Abstract
Small-conductance Ca2+-activated K+ (SK) currents are important in the repolarization of normal atrial (but not ventricular) cardiomyocytes. However, recent studies showed that the SK currents are upregulated in failing ventricular cardiomyocytes, along with increased SK channel protein expression and enhanced sensitivity to intracellular Ca2+. The SK channel activation may be either antiarrhythmic or proarrhythmic, depending on the underlying clinical situations. While the SK channel is a new target of antiarrhythmic therapy, drug safety is still one of the major concerns.
Keywords: SK channels, heart failure, repolarization
Introduction
Heart failure (HF) is associated with increased risk of sudden cardiac death (SCD), which accounts for up to 50% of death in patients with HF (Tomaselli and Zipes, 2004). The mechanisms of ventricular tachyarrhythmias in HF are complicated, involving anatomic remodeling, impaired conduction system, ion channel alteration, Ca2+ homeostasis, changes in neurohumoral signaling and genetic factors. The hallmark of electrophysiological remodeling is prolonged action potential duration (APD). Downregulation of most major K+ currents, increasing of late Na+ current and alteration of Ca2+ homeostasis contribute to prolongation of APD (Aiba and Tomaselli, 2010). Failing hearts are prone to electrical storm. Acute shortening of APD after termination of ventricular fibrillation (VF) with persistently elevated intracellular Ca2+ (Cai) leads to development of late phase 3 early afterdepolarizations (EADs), which is also known to promote immediate recurrence of atrial fibrillation (AF) in isolated canine atrium (Burashnikov and Antzelevitch, 2003). Ogawa et al (Ogawa et al., 2009) subsequently documented acute but reversible APD shortening after defibrillation during episodes of electrical storm in failing rabbit hearts. The mechanisms of electrical storm in the rabbit model of HF remained unclear until Chua et al (Chua et al., 2011) discovered that upregulation of apamin-sensitive small-conductance Ca2+-activated K+ (SK) current (IKAS) in failing hearts was responsible for the post-shock APD shortening (Figure 1). Apamin, a specific SK channel blocker (Adelman et al., 2012), prevented acute shortening of APD and recurrent spontaneous VF. These findings showed that SK currents were important in the electrical storm in this rabbit model of HF.
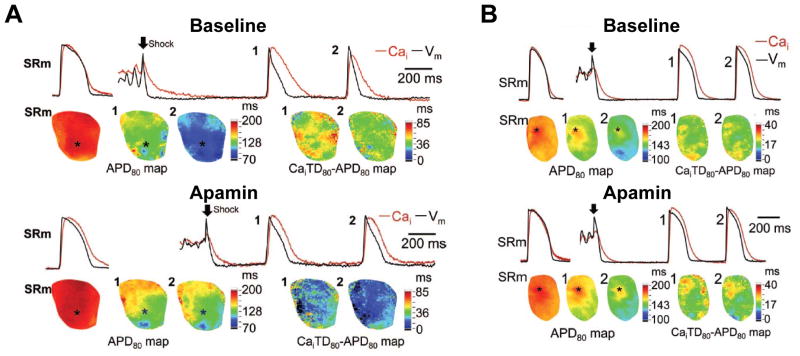
Figure 1.
Effects of apamin (1μmol/L) on APD80 and the differences between Ca2+ transient duration (CaiTD80) and APD80 in failing and normal rabbit hearts. Optical traces (top) of Vm (black line) and Cai (red line) were recorded from site marked by an asterisk in APD80 map (bottom). A. Failing heart. Top subpanel shows epicardial optical traces of Vm and Cai and APD80 map during sinus rhythm (SRm) before pacing-induced VF (left). Top right shows beats 1 and 2 had acute shortening of APD in the immediate post-shock period, resulting in the Cai elevation during late phase 3 and phase 4. Bottom right shows the corresponding APD80 maps, and the maps of the difference between CaiTD80 and APD80 in beats 1 and 2. After apamin (bottom subpanel), the postshock beats 1 and 2 had longer APD80 than those at baseline. The CaiTD80 was similar to that in A, and the differences between CaiTD80 and APD80 in beats 1 and 2 were reduced. B. Normal heart. As compared with the baseline, there were little changes of APD and CaiTD after defibrillation when the tissues were pretreated with apamin. Arrow indicates defibrillation. PI VF, pacing-induced VF; SRm indicates sinus rhythm. From Chua et al (Chua et al., 2011), with permission.
The discovery of SK channels
The research of apamin, a polypeptide bee venom, led to the identification of SK channels (Adelman et al., 2012). It has been found that lethal dose of apamin results in tonic convulsion and respiratory failure through its neurologic toxic effects. The SK channels were not well known until Kohler et al cloned the genes and detected the abundant expression of the mRNAs in the rat brain, heart, and other organs (Kohler et al., 1996). Electrophysiological study further showed that the SK channels are the only target of apamin (Adelman et al., 2012; Yu et al., 2014). In neuronal cells, the SK channels account for the slow component of afterhyperpolarization, which regulates neuronal discharges. SK channels contribute to repolarization of action potential in atrial cardiomyocytes and diseased ventricular cells (Bonilla et al., 2014; Chang et al., 2013a; Chua et al., 2011; Lee et al., 2013; Xu et al., 2003). The activation of SK channels also plays important roles in the function of a range of other tissues, such as the endothelium, intestine and urinary bladder (Feher et al., 2014; Hougaard et al., 2009; Ro et al., 2001). The SK channel family consists of 3 major subtypes: SK1, SK2 and SK3. In normal hearts, SK1 and SK2 are expressed predominantly in atria, and SK3 is expressed in both atria and ventricles (Tuteja et al., 2005). Among these 3 subtypes, SK2 is the most sensitive to apamin (EC50 ~ 40 pM), SK1 is the least sensitive (EC50 ~ 10 nM), and SK3 has intermediate sensitivity (EC50 ~ 1 nM) (Adelman et al., 2012). Apamin is a highly selective SK channel blocker and does not affect human cardiac Na+, L-type Ca2+ and major K+ currents (Yu et al., 2014). In addition to apamin, many other compounds also inhibit SK channels, including tamapin, UCL-1684, UCL-1848, NS8593, d-tubocurarine, dequalinium, etc (Weatherall et al., 2010). Tamapin and UCL-1684 have been shown to selectively block SK channels (Fanger et al., 2001; Pedarzani et al., 2002). The selectivity of other compounds has not yet been thoroughly investigated.
The role of SK channels in cardiomyocyte repolarization
In cardiomyocytes, trans-sarcolemmal Ca2+ influx through L-type Ca2+ channels, Ca2+ release from sarcoplasmic reticulum (SR), or combination of both, regulates the gating of SK channels (Lu et al., 2007; Terentyev et al., 2013). SK channels are coupled to L-type Ca2+ channels, and Ca2+ influx through L-type Ca2+ channels directly activates SK channels (Lu et al., 2007; Maingret et al., 2008). Ca2+-induced Ca2+ release (CICR) also triggers the activation of SK channels (Terentyev et al., 2013). The gating is endowed by the interaction between the pore-forming subunits and calmodulin. The Cai concentration required for half-maximal activation of SK channels is around 300–700 nM. SK channels are activated during the systolic phase when the Cai increases, and thereby the activation of SK channels repolarizes cardiomyocytes and shortens APD. Longer APD leads to longer Ca2+ wave and enhances activation of SK channels (Chang et al., 2013b), which helps shorten the APD. This mechanism is a negative feedback system and helps prevent excessive prolongation of action potential.
Recently, Terentyev et al reported that SR Ca2+ release is also necessary for SK channel activation (Terentyev et al., 2013). A spontaneous SR Ca2+ release wave activates SK currents, which contribute to repolarization during action potentials and attenuate delayed afterdepolarizations (DADs) driven by spontaneous Ca2+ waves. Thus, SK upregulation in HF may have an anti-arrhythmic effect by reducing triggered activity.
While SK channel proteins are present in both the atria and ventricles, the magnitudes of the SK currents are not uniformly expressed throughout the heart. Before the cloning of SK channels, Giles et al noted that rabbit atrial myocytes expressed more Ca2+-activated K+ currents than ventricular myocytes (Giles and Imaizumi, 1988). After the identification of SK channels, Xu et al (Xu et al., 2003) showed that apamin prolonged APD in atrial cardiomyocytes but had little effects in ventricular cells. The expression of SK channel protein and mRNA in atrial tissues is more pronounced than that in ventricular ones (Tuteja et al., 2005; Xu et al., 2003). Therefore, specific SK blockade, such as apamin (Yu et al., 2014), was once thought to be atrial selective. SK channel modulation might be an ideal solution to manage atrial tachyarrhythmia because the ion channel blocking effects on ventricular cardiomyocytes were thought to be minimal. These observations led to significant enthusiasm of developing SK channel blockers for the management of atrial arrhythmias.
Multiple studies confirmed the importance of SK channels in atrial arrhythmogenesis. Li et al (Li et al., 2009) demonstrated more frequent EADs and enhanced inducibility of AF in SK2 knock-out mice than in wild type mice. Hsueh et al (Hsueh et al., 2013) also reported that SK channel blockers increased inducibility of atrial arrhythmia. The proarrhythmic mechanisms of SK channel blockade might be due to the prolonged APD and more pronounced APD heterogeneity, which facilitates wave breaks. On the other hand, Diness et al (Diness et al., 2010) demonstrated that SK channel blockers prevented and terminated AF in the guinea pigs, rats and rabbit models. They also noted that SK channel blockade lengthened atrial effective refractory period without affecting QT interval. The results were compatible with previous findings that SK channel blockers affected only the APD of atrial but not ventricular cardiomyocytes. Ozgen et al (Ozgen et al., 2007) showed that atrial burst pacing led to SK2 trafficking to the cell membrane, which shortened APD in pulmonary vein cells. These studies suggest that SK channel blockade might be both proarrhythmic and antiarrhythmic in the atria, depending on the experimental models and study protocols. In humans, genome-wide association studies of lone AF patients showed a significant association to AF on chromosome 1q21 (rs13376333), which is intronic to KCNN3 (SK3) (Ellinor et al., 2012; Ellinor et al., 2010). Overexpression of the KCNN3 in mice causes an increased risk of sudden death associated with bradyarrhythmias and heart block, possibly due to atrioventricular (AV) nodal dysfunction (Mahida et al., 2014). Those mice also are more susceptible to pacing-induced atrial arrhythmias. However, the exact mechanisms by which lone AF was associated with rs13376333 remain unclear.
Differential expression of SK channels in normal and diseased ventricles
In contrast to the reports that showed SK channels are important in the repolarization of atrial myocytes, the importance of SK channels in the repolarization of ventricular cells has been debated. Xu et al (Xu et al., 2003) showed that APD prolongation induced by apamin was much more pronounced