Abstract
Cells rely on the coordinated action of diverse signaling molecules to sense, interpret, and respond to their highly dynamic external environment. To ensure the specific and robust flow of information, signaling molecules are often spatially organized to form distinct signaling compartments, and our understanding of the molecular mechanisms that guide intracellular signaling hinges on the ability to directly probe signaling events within these cellular microdomains. Ca2+ signaling in particular owes much of its functional versatility to this type of exquisite spatial regulation. As discussed below, a number of methods have been developed to investigate the mechanistic and functional implications of microdomains of Ca2+ signaling, ranging from the application of Ca2+ buffers to the direct and targeted visualization of Ca2+ signaling microdomains using genetically encoded fluorescent reporters.
Keywords: Biosensors, FRET, Live-cell imaging, Compartmentalized signaling, Calmodulin, Calcineurin
1. Introduction
All living cells must continually sense and respond to changes in their external chemical environment. During intracellular signaling, information regarding the conditions outside the cell is passed along from the cell surface to the appropriate response machinery inside the cell. Yet although cells contain diverse signaling pathways that specifically control the myriad biological processes that are essential to life, the pool of signaling molecules that comprise these pathways is limited, and specificity cannot be intrinsically encoded into individual pathways. Rather, in order to both ensure the specificity and promote the diversity of signaling outcomes, cells must carefully coordinate the actions of signaling molecules as they participate in a dynamic network of highly integrated signaling pathways. One way for cells to achieve this level of coordination is through the spatial compartmentalization of the cell interior into local signaling domains of various sizes, and intracellular Ca2+ signaling offers a striking example of this process.
Ca2+ signaling regulates many fundamental biological processes, including neurotransmission, muscle contraction, gene expression, cell proliferation, and cell death [1], often regulating multiple cellular processes in parallel. The remarkable versatility of Ca2+ as an intracellular messenger stems from the exquisite spatial and temporal regulation of elevations in intracellular Ca2+ concentrations, especially through the formation of discrete microdomains of Ca2+ signaling. Often, these microdomains involve so-called “elementary” Ca2+-release events that result from the opening of individual Ca2+ channels [2]. Broadly speaking, however, the term Ca2+ microdomain can apply not only to zones of high Ca2+ concentration that occur near the mouths of Ca2+ channels but to any Ca2+ signaling event that is confined to a particular region of the cell – be it the plasma membrane, a specific part of the cytosol, or Ca2+-storing organelles – as opposed to the cytoplasm as a whole [3,4].
As with the compartmentalization of other signaling molecules, such as cAMP (see [5]), the existence of Ca2+ microdomains was originally proposed to account for experimental observations that lacked a clear mechanistic foundation. Various theoretical studies (e.g., [6,7]) and indirect observations [8] bolstered this idea, but it was not until the development of optical (e.g., fluorescent) techniques to visualize Ca2+ dynamics in intact cells that direct experimental evidence of Ca2+ microdomains was obtained. Following the adoption of fluorescent probes to study local Ca2+ dynamics, the more recent development of genetically encoded fluorescent reporters based on green fluorescent protein (GFP) and related fluorescent proteins has completely revolutionized the study of spatially confined signaling events. In this review, we provide a brief primer on the design and development of genetically encoded fluorescent reporters and discuss the application of these biosensors to the study of spatially compartmentalized signaling events in living cells, using Ca2+ signaling as an example.
2. Fluorescent biosensors
Understanding the functional and mechanistic properties of signaling microdomains depends on our ability to assay molecular events specifically within these confined cellular regions. In other words, the more selectively we can target these microdomains, the more precisely we can study local signaling. These efforts have been greatly facilitated by advances in live-cell fluorescence imaging approaches, which have deep ties to the study of Ca2+ signaling. For example, the use of fluorescent indicator dyes for live-cell imaging was popularized by the success of Ca2+ indicators such as quin-2, fluo-3, and fura-2 (Fig. 1A) [9], and it was the isolation of the Ca2+-dependent photoprotein aequorin – itself a useful tool for monitoring Ca2+ inside cells (Fig. 1B) [10,11] – that ultimately led to the discovery of Aequoria victoria GFP (reviewed in [12,13]) and the development of genetically encoded fluorescent reporters. These genetically encoded molecular tools can easily be expressed in cells using recombinant DNA technology and can further be targeted throughout the cell via the incorporation of endogenous subcellular targeting motifs or via fusion to endogenous proteins that natively localize to specific regions of the cell. This relatively straightforward targetability enables the specific and detailed investigation of signaling processes occurring within all manner of cellular microdomains and compartments [14,15].
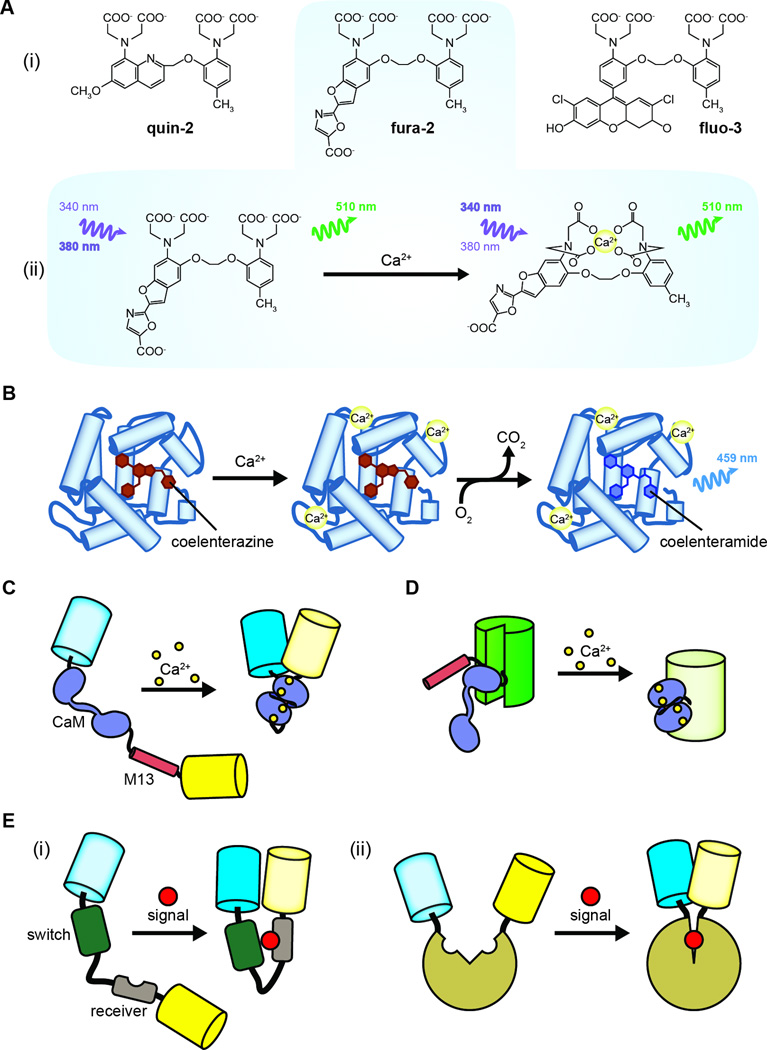
Figure 1. Tools for visualizing signaling in live cells.
