Check-Up (n = 37) or No Check-Up (NCU; n = 37) intervention condition (see Figure 1). Both conditions initially received nine sessions of a MET/CBT found effective in previous trials (Marjuana Treatment Project Research Group, 2004; Steinberg et al., 2005). The initial nine sessions occurred within a 12 week timespan (i.e. by the 3 month anniversary of the participant’s baseline assessment). MCU participants received two additional MET-based check-up sessions 1 and 4 months following the completion of the initial treatment (i. e., at the 4 and 7 month anniversaries of the baseline assessment). The NCU participants received no further contact at these time points. Participants in both conditions were able to schedule additional CBT sessions on an as-needed basis throughout the follow-up period. Follow-up assessments 3 months and 9 months after baseline corresponded approximately to the end of the initial nine sessions of treatment and to six months following the completion of the initial treatment. As Figure 1 shows, follow-up assessments were timed to assess the effect of the initial treatment (i.e. 3 Month Follow-Up) and the effect of the Maintenance Check-Ups (9 Month Follow-Up).
Figure 1.
2.2 Participants
Of the 224 callers screened, 98 were ineligible because they met one or more of the exclusion criteria: used cannabis on less than 50 of the last 90 days or did not meet diagnostic criteria for dependence on cannabis (n = 45), were already involved in treatment or self-help groups (n = 34), were dependent on alcohol or other drugs (n = 38), were planning on relocating within the next year (n = 15), lived more than 60 miles from the treatment offices (n = 4), showed signs of psychosis (n = 1), or were under 18 years of age (n = 1). Of those who were eligible (n = 126), 40% (n = 51) declined to participate. One participant was misassigned to condition due to clerical error and subsequently derandomized. The 74 participants randomly assigned to one of two treatment conditions were primarily male (66%), white (78%), 37.73 (SD = 12.08) years old, and had 14.19 (SD = 2.63) years of education. Most had never married (46%) or were divorced or separated (23%).
2.3 Procedures
Procedures were approved by institutional review boards at the University of Washington and Virginia Tech. Participants were recruited from the Seattle, Washington metropolitan area using newspaper and radio advertisements offering treatment for cannabis use. The study began recruitment in February 2010 and completed enrollment in July 2010. All follow-up assessments and data collection were completed by April 2011. After providing verbal consent to participate, interested participants were screened for eligibility criteria via phone and then invited to complete an in-person baseline interview to finalize eligibility. At the baseline assessment session, participants gave written informed consent and were administered a structured interview to determine substance use diagnoses and recent substance use by trained research staff. They then completed self-report questionnaires. Eligible participants were randomly assigned to treatment condition and scheduled for the first treatment sessions. The same research staff, blind to condition, conducted in-person follow-up assessments 3 and 9 months following random assignment. Participants were paid $50 for completion of each assessment and a bonus of $50 for completing both follow-up assessments.
Participants in both conditions received 9 individual sessions of MET/CBT treatment. The first two treatment sessions used motivational interviewing techniques in the review of a personalized feedback report based on information collected in the baseline assessment. Treatment was manual guided (Steinberg et al., 2005) and delivered by three master’s level therapists. Therapists trained for 40 hours under the supervision of an experienced MET/CBT therapist and then participated in ongoing weekly supervision of taped therapy sessions throughout the treatment period.
Participants in both conditions were told that they could have additional, optional MET/CBT treatment sessions as needed during the six month follow-up period by calling to schedule an appointment. Participant and therapist were yoked throughout the project for all therapeutic interactions: the initial 9 sessions, any optional support sessions, and the check-ups where applicable. For the additional MET/CBT sessions, therapists selected modules from the same manual appropriate to the participant’s ongoing concerns and the antecedents maintaining cannabis use and related problems.
Participants assigned to the NCU condition received no further contact from the treatment staff after the initial 9 sessions unless they self-initiated additional treatment by calling the office. Participants in the MCU condition were scheduled for check-up sessions 1 and 3 months following the completion of the initial nine sessions. Check-up sessions consisted of completing a brief computerized assessment and then receiving feedback within the context of a MET session. The MCU assessment and personal feedback report covered recent cannabis use, related problems, and dependence symptoms presented visually in relation to pretreatment data in order to illustrate the amount of change that had taken place. The report reviewed important life goals identified pretreatment and the role cannabis played in hampering progress toward them. Finally, the participants’ updated goals for cannabis use (abstinence versus moderated use) and the importance and confidence they assigned to achieving them were presented and discussed. Sessions were designed to reinforce reduced cannabis use and improvement in life functioning and to identify ongoing concerns when present. In the latter case, participants were encouraged to consider returning to additional MET/CBT treatment. Although MCU sessions were conducted with a motivational interviewing style designed to elicit self-motivation, an advice-giving component was included. Therapists were instructed to explicitly encourage additional treatment sessions if the MCU assessment revealed ongoing problems or dependence symptoms, use of cannabis on 13 or more days in the past month, reports that current cannabis use was impeding progress toward important life goals, or low confidence in being able to attain and maintain abstinence or moderate use levels. Therapists completed a rating scale after each check-up session indicating the degree to which they encouraged participants to schedule additional MET/CBT therapy sessions (1 = Not at all; 5 = Strongly). Therapists reported encouraging 89% and 97% of the participants at least “a little” to schedule additional therapy at the 1 and 3 month posttreatment check-ups, respectively.
2.4 Measures
Cannabis use was assessed with an interviewer-administered Timeline Follow-Back (TLFB; Sobell & Sobell, 1992) at the initial baseline session and at each subsequent follow-up for a 90-day window. Percent days of use was calculated for the past 30 days at each assessment as the primary outcome measure. Urines were also collected at each follow-up and assayed for cannabinoids as a check on the validity of self-reported abstinence.
Problems commonly associated with cannabis use were assessed with the Marijuana Problems Scale (MPS; Stephens et al., 2000). The MPS assesses 19 negative consequences of use experienced over the past 90 days. Items endorsed as either a minor or major problem were counted to create a score of total problems. Alpha reliabilities were .87 at baseline and .94 at both follow-ups.
The Structured Clinical Interview for DSM-IV (SCID-I; First, Spitzer, Gibbon, & Williams, 1996) was utilized at baseline and all follow-up assessments to determine eligibility and to create a measure of dependence severity based on the seven DSM-IV dependence symptoms. Symptoms scored above threshold were counted to create a total score with alphas ranging from .37 at baseline to .82 at the 9-month follow-up. Internal consistency at baseline appeared to be suppressed by limited variance because ceiling effects on the endorsement of symptoms.
Self-efficacy for avoiding cannabis use was assessed with a 20-item scale that asked participants to rate how confident they felt resisting the temptation to smoke marijuana in various high-risk situations (Stephens, Wertz, & Roffman, 1995). Ratings were made on a 7-point scale (1 = not at all confident; 7 = very confident) and averaged across all items (alphas ranged from .92 at baseline to .96 at both follow-ups).
3. Results
3.1 Preliminary Analyses
Comparisons on baseline sociodemographic and substance use and abuse variables did not reveal any significant differences by condition. Rates of attrition at the 3-month (X = 7.40, p < .01) and 9-Month (X = 5.23; p < .05) follow-ups differed significantly by condition. MCU condition participants had greater rates of follow-up attendance than the NCU condition participants at both the 3-month (97% vs 76%) and 9-month (95% vs 76%) assessment points. However, examination of baseline sociodemographic and substance use characteristics for follow-up attenders versus non-attenders did not reveal any significant differences. In addition, there were no significant interactions between treatment condition and follow-up status at either the 3 or 9 month assessment to suggest treatment condition contributed to differential attrition. Comparisons of urinalyses with self-reported abstinence showed high levels of agreement at 3-months (87%) and 9-months (95%). Most disagreements were due to self-reported use when urinalyses were negative, providing confidence in the validity of self-reports.
3.2 Treatment Attendance
Of the nine initial MET/CBT sessions, the average number attended was 6.92 (SD =3.15) and did not differ significantly by treatment condition. In the MCU condition, attendance of the first checkup was 78% and attendance of the second checkup was 81%. More than half of MCU participants (62%) attended at least one optional MET/CBT session compared to 46% of NCU participants, but the difference was not statistically significant. Of those who attended optional CBT sessions, the average number of optional CBT sessions attended was 4.30 (SD = 6.14) for MCU and 2.81 (SD=4.79) for NCU, but did not differ significantly (p = .25; d = .27).
3.3 Outcome analyses
First, we tested the hypotheses that both treatment conditions would result in increased abstinence and reduced overall rates of cannabis use and associated problems. Abstinence rates for the month preceding assessment favored the MCU condition at both the 3-month (36% vs 13%; Χ2 (66) = 4.44; p < .05) and 9-month (26% vs 7%; Χ2 (63) = 3.72; p < .06) follow-ups. A 2 (Condition) × 3 (Time) general linear model analyses performed on the percentage of days of cannabis use during the month preceding the start of treatment and the month preceding each of the follow-ups, showed a significant effect of time (F = 46.56; p < .001) but the condition by time interaction did not reach conventional levels of significance (F = 2.76, p < .08). Table 1 shows that the percentage days of cannabis use were reduced significantly at both follow-ups relative to baseline (ps < .001). Post-hoc comparisons showed that participants in the MCU condition reported fewer days of use than those in the NCU condition at 3-months (p < .05; d = 0.88) but not at 9- months (p > .15; d = 0.51). Parallel analyses performed on dependence symptom and cannabis problem counts revealed only significant time effects (F = 41.20, p < .001 and F = 34.84, p < .001). Dependence symptoms and problems were reduced at both follow-ups relative to baseline (p < .001), but did not differ between conditions.
Table 1.
Cannabis use and negative consequences before and after treatment
| Baseline | 3 Months | 9 Months | ||||
|---|---|---|---|---|---|---|
| Measures | MCU | NCU | MCU | NCU | MCU | NCU |
| Percent Days of Use | 84.41 a (26.31) |
85.55 a (25.74) |
25.52 a (33.57) |
50.37 b (43.62) |
43.10 a (41.26) |
57.36 a (37.19) |
| Dependence Symptoms | 5.86 a (1.26) |
5.62 a (1.13) |
3.40 a (2.40) |
3.38 a (2.28) |
3.03 a (2.68) |
3.46 a (2.34) |
| Marijuana Problems | 10.37 a (4.36) |
9.81 a (4.10) |
5.69 a (5.13) |
5.65 a (5.08) |
5.89 a (5.47) |
5.69 a (4.98) |
| Self-Efficacy | 3.19 a (1.07) |
3.86 a (1.27) |
5.17 a (1.54) |
4.78 b (1.36) |
4.86 a (1.53) |
4.45 a (1.45) |
Note. MCU = Maintenance Check Up (n = 35); NCU = No Check-Up (n = 28). Parentheses indicate standard deviations. Means with different superscripts at a given assessment point differ significantly, p < .05.
The significant differences in cannabis use at the 3 month follow-up were not expected because at this point both treatment conditions had received the same 9-session intervention. Therefore, in order to test the hypothesis that participation in the MCU condition check-ups would lead to greater abstinence and reductions in cannabis use at the 9-month follow-up we controlled for cannabis use at the 3-month follow-up when testing the effect of condition. Condition was dummy coded (MCU = 1; NCU = 0). A logistic regression predicting abstinence from cannabis use at the 9-month follow-up showed a significant effect of abstinence status at the 3-month follow-up (Adjusted OR = 24.95; 95% CI = 4.34, 143.48; p < .001). However, the effect of condition was not significant (Adjusted OR = 2.94; 95% CI = 0.45, 19.44; p = .26). Similar linear regressions analyses were used to test differences at the 9-month follow-up in days of cannabis use, dependence symptoms, and problem counts after controlling for the corresponding indices at the 3-month follow-up. In each equation the corresponding 3-month follow-up index was a significant predictor of the 9-month follow-up variable. Standardized regression coefficients (Betas) were .54 for days of use, .60 for dependence symptoms, and .69 for problems (all ps < .001). Again, condition was not a significant predictor of 9-month outcomes after controlling for 3-month outcomes, accounting for less than 1% of the variance in each equation (Betas = − 0.01 for days of use; − .09 for dependence symptoms; and .02 for problem counts ; all ps > .40).
We did not test the hypothesis that treatment condition differences in outcomes would be partially mediated by greater attendance of additional treatment because there were no significant differences in additional treatment utilization. Further, significant differences in cannabis use between conditions emerged at the 3-month follow-up, before the first checkup sessions for the MCU conditions. This effect led us to explore whether differences in expectancies may have accounted for this difference. A 2 (Condition) × 3 (Time) general linear model analyses performed on the measure of self-efficacy for avoiding cannabis use showed a significant effect of time (F = 32.48; p < .001) and a significant condition by time interaction (F = 5.43; p < .01). Table 1 shows that MCU condition participants reported significantly greater self-efficacy at the 3-month follow-up (p < .05).
4. Discussion
The present study tested whether posttreatment MET-based check-ups would enhance outcomes of treatment for cannabis dependence by reinforcing gains and encouraging a return to treatment when indicated. Both treatment conditions led to significant reductions in cannabis use and related negative consequences at follow-ups, replicating findings from previous trials of the same 9-session treatment (Marijuana Treatment Project Research Group, 2004). Participants assigned to the MCU condition reported greater rates of abstinence at the end of treatment and at the follow-up six months later. In contrast to hypotheses, MCU participants did not use additional treatment sessions significantly more than those in the comparison treatment and differences in cannabis use between conditions emerged before the first check-up session.
Overall, the results do not directly support the efficacy of MCUs as a means to improve treatment outcomes for cannabis dependent adults, at least not as originally conceptualized. Unexpectedly, greater abstinence rates and greater reductions in the frequency of cannabis were evident in the MCU condition at the end of the initial treatment as assessed at the 3-month follow-up. At this assessment, participants in both conditions had received the same 9-session MET/CBT treatment administered by the same therapists. Outcomes occurring after participation in the check-ups at the 9-month follow-up were not affected by treatment condition once the earlier 3-month outcomes were considered. MCUs also did not clearly foster significantly greater re-engagement with treatment as hypothesized. Nevertheless, greater abstinence and reductions in cannabis use in the MCU condition remained at the 9-month follow-up.
Therapists could not be kept unaware of condition assignment because they were responsible for introducing the rationale and process for the MCUs. Thus, it is possible that unintended differences in therapist behavior during the initial nine sessions account for the differential outcomes. However, the same therapists conducted both treatment conditions and treatment session checklists and ratings completed by therapists following each intervention session did not show differences by condition. It seems more likely that the promise of future check-ups promoted greater expectancy for success in reducing cannabis use and that these expectancy effects translated to actual behavior. This explanation is consistent with the finding of greater self-efficacy for avoiding cannabis use in the MCU condition at the 3-month follow-up. Although self-efficacy does not directly measure expectancy for success from treatment it has consistently been shown to be influenced by treatment and is one of the strongest predictors of outcomes in the treatment of cannabis dependence (Litt, Kadden, & Stephens, 2005; Stephens et al., 1995). The promise of additional support and encouragement via anticipated check-ups may have increased confidence in being able to successfully avoid cannabis use.
Despite the limited support for the efficacy of the MCUs in producing superior outcomes via enhanced motivation for change and treatment utilization, further study of interventions designed to monitor posttreatment outcomes and encourage treatment re-entry as needed is warranted. The current study was limited by a small sample size and reduced power. Further, the follow-up period, and hence the period available for monitoring and treatment re-entry, was relatively short. It is possible that larger effects of the MCUs may have been evident at later points in time or as the result of cumulative effects of multiple check-up experiences. It is encouraging to note that attendance of the MCU sessions was excellent, suggesting the appeal of ongoing interaction with treatment providers. Similarly, over 50% of participants, regardless of treatment condition, made use of additional treatment sessions. These data indicate that a substantial subset of treatment seekers found the standard 9-session MET/CBT insufficient to meet their needs. Conceptualizing addiction as a chronic disorder requires novel ways of providing treatment over time and research designs that are less bound by standardization of the duration and intensity of treatment for all participants.
Highlights.
Post-treatment Maintenance Check-Ups were related to better abstinence rates at 3 and 9-month follow-ups.
Maintenance Check-Ups did not increase optional treatment attendance.
Post-treatment Maintenance Check-Ups reduced days of use relative to a control condition at 3-months.
Acknowledgments
This study was made possible by funding from the National Institute on Drug Abuse: 2RO1DA14050-06A2. We thank the participants who gave of their time to be involved in this research. We also want to offer our sincere appreciation to the research staff on this project including Cynthia Shaw (Project Director), and Lauren Matthews (Research Assistant), as well as the counseling staff Jocelyn Savage, Ernie McGarry, Jodi Pierce, and Clinical Supervisor, Jonnae Tillman for their caring work with the participants in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Denise D. Walker, Email: ddwalker@u.washington.edu.
Robert S. Stephens, Email: stephens@vt.edu.
Sheri Towe, Email: towe@vt.edu.
Kelsey Banes, Email: kbanes@vt.edu.
Roger Roffman, Email: roffman@uw.edu.
References
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2(3):244–268. [Google Scholar]
- Brown TG, Seraganian P, Tremblay J, Annis H. Matching substance abuse aftercare treatments to client characteristics. Addictive Behaviors. 2002;27(4):585–604. doi: 10.1016/s0306-4603(01)00195-2. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgens ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68(6):1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Radonovich KJ, Higgins ST, Wong CJ. Adults seeking treatment for marijuana dependence: A comparison with cocaine-dependent treatment seekers. Experimental and Clinical Psychopharmacology. 1998;6(4):419–426. doi: 10.1037//1064-1297.6.4.419. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive–behavioral interventions for cannabis use disorder. Journal of Substance Abuse Treatment. 2001;21:55–64. doi: 10.1016/s0740-5472(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Dennis M, Scott CK, Funk R. An experimental evaluation of recovery management checkups (RMC) for people with chronic substance use disorders. Evaluation and Program Planning. 2003;26(3):339–352. doi: 10.1016/S0149-7189(03)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C.: American Psychiatric Press Inc.; 1996. [Google Scholar]
- Marijuana Treatment Project Research Group. Brief treatments for cannabis dependence: findings from a randomized multisite trial. Journal of Consulting and Clinical Psychology. 2004;72(3):455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2014 doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- Lozano BE, Stephens RS, Roffman RA. Abstinence and moderate use goals in the treatment of marijuana dependence. Addiction. 2006;101(11):1589–1597. doi: 10.1111/j.1360-0443.2006.01609.x. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Stephens RS The Marijuana Treatment Project Research Group. Coping and self-efficacy in marijuana treatment: Results from the Marijuana Treatment Project. Journal of Consulting and Clinical Psychology. 2005;73:1015–1025. doi: 10.1037/0022-006X.73.6.1015. [DOI] [PubMed] [Google Scholar]
- McKay JR. Is there a case for extended interventions for alcohol and drug use disorders? Addiction. 2005;100(11):1594–1610. doi: 10.1111/j.1360-0443.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- McKay JR. Continuing care in the treatment of addictive disorders. Current Psychiatry Reports. 2006;8(5):355–362. doi: 10.1007/s11920-006-0036-9. [DOI] [PubMed] [Google Scholar]
- McKay JR, Van Horn DHA, Oslin DW, Lynch KG, Ivey M, Ward K, Covilello DM. A randomized trial of extended telephone-based continuing care for alcohol dependence: Within-treatment substance use outcomes. Journal of Consulting and Clinical Psychology. 2010;78(6):912–923. doi: 10.1037/a0020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CK, Dennis ML, Foss MA. An experimental evaluation of recovery management checkups (RMC) for people with chronic substance use disorders. Drug and Alcohol Dependence. 2005;78(3):325–338. doi: 10.1016/j.drugalcdep.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten Rz, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Steinberg KL, Babor TF, Miller M, Kadden R, Duresky D, Stephens R. Brief counseling for marijuana dependence: A manual for treating adults. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2005. [Google Scholar]
- Stephens RS, Banes KE. Cannabis and Hallucinogens. In: McCrady BS, Epstein EE, editors. Addictions: A Comprehensive Guidebook. 2nd Ed. New York: Oxford University Press; 2013. pp. 215–239. [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68(5):898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: A test of the relapse prevention model. Journal of Consulting and Clinical Psychology. 1994;62(1):92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Wertz JS, Roffman RA. Self-Efficacy and Marijuana Cessation : A Construct Validity Analysis. Journal of Consulting and Clinical Psychology. 1995;63(6):1022–1031. doi: 10.1037//0022-006x.63.6.1022. [DOI] [PubMed] [Google Scholar]
- Stout R, Rubin A, Zwick W, Zywiak W, Bellino L. Optimizing the cost-effectiveness of alcohol treatment: A rationale for extended case monitoring. Addictive Behaviors. 1999;24(1):17–35. doi: 10.1016/s0306-4603(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Van der Pol P, Liebregts N, de Graaf R, Korf DJ, van den Brink W, van Laar M. Predicting the transition from frequent cannabis use to cannabis dependence: A three-year prospective study. Drug and Alcohol Dependence. 2013;133(2):352–359. doi: 10.1016/j.drugalcdep.2013.06.009. [DOI] [PubMed] [Google Scholar]