(A) Fluorescent Ca2+ indicators. (i) The chemical structures of the Ca2+ indicators quin-2, fura-2, and fluo-3 in their Ca2+-free states. (ii) Fura-2 emits 510-nm light upon excitation at either 340 nm or 380 nm. In its Ca2+-free state, fura-2 is preferentially excited at 380 nm, whereas Ca2+-bound fura-2 is preferentially excited at 340 nm. The fluorescence intensity at each excitation wavelength is directly proportional to the concentration of Ca2+. (B) Aequorin consists of an apo-protein bound to a coelenterazine co-factor (left). The binding of Ca2+ (middle) results in the enzymatic conversion of coelenterazine to coelenteramide (right) and the emission of blue (459 nm) light. (C) The FRET-based Ca2+ sensor cameleon consists of a tandem fusion of calmodulin (CaM) and the CaM-binding M13 peptide sandwiched between a FRET pair (shown here: CFP and YFP). The binding of Ca2+ induces CaM to form a complex with the M13 peptide, thereby causing a conformational rearrangement that increases FRET between the two fluorescent proteins. (D) GCaMP also uses CaM and the M13 peptide as its molecular switch; however, rather than modulating FRET between a pair of fluorescent proteins, in GCaMP, CaM and M13 are inserted into a single fluorescent protein (e.g., GFP), wherein they modulate fluorescence intensity. (E) Genetically encoded fluorescent reporters are a highly versatile set of molecular tools, owing to their highly modular design scheme. The key component is the molecular switch, which can be (i) assembled from protein fragments that function as a “receiver” for the input signal and a “switch” that drives the conformational change or (ii) taken directly from an endogenous protein that undergoes a native conformational change in response to a known stimulus.
Genetically encoded fluorescent reporters come in a variety of forms (reviewed extensively in [16]) that all share a highly generic, modular design in which a sensing unit capable of detecting a specific biochemical signal is coupled to a reporting unit composed of one or more fluorescent proteins. In the most versatile class of biosensors developed thus far, the sensing unit encompasses a protein or protein fragments in the form of a molecular switch that undergoes a conformational change in response to a particular input signal. The conformational change in the molecular switch then alters the distance and/or orientation of a pair of fluorescent proteins capable of undergoing Förster resonance energy transfer (FRET) [17]. The first such FRET-based biosensor, cameleon, was generated by sandwiching the Ca2+ sensor calmodulin (CaM) and a CaM-binding peptide from myosin light-chain kinase (M13) between a FRET pair [18,19], again highlighting the close ties between Ca2+ signaling and live-cell fluorescence imaging. In the presence of Ca2+, CaM binds the M13 peptide and induces a conformational change that alters the FRET signal from cameleon (Fig. 1C). In an alternative design that eschews FRET, CaM and M13 are instead inserted within a single fluorescent protein and used to directly control the fluorescence intensity, as in the popular GCaMP series of Ca2+ sensors (Fig. 1D) [20,21].
Molecular switches have proven to be an extremely versatile solution to the problem of monitoring signaling in living cells, as they are readily adaptable to detecting a variety of biochemical events associated with signaling. As demonstrated with cameleon, molecular switches can often be engineered from protein components that will interact and induce a conformational change in response to a given signal. These bipartite switches comprise a receiving domain that is modified by the input signal and a switching domain that interacts with the receiver to drive the conformational change (Fig. 1E). For example, numerous kinase activity reporters have been generated by fusing a consensus substrate sequence and a phosphoamino acid-binding domain (PAABD), which are then sandwiched between a FRET pair [22]. Binding of the phosphorylated substrate by the PAABD leads to a conformational change that produces a FRET change. Similarly, fusing a small GTPase to a binding partner that only interacts with the active, GTP-bound form of the enzyme results in a molecular switch that underlies many GTPase activation sensors [23]. Molecular switches can also be derived from the intrinsic conformational dynamics of native proteins (Fig. 1E). The most commonly used cAMP biosensors, for instance, use the intrinsic conformational change that occurs when cAMP binds exchange protein activated by cAMP (Epac) to drive a FRET change [24,25]. In theory, any protein whose conformation is directly modulated by an upstream signal can be inserted between a FRET pair to construct a biosensor, though this frequently involves trial and error. This generalizable design scheme has inspired the development of a multitude of biosensors capable of detecting a broad spectrum of biochemical processes (e.g., second messenger production, ion and metabolite concentrations, enzyme activity, and enzyme activation), making genetically encoded fluorescent reporters an invaluable asset in the study of intracellular signaling [15,16].
3. Investigating microdomains of Ca2+ signaling
Many approaches have been employed over the years to investigate local Ca2+ signaling processes within cells. These range from indirect measurements based on Ca2+ buffering to highly selective, direct visualization of Ca2+ signals using genetically encoded biosensors. As more targeted and versatile methods have become available, the ability to probe Ca2+ signaling has also expanded to include not only Ca2+ itself but also a number of Ca2+-dependent signaling proteins, thus fueling the development of a much more sophisticated understanding of Ca2+ signaling microdomains.
3.1 Ca2+ microdomains at the plasma membrane
Ca2+ influx across the plasma membrane is an essential signaling mechanism in all cell types, especially in electrically excitable cells such as neurons and muscle cells. Hence, early studies of local Ca2+ signaling often focused on Ca2+ microdomains near the plasma membrane. Plasma membrane Ca2+ microdomains were initially proposed to help explain the very rapid release of neurotransmitter in response to Ca2+ influx, under the assumption that the exocytotic machinery must be closely coupled to Ca2+ channels [26]. The existence of these microdomains could be observed indirectly by loading cells with different chelators to buffer global Ca2+ increases [8]. Typically, cells are loaded with either the “slow” chelator EGTA or the “fast” chelator BAPTA, the idea being that given a very short (<100 nm) distance between the Ca2+ channel and the target, only BAPTA will be able to capture Ca2+ quickly enough to disrupt the microdomain (Fig. 2A) [8,27]. This is a powerful technique that is still used to study Ca2+ microdomains in the vicinity of plasma membrane Ca2+ channels to this day. For example, both BAPTA and EGTA were recently shown to disrupt transmission in mature hippocampal mossy fiber synapses, suggesting that synaptic vesicles are only loosely coupled to Ca2+ channels in these plastic synapses, in contrast to the tight coupling that is often observed in the mature nervous system [28]. In addition, Selway et al. recently used cell-permeable versions of these chelators to demonstrate that Ca2+ microdomains formed by L-type voltage-gated Ca2+ channels (VGCCs) were sufficient to activate ERK signaling in response to GLP-1 stimulation in MIN6 pancreatic β-cells [29].
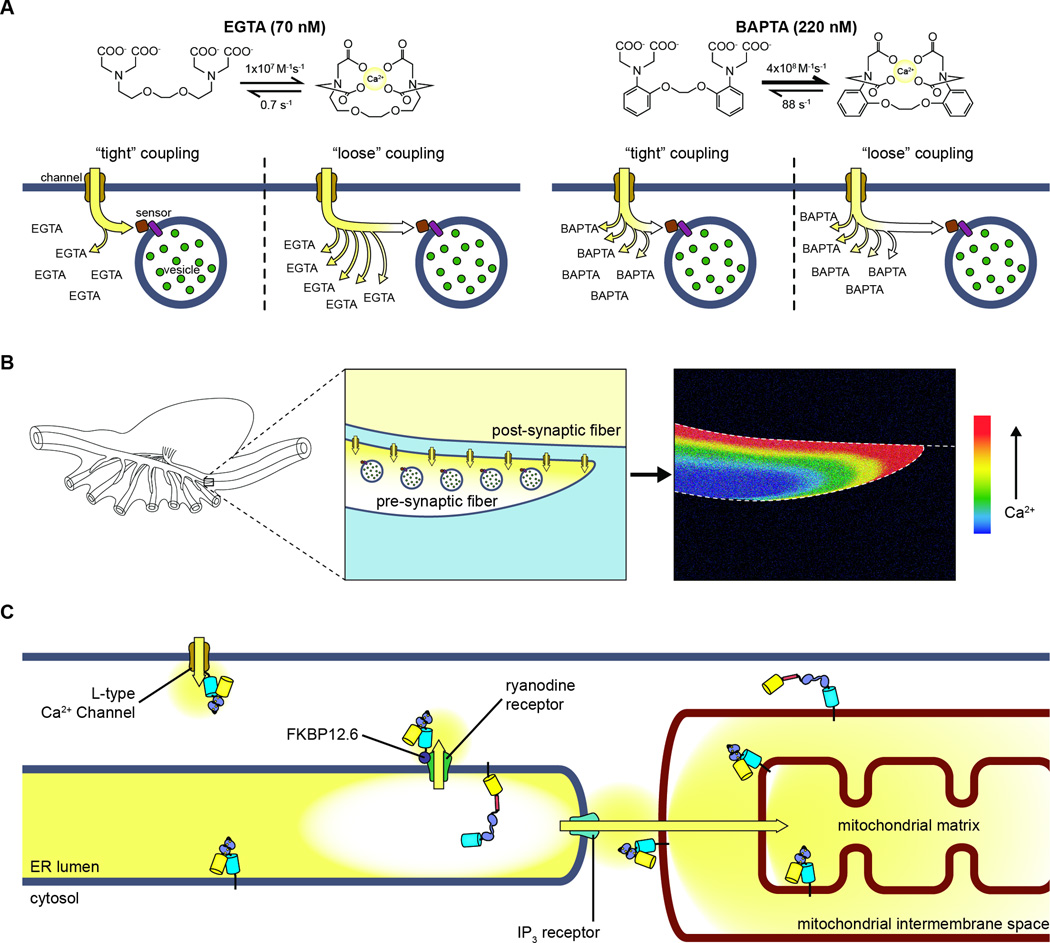
Figure 2. Dynamically probing microdomains of Ca2+ signaling.
(A) The extent to which different Ca2+ chelators are able to disrupt downstream signaling can provide information about how closely linked a given process is to Ca2+ microdomains. As shown here, if a Ca2+ target (e.g., synaptic vesicle) is located within a Ca2+ microdomain (“tight” coupling), EGTA will not be able to chelate Ca2+ before it reaches the target. Only a target that is further away (“loose” coupling) will be affected. BAPTA binds Ca2+ far more quickly, however, and is able to chelate Ca2+ rapidly enough to prevent it from reaching even the tightly coupled target. (B) In some cases, Ca2+ gradients can be directly visualized using diffusible probes. For example, in the squid giant synapse (left), electrical stimulation induces localized Ca2+ influx in the pre-synaptic fiber (middle). This can be visualized by loading the axon with a diffusible Ca2+ indicator; the cytosolic Ca2+ gradient appears as a clearly visible gradient in the fluorescent signal from the indicator (right) due to the relatively large size of the cells (as in [33]). (C) Ca2+ signaling compartments can also be directly visualized using genetically encoded biosensors (generically illustrated here in the form of cameleon) targeted to various subcellular locations. Targeting involves appending a specific DNA sequence that encodes an endogenous localization signal onto the DNA sequence of the reporter. For example, these sequences can be used to tether a Ca2+ biosensor to Ca2+ channels (e.g., L-type Ca2+ channel [44] or ryanodine receptor [51]) to probe discrete Ca2+ release domains. Similarly, Ca2+ sensors can be targeted to the interior or exterior of various organelles, such as the ER lumen to monitor store heterogeneity during Ca2+ release [55] or to the mitochondrial inner and outer membranes to investigate Ca2+ uptake by mitochondria [3,66–68].
Detailed studies of Ca2+ microdomains, however, require more direct methods for monitoring local Ca2+ signaling events in cells. A number of optical detection methods have been developed to permit the direct visualization of intracellular Ca2+ dynamics in living cells, and many of these had already begun to see widespread adoption by the late 1980s [30]. In many cases, these diffusible probes can be introduced into cells and used to directly image discrete concentration changes that are associated with Ca2+ microdomains located at the plasma membrane. Indicator dyes such as fura-2 and fluo-3, for example, could often be used to resolve minute gradients of Ca2+ influx [31–33]. Along with fluorescent indicators, the bioluminescent protein aequorin, which has been used to study Ca2+ signaling in live cells for nearly half a century [34], has also been used to visualize Ca2+ microdomains. In particular, injecting cells with a low-sensitivity derivative of aequorin (n-aequorin-J) enabled the detection of discrete Ca2+ blips, or quantum emission domains (QEDs) [26,35–37], which are highly localized sites of Ca2+ influx that are attributed to the opening of a small number of plasma membrane Ca2+ channels. Such elementary Ca2+ release events have since taken on many names to reflect the various cellular contexts in which they have been described [2,38]. More recently, the fluorescent Ca2+ indicator fluo-4 was used in combination with total internal reflection fluorescence (TIRF) microscopy to visualize elementary Ca2+ release events associated with the vanilloid transient receptor potential (TRP) channel TRPV4 in endothelial cells [39], while Sonkusare et al. performed confocal imaging of diffusible GCaMP2 to visualize TRPV4-dependent Ca2+ sparklets and investigate the Ca2+-dependent regulation of smooth muscle tone [40].
Another essential consideration in the study of signaling microdomains is selectivity. In the preceding examples, Ca2+ microdomains were observed by imaging particular cells in which gradients can be clearly resolved (e.g., giant squid axons) (Fig. 2B) or with the aid of selective illumination to distinguish local Ca2+ signals from the diffuse background. Alternatively, with n-aequorin-J selectivity is achieved via reduced Ca2+ sensitivity compared with unmodified aequorin [26,34,35]. This reduced sensitivity was advantageous for visualizing Ca2+ specifically within microdomains, as these confined regions were predicted to contain very high Ca2+ concentrations [35,41]; using n-aequorin-J ensured that only these high-concentration Ca2+ compartments will be visualized. Even greater selectivity can be achieved by physically targeting a probe to the specific compartment under investigation. For example, expressing aequorin directly in cells as a chimeric fusion with various targeting sequences enabled the selective detection of Ca2+ microdomains in the vicinity of the plasma membrane [42], as well as the first direct visualization of the local Ca2+ dynamics associated with intracellular organelles (see Section 3.2). Subcellular targeting is also one of the major hallmarks of genetically encoded fluorescent reporters. In an early example, Emmanouilidou et al. targeted the probe yellow cameleon to the secretory vesicle surface and observed a subset of vesicles within 1 °m of the plasma membrane that experienced significantly higher Ca2+ elevations than did more distant vesicles or the overall cytoplasm [43]. Genetically encoded fluorescent reporters have even been localized directly to the mouths of Ca2+ channels, as was done recently by Tay and colleagues with the VGCC Cav2.2 [44], or to distinct plasma membrane subdomains such as caveolae [45], thereby offering an exquisitely detailed look at Ca2+ microdomains that cannot be resolved using more traditional approaches.
3.2 Local Ca2+ domains within the cell
Resting cytosolic Ca2+ concentrations are kept low by the active extrusion of Ca2+ across the plasma membrane as well as by the uptake of Ca2+ into intracellular organelles, which act as internal Ca2+ stores that can be called upon to release Ca2+ to regulate various cellular processes [4,46]. Internal Ca2+ stores thus represent another domain of local Ca2+ signaling, in terms of both localized Ca2+ release from organelles and Ca2+ handling within organelles, that have long been the subject of intense scrutiny. The endoplasmic/sarcoplasmic reticulum (ER/SR) in particular often comprises the largest intracellular Ca2+ store and serves as a major source of cytosolic Ca2+ release and signaling in non-excitable cell types as well as in excitable cells, where it plays essential roles in, for example, muscle contraction. Diffusible Ca2+ indicators have been used to image microdomains of Ca2+ release from the ER in combination with pharmacological agents known to specifically promote ER Ca2+ release, such as caffeine [31,47] or histamine [48]. The inositol (1,4,5)-triphosphate (IP3) receptor (IP3R) can also be directly activated by the application of IP3 or a caged derivative [49]. In an elegant example of this technique, Smith and Parker used TIRF imaging to visualize single-channel flux through IP3Rs in fluo-4-loaded SH-SY5Y neuroblastoma cells upon photo-uncaging of IP3 [50]. Notably, the cells were also loaded with EGTA to prevent the build-up of Ca2+ waves while simultaneously preserving elementary Ca2+ blips, a twist on the Ca2+-buffering approach discussed in Section 3.1
Targeted approaches using genetically encoded reporters are also apt for probing ER Ca2+ release microdomains. For example, Despa et al. recently targeted GCaMP to the junctional cleft in cardiac myocytes in order to directly measure local Ca2+ dynamics in this functionally important microdomain [51]. The authors ensured the correct localization of their probe by fusing it to FKBP12.6, which tightly and selectively binds the cardiac ryanodine receptor (RyR2) [52,53]. Yet where targeted approaches truly shine is in examining Ca2+ dynamics within organelles, which represents a significant technical challenge to diffusible probes such as fluorescent indicator dyes (Fig. 2C). However, it is important to make sure that the sensitivity of the probe is sufficiently tuned so as to accurately report high Ca2+ concentrations within stores. Montero et al. targeted a low-affinity aequorin to the ER lumen to measure Ca2+ accumulation, yet even this probe was readily saturated by the high ER Ca2+ concentration ([Ca2+]ER). Instead, the authors used Sr2+ as a Ca2+ surrogate in their measurements [54]. Recently, another low-affinity aequorin mutant was used to study ER Ca2+ homeostasis in detail, revealing significant heterogeneity in [Ca2+]ER, as well as high-concentration areas that were unaffected by Ca2+-release agents. Buffering the cytosol with either EGTA or BAPTA also revealed that Ca2+ microdomains play a crucial role in store refilling [55]. The modified cameleon D1ER also exhibits a lower Ca2+-binding affinity that is suitable for probing [Ca2+]ER [56] and was recently used by the Delbono group to show that physiological stimuli do not induce significant SR Ca2+ depletion in skeletal muscle fibers [57]. This same group also used a novel Ca2+ probe, CatchER, that consists of a directly Ca2+-sensitive GFP [58] to show that this residual SR Ca2+ is higher in older mice than in younger mice, providing evidence for excitation-contraction uncoupling in aging muscle [59].
Alongside the ER, numerous other organelles are attracting interest regarding their roles in shaping local Ca2+ signaling domains. Recent studies have used cameleon-based fluorescent biosensors to study Ca2+ dynamics within both the trans- and medial-Golgi [60,61], as well as in lysosomes [62]. This ability to target probes to specific subcellular locations means investigators are largely unrestrained in their efforts to study these domains in minute detail. For example, mitochondrial Ca2+ uptake has been shown to modulate cytosolic Ca2+ signals [63,64], and the expression of a COX8-aequorin fusion localized to the inner mitochondrial membrane revealed that Ca2+ concentrations in the mitochondrial matrix ([Ca2+]m) change rapidly in response to cytosolic Ca2+ signals (Fig. 2C) [65]. Further investigations using matrix-targeted aequorin indicated that [Ca2+]m increased in response to IP3-mediated ER Ca2+ release but was unaffected by diffuse Ca2+ elevations of a similar magnitude, thus implicating ER Ca2+ microdomains in mitochondrial uptake [66]. Labeling the ER and mitochondria with distinctly colored fluorescent proteins highlighted the close physical association between these two organelles, and aequorin targeted to the mitochondrial intermembrane space showed that the opening of IP3Rs exposes mitochondria to very high local Ca2+ concentrations and even hinted at the existence of Ca2+ hotspots on the exterior of the mitochondria [67]. Recently, fluorescence imaging of an enhanced cameleon probe (D1cpv) targeted to the cytosolic face of the outer mitochondrial membrane was combined with detailed, pixel-by-pixel analyses to directly visualize these Ca2+ hotspots, in which Ca2+ concentrations were up to 10 times higher compared with the bulk cytosol [68].
3.3 Compartmentalized signaling by Ca2+ targets
Ca2+ acts by modulating the activities of numerous Ca2+-sensitive enzymes and regulatory proteins that participate in various cellular processes. The Ca2+ signaling machinery also engages in extensive interactions with the components of other intracellular signaling pathways. Hence, building a complete picture of the role of cellular microdomains in regulating Ca2+ signaling also requires studying the spatial regulation of Ca2+-dependent signaling molecules and investigating how Ca2+ signaling microdomains impinge on other signaling pathways. It is well known, for example, that Ca2+ plays a major role in regulating cAMP-dependent signaling pathways, and vice versa [69–71]. In particular, Ca2+ signals can both stimulate and inhibit the production of cAMP by modulating the activities of a subset of adenylyl cyclase (AC) isoforms [72]. These Ca2+-sensitive ACs have been shown to respond almost exclusively to Ca2+ signals generated during capacitative Ca2+ entry (CCE; also known as store-operated Ca2+ entry, or SOCE) [73,74], suggesting that these enzymes might be specifically responding to CCE-induced Ca2+ microdomains. Nakahashi and colleagues were able to test this idea directly by fusing aequorin to the C-terminus of the Ca2+-inhibited AC isoform AC5 and comparing the response from this probe with that of cytosolic aequorin under various Ca2+-elevating conditions [75]. Whereas AC5-aequorin was less responsive to general Ca2+ release from intracellular stores than cytosolic aequorin, the targeted probe reported much higher Ca2+ concentrations in response to the induction of CCE than were detected in the cytosol. More recently, Willoughby et al. performed similar experiments by fusing GCaMP to either AC8, a Ca2+-stimulated AC, or AC2, which is Ca2+ insensitive [76]. The authors confirmed that AC8 activity is specifically stimulated by CCE and found that AC8-GCaMP specifically sensed Ca2+ from CCE, being virtually insensitive to general Ca2+ release. On the other hand, the AC2-GCaMP response mirrored that of GCaMP expressed throughout the plasma membrane or in the cytosol. In fact, so close is the coupling between ER stores and cAMP that store depletion alone can activate cAMP signaling, independent of cytosolic Ca2+ influx: Using cAMP biosensors (see Section 2), Lefkimmiatis and coworkers recently demonstrated that STIM1, which monitors ER Ca2+ stores [77–79], directly activates cAMP production upon store depletion [80].
However, although they illuminate an important facet of intracellular signaling, at present, studies of the spatial regulation of Ca2+ targets remain somewhat rare, especially compared with the vast body of work that has been built around the characterization of Ca2+ microdomains themselves. In most cases, this stems from the need to develop appropriate tools (e.g., genetically encoded biosensors) for directly probing the compartmentalized activities of the Ca2+ signaling machinery. Though this is not to say that spatially regulated signaling by Ca2+ targets cannot be investigated by other means. For example, the Ca2+/CaM-dependent protein phosphatase calcineurin (CaN) is known to physically interact with L-type VGCCs [81–83], and studies have shown that local activation by L-type channels is essential for CaN signaling. In particular, Nieves-Cintrón et al. found that BAPTA treatment was unable to disrupt the local activation of CaN by Ca2+ sparklets (visualized using the Ca2+ indicator fluo-5F) generated by Ltype VGCCs [84]. Nevertheless, genetically encoded fluorescent reporters remain among the most powerful and versatile means of assaying the spatial patterns of intracellular signaling. Wu et al. recently described a FRET-based biosensor, based on the interaction between CaN and Ca2+/CaM, which they used to monitor the spatial dynamics of CaN activation in murine primary cortical neurons treated with oligomeric amyloid-β (Aβ) [85]. This reporter, which exhibits a FRET increase when Ca2+/CaM binds and activates CaN, revealed that Aβ treatment rapidly activates CaN localized in dendritic spines, followed within minutes by CaN activation in the cytosol and hours later in the nucleus.
Our group also recently investigated the spatial regulation of CaN signaling in pancreatic β-cells [86]. Insulin secretion by pancreatic β-cells is known to be controlled by oscillatory changes in cytosolic Ca2+ concentrations ([Ca2+]c) [87], which we previously linked to the spatial regulation of cAMP-dependent protein kinase (PKA) signaling in these cells [88]. Therefore, we used a FRET-based CaN activity reporter (CaNAR), based on conformational changes associated with the CaN-dependent dephosphorylation of nuclear factor of activated T-cells (NFAT) [89], to examine whether CaN activity in different subcellular regions is differentially regulated by these Ca2+ oscillations. Using improved, subcellularly targeted versions of CaNAR, we identified two distinct subcellular “zones” with unique CaN activity patterns: In response to Ca2+ oscillations, CaN activity in the cytosol increased in an integrative fashion, with each Ca2+ peak leading to a step-increase in CaN activity, whereas CaN activity measured near the ER surface oscillated in tandem with [Ca2+]c (Fig. 3). To elucidate the mechanism underlying these spatially distinct CaN activity patterns, we first explored the role of kinases in antagonizing CaN. Specifically, we found that inhibiting PKA activity dramatically altered ER CaN activity dynamics, causing them to adopt the step-like, integrative pattern seen in the cytosol [86]. However, increasing PKA to saturating levels did not similarly induce an oscillatory CaN activity pattern in the cytosol, suggesting that additional factors were rendering ER CaN activity more susceptible to PKA.
Figure 3. Spatial regulation of CaN signaling in pancreatic β-cells.
(A) A FRET-based CaN activity reporter (CaNAR), which consists of the N-terminal 297 amino acids from NFAT1 sandwiched between CFP and YFP. Dephosphorylation of the reporter by CaN leads to a conformational change, and thus a FRET change. (B) In MIN6 pancreatic β-cells, the FRET response from CaNAR (black curves) reveals that CaN activity increases in a step-like fashion in the cytosol (left), whereas CaN activity appears to oscillate near the ER surface (right), in relation to cytosolic Ca2+ oscillations (red curves). (C) A CaN activation ratiometric indicator (CaNARi), which consists of the catalytic subunit of CaN (CNA) sandwiched between CFP and YFP. CaN activation via Ca2+/CaM binding induces a conformational change, leading to altered FRET between CFP and YFP. (D) The FRET response from CaNARi (black curves) reveals that CaN exhibits an oscillatory activation pattern both in the cytosol (left) and at the ER surface (right), in contrast to the CaN activity pattern revealed by CaNAR. (E) As described in [86], these data are consistent with a model in which free Ca2+/CaM is less abundant near the ER surface, thereby leading to weaker CaN activation and CaN activity oscillations in this compartment. Adapted from [86].
We therefore used another FRET biosensor, based on the Ca2+/CaM-induced conformational change that occurs during CaN activation, to test whether CaN activation differs subcellularly in pancreatic β-cells [86]. Interestingly, although we observed that CaN activation was uniformly oscillatory throughout these cells in response to Ca2+ oscillations (Fig. 3), our results did suggest that less CaN was being activated near the ER surface. The activity of CaN is tightly regulated by Ca2+/CaM, which serves as a central node in the Ca2+ signaling network, mediating diverse cellular processes through its many targets. Free Ca2+/CaM that is available to transiently interact with targets, rather than being bound as a dedicated subunit, is considered to be a limiting resource in cells, and given its limited diffusibility, free Ca2+/CaM may impart another layer of spatial control over Ca2+ signaling [90,91]. We therefore used a FRET-based biosensor that specifically detects free Ca2+/CaM [92] to test the hypothesis that limiting amounts of Ca2+/CaM at the ER surface in β-cells were contributing to weaker CaN activation and thus causing CaN activity oscillations in this compartment. This probe was previously used to compare free Ca2+/CaM levels in the cytosol and nucleus in rat basophilic leukemia cells [93], and differences in free Ca2+/CaM levels detected using this biosensor have been confirmed to correspond to physiologically meaningful differences in Ca2+/CaM target activity [94]. Using this sensor, we found that free Ca2+/CaM was indeed less abundant at the ER compared with the cytosol. Furthermore, overexpressing additional CaM not only rescued this difference but also led to integrative ER CaN activity. Conversely, treating the cells with a CaM antagonist also endowed the cytosol with oscillating CaN activity [86].
Diffusible reaction systems form the backbone of spatially organized signaling [95]. The formation of Ca2+ microdomains, for instance, relies on the interplay between influx, efflux, and buffering to restrict the diffusion of Ca2+ [3]. Our study revealed that Ca2+/CaM can also impose a spatial signal atop the transient but global Ca2+ rises that accompany cytosolic Ca2+ oscillations. Ca2+ transients have similarly been shown to produce large local increases in free Ca2+/CaM but only minimal increases at distal locations (e.g., the nucleus) due to the limited diffusibility of Ca2+/CaM [93]. In fact, long-range Ca2+/CaM signaling was recently shown to require a dedicated carrier protein [96]. Seen in this light, it is clear that the diffusion of free Ca2+/CaM adds another dimension to the organization of Ca2+ signaling microdomains.
4. Concluding remarks
The idea that signaling pathways are spatially compartmentalized is not new, though it emerged in the absence of tools for directly probing signaling compartments in living cells, and we have essentially spent the last few decades catching up. Ca2+ signaling is somewhat unique in this regard, as specialized tools for visualizing Ca2+ in living cells were developed quite quickly based on existing in vitro techniques. Thus, Ca2+ has long been able to serve as a model for the study of compartmentalized signaling. The advent of genetically encoded fluorescent reporters has leveled the playing field, so to speak, and has allowed researchers to move into new realms and study the compartmentalization of more and more signaling processes. We now have access to a powerful and versatile arsenal of tools that can be used to probe signaling microdomains from a variety of angles, from unraveling the molecular mechanisms and biological roles of known microdomains to identifying and characterizing entirely new signaling domains. Along these lines, Matsuda and colleagues recently developed a “caged” FRET-based Ca2+ indicator whose fluorescence can be activated at specific locations and times via UV illumination [97], thus enabling the precise visualization of Ca2+ signals in cellular microdomains that are not currently accessible by approaches that rely on the use of targeting sequences. Like the first FRET-based Ca2+ sensor, cameleon, such novel designs will likely serve as prototypes for the development of new probes for other signaling molecules, thus continuing to advance the study of signaling microdomains into new frontiers.
We review the application of fluorescent biosensors to study local calcium signaling in live cells
Acknowledgements
This work was supported by National Institutes of Health grants R01 DK073368 and DP1 CA174423 to J.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 4.Laude AJ, Simpson AWM. Compartmentalized signalling: Ca2+ compartments, microdomains and the many facets of Ca2+ signalling. Febs J. 2009;276:1800–1816. doi: 10.1111/j.1742-4658.2009.06927.x. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu. Rev. Pharmacol. Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- 6.Chad JE, Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of Ca-dependent responses. Biophys J. 1984;45:993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon SM, Llinás RR. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985;48:485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsien RY. Fluorescent probes of cell signaling. Annu. Rev. Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- 10.Shimomura O, Musicki B, Kishi Y. Semi-synthetic aequorin. An improved tool for the measurement of calcium ion concentration. Biochem J. 1988;251:405–410. doi: 10.1042/bj2510405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimomura O, Inouye S, Musicki B, Kishi Y. Recombinant aequorin and recombinant semi-synthetic aequorins. Cellular Ca2+ ion indicators. Biochem J. 1990;270:309–312. doi: 10.1042/bj2700309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J. The Colorful Journey of Green Fluorescent Protein. ACS Chem Biol. 2009;4:85–88. doi: 10.1021/cb900027r. [DOI] [PubMed] [Google Scholar]
- 13.Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Zhang J. FRET-based activity biosensors to probe compartmentalized signaling. Chembiochem. 2010;11:147–151. doi: 10.1002/cbic.200900594. [DOI] [PubMed] [Google Scholar]
- 15.Mehta S, Zhang J. Reporting from the Field: Genetically Encoded Fluorescent Reporters Uncover Signaling Dynamics in Living Biological Systems. Annu Rev Biochem. 2010;80 doi: 10.1146/annurev-biochem-060409-093259. 110301095147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman RH, Fosbrink MD, Zhang J. Genetically Encodable Fluorescent Biosensors for Tracking Signaling Dynamics in Living Cells. Chem Rev. 2011;111:3614–3666. doi: 10.1021/cr100002u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell RE. Fluorescent-protein-based biosensors: modulation of energy transfer as a design principle. Anal. Chem. 2009;81:5972–5979. doi: 10.1021/ac802613w. [DOI] [PubMed] [Google Scholar]
- 18.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 19.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci USA. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 21.Akerboom J, Rivera JDV, Guilbe MMR, Malavé ECA, Hernandez HH, Tian L, et al. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem. 2009;284:6455–6464. doi: 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbst KJ, Ni Q, Zhang J. Dynamic visualization of signal transduction in living cells: From second messengers to kinases. IUBMB Life. 2009;61:902–908. doi: 10.1002/iub.232. [DOI] [PubMed] [Google Scholar]
- 23.Kiyokawa E, Aoki K, Nakamura T, Matsuda M. Spatiotemporal Regulation of Small GTPases as Revealed by Probes Based on the Principle of Förster Resonance Energy Transfer (FRET): Implications for Signaling and Pharmacology. Annu. Rev. Pharmacol. Toxicol. 2011;51:337–358. doi: 10.1146/annurev-pharmtox-010510-100234. [DOI] [PubMed] [Google Scholar]
- 24.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 26.Llinás R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 27.Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci. 2011;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyleta NP, Jonas P. Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science. 2014;343:665–670. doi: 10.1126/science.1244811. [DOI] [PubMed] [Google Scholar]
- 29.Selway J, Rigatti R, Storey N, Lu J, Willars GB, Herbert TP. Evidence that Ca2+ within the microdomain of the L-type voltage gated Ca2+ channel activates ERK in MIN6 cells in response to glucagon-like peptide-1. PLoS ONE. 2012;7:e33004. doi: 10.1371/journal.pone.0033004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cobbold PH, Rink TJ. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987;248:313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipscombe D, Madison DV, Poenie M, Reuter H, Tsien RW, Tsien RY. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988;1:355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 32.López-López JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- 33.Smith SJ, Buchanan J, Osses LR, Charlton MP, Augustine GJ. The spatial distribution of calcium signals in squid presynaptic terminals. J Physiol (Lond) 1993;472:573–593. doi: 10.1113/jphysiol.1993.sp019963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridgway EB, Ashley CC. Calcium transients in single muscle fibers. Biochem Biophys Res Commun. 1967;29:229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- 35.Llinás R, Sugimori M, Silver RB. Imaging Preterminal Calcium Concentration Microdomains in the Squid Gaint Synapse. Biol. Bull. 1991;181:316–317. doi: 10.1086/BBLv181n2p316. [DOI] [PubMed] [Google Scholar]
- 36.Silver RB, Sugimori M, Lang EJ, Llinás R. Time-resolved imaging of Ca(2+)-dependent aequorin luminescence of microdomains and QEDs in synaptic preterminals. Biol. Bull. 1994;187:293–299. doi: 10.2307/1542285. [DOI] [PubMed] [Google Scholar]
- 37.Sugimori M, Lang EJ, Silver RB, Llinás R. High-resolution measurement of the time course of calcium-concentration microdomains at squid presynaptic terminals. Biol. Bull. 1994;187:300–303. doi: 10.2307/1542286. [DOI] [PubMed] [Google Scholar]
- 38.Berridge MJ. Elementary and global aspects of calcium signalling. 1997 doi: 10.1242/jeb.200.2.315. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan MN, Francis M, Pitts NL, Taylor MS, Earley S. Optical recording reveals novel properties of GSK1016790A-induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Molecular Pharmacology. 2012;82:464–472. doi: 10.1124/mol.112.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Augustine GJ, Neher E. Neuronal Ca2+ signalling takes the local route. Curr Opin Neurobiol. 1992;2:302–307. doi: 10.1016/0959-4388(92)90119-6. [DOI] [PubMed] [Google Scholar]
- 42.Marsault R, Murgia M, Pozzan T, Rizzuto R. Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. Embo J. 1997;16:1575–1581. doi: 10.1093/emboj/16.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emmanouilidou E, Teschemacher AG, Pouli AE, Nicholls LI, Seward EP, Rutter GA. Imaging Ca2+ concentration changes at the secretory vesicle surface with a recombinant targeted cameleon. Curr Biol. 1999;9:915–918. doi: 10.1016/s0960-9822(99)80398-4. [DOI] [PubMed] [Google Scholar]
- 44.Tay LH, Dick IE, Yang W, Mank M, Griesbeck O, Yue DT. Nanodomain Ca2+ of Ca2+ channels detected by a tethered genetically encoded Ca2+ sensor. Nat Commun. 2012;3:778. doi: 10.1038/ncomms1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isshiki M, Nishimoto M, Mizuno R, Fujita T. FRET-based sensor analysis reveals caveolae are spatially distinct Ca2+ stores in endothelial cells. Cell Calcium. 2013;54:395–403. doi: 10.1016/j.ceca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 47.Lipscombe D, Madison DV, Poenie M, Reuter H, Tsien RY, Tsien RW. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci USA. 1988;85:2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bootman M, Niggli E, Berridge M, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol (Lond) 1997;499(Pt 2):307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol (Lond) 1995;482(Pt 3):533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith IF, Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc Natl Acad Sci USA. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Despa S, Shui B, Bossuyt J, Lang D, Kotlikoff MI, Bers DM. Junctional cleft [Ca2+]i measurements using novel cleft-targeted Ca2+ sensors. Circ Res. 2014;115:339–347. doi: 10.1161/CIRCRESAHA.115.303582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, et al. Selective Binding of FKBP12.6 by the Cardiac Ryanodine Receptor. J Biol Chem. 1996;271:20385–20391. doi: 10.1074/jbc.271.34.20385. [DOI] [PubMed] [Google Scholar]
- 53.Timerman AP, Ogunbumni E, Freund E, Wiederrecht G, Marks AR, Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. Dissociation and reconstitution of FKBP-12 to the calcium release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993;268:22992–22999. [PubMed] [Google Scholar]
- 54.Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, et al. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. Embo J. 1995;14:5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de la Fuente S, Fonteriz RI, Montero M, Alvarez J. Ca2+ homeostasis in the endoplasmic reticulum measured with a new low-Ca2+-affinity targeted aequorin. Cell Calcium. 2013;54:37–45. doi: 10.1016/j.ceca.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Palmer AE. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiménez-Moreno R, Wang Z-M, Messi ML, Delbono O. Sarcoplasmic reticulum Ca2+ depletion in adult skeletal muscle fibres measured with the biosensor D1ER. Pflugers Arch. 2010;459:725–735. doi: 10.1007/s00424-009-0778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang S, Wong H-C, Wang Z-M, Huang Y, Zou J, Zhuo Y, et al. Design and application of a class of sensors to monitor Ca2+ dynamics in high Ca2+ concentration cellular compartments. Proc Natl Acad Sci USA. 2011;108:16265–16270. doi: 10.1073/pnas.1103015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z-M, Tang S, Messi ML, Yang JJ, Delbono O. Residual sarcoplasmic reticulum Ca2+ concentration after Ca2+ release in skeletal myofibers from young adult and old mice. Pflugers Arch. 2012;463:615–624. doi: 10.1007/s00424-012-1073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lissandron V, Podini P, Pizzo P, Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci USA. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong AKC, Capitanio P, Lissandron V, Bortolozzi M, Pozzan T, Pizzo P. Heterogeneity of Ca2+ handling among and within Golgi compartments. J Mol Cell Biol. 2013;5:266–276. doi: 10.1093/jmcb/mjt024. [DOI] [PubMed] [Google Scholar]
- 62.McCue HV, Wardyn JD, Burgoyne RD, Haynes LP. Generation and characterization of a lysosomally targeted, genetically encoded Ca2+-sensor. Biochem J. 2013;449:449–457. doi: 10.1042/BJ20120898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994;14:348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 65.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 66.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 67.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 68.Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Zaccolo M, Pozzan T. cAMP and Ca2+ interplay: a matter of oscillation patterns. Trends Neurosci. 2003;26:53–55. doi: 10.1016/s0166-2236(02)00017-6. [DOI] [PubMed] [Google Scholar]
- 70.Bruce JIE, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 71.Borodinsky LN, Spitzer NC. Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci STKE. 2006;2006:pe22. doi: 10.1126/stke.3362006pe22. [DOI] [PubMed] [Google Scholar]
- 72.Cooper DM, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 73.Chiono M, Mahey R, Tate G, Cooper DM. Capacitative Ca2+ entry exclusively inhibits cAMP synthesis in C6-2B glioma cells. Evidence that physiologically evoked Ca2+ entry regulates Ca2+-inhibitable adenylyl cyclase in non-excitable cells. J Biol Chem. 1995;270:1149–1155. doi: 10.1074/jbc.270.3.1149. [DOI] [PubMed] [Google Scholar]
- 74.Fagan KA, Mahey R, Cooper DM. Functional co-localization of transfected Ca2+-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem. 1996;271:12438–12444. doi: 10.1074/jbc.271.21.12438. [DOI] [PubMed] [Google Scholar]
- 75.Nakahashi Y, Nelson E, Fagan K, Gonzales E, Guillou JL, Cooper DM. Construction of a full-length Ca2+-sensitive adenylyl cyclase/aequorin chimera. J Biol Chem. 1997;272:18093–18097. doi: 10.1074/jbc.272.29.18093. [DOI] [PubMed] [Google Scholar]
- 76.Willoughby D, Wachten S, Masada N, Cooper DMF. Direct demonstration of discrete Ca2+ microdomains associated with different isoforms of adenylyl cyclase. J Cell Sci. 2010;123:107–117. doi: 10.1242/jcs.062067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Storeoperated cyclic AMP signalling mediated by STIM1. Nat Cell Biol. 2009;11:433–442. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]
- 81.Tandan S, Wang Y, Wang TT, Jiang N, Hall DD, Hell JW, et al. Physical and functional interaction between calcineurin and the cardiac L-type Ca2+ channel. Circ Res. 2009;105:51–60. doi: 10.1161/CIRCRESAHA.109.199828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 Anchoring of Calcineurin Controls Neuronal L-Type Ca2+ Channel Activity and Nuclear Signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliveria SF, Dittmer PJ, Youn DH, Dell'Acqua ML, Sather WA. Localized Calcineurin Confers Ca2+-Dependent Inactivation on Neuronal L-Type Ca2+ Channels. J Neurosci. 2012;32:15328–15337. doi: 10.1523/JNEUROSCI.2302-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nieves-Cintrón M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci USA. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu HY, Hudry E, Hashimoto T, Uemura K, Fan ZY, Berezovska O, et al. Distinct Dendritic Spine and Nuclear Phases of Calcineurin Activation After Exposure to Amyloid-β Revealed by a Novel Fluorescence Resonance Energy Transfer Assay. J Neurosci. 2012;32:5298–5309. doi: 10.1523/JNEUROSCI.0227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehta S, Aye-Han N-N, Ganesan A, Oldach L, Gorshkov K, Zhang J. Calmodulin-controlled spatial decoding of oscillatory Ca2+ signals by calcineurin. Elife. 2014:e03765. doi: 10.7554/eLife.03765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tengholm A, Gylfe E. Oscillatory control of insulin secretion. Mol. Cell. Endocrinol. 2008;297:58–72. doi: 10.1016/j.mce.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Ni Q, Ganesan A, Aye-Han N-N, Gao X, Allen MD, Levchenko A, et al. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol. 2010;7:34–40. doi: 10.1038/nchembio.478. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newman RH, Zhang J. Visualization of phosphatase activity in living cells with a FRET-based calcineurin activity sensor. Mol Biosyst. 2008;4:496–501. doi: 10.1039/b720034j. [DOI] [PubMed] [Google Scholar]
- 90.Persechini A, Stemmer PM. Calmodulin is a limiting factor in the cell. Trends Cardiovasc Med. 2002;12:32–37. doi: 10.1016/s1050-1738(01)00144-x. [DOI] [PubMed] [Google Scholar]
- 91.Saucerman JJ, Bers DM. Calmodulin binding proteins provide domains of local Ca2+ signaling in cardiac myocytes. J Mol Cell Cardiol. 2012;52:312–316. doi: 10.1016/j.yjmcc.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Persechini A, Cronk B. The relationship between the free concentrations of Ca2+ and Ca2+-calmodulin in intact cells. J Biol Chem. 1999;274:6827–6830. doi: 10.1074/jbc.274.11.6827. [DOI] [PubMed] [Google Scholar]
- 93.Teruel MN, Chen W, Persechini A, Meyer T. Differential codes for free Ca2+-calmodulin signals in nucleus and cytosol. Curr Biol. 2000;10:86–94. doi: 10.1016/s0960-9822(00)00295-5. [DOI] [PubMed] [Google Scholar]
- 94.Tran Q-K, Black DJ, Persechini A. Intracellular coupling via limiting calmodulin. J Biol Chem. 2003;278:24247–24250. doi: 10.1074/jbc.C300165200. [DOI] [PubMed] [Google Scholar]
- 95.Dehmelt L, Bastiaens PIH. Spatial organization of intracellular communication: insights from imaging. Nat Rev Mol Cell Biol. 2010:1–13. doi: 10.1038/nrm2903. [DOI] [PubMed] [Google Scholar]
- 96.Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, et al. γCaMKII Shuttles Ca2+/CaM to the Nucleus to Triggerger CREB Phosphorylation and Gene Expression. Cell. 2014;159:281–294. doi: 10.1016/j.cell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsuda T, Horikawa K, Saito K, Nagai T. Highlighted Ca2+ imaging with a genetically encoded “caged” indicator. Sci Rep. 2013;3:1398. doi: 10.1038/srep01398. [DOI] [PMC free article] [PubMed] [Google Scholar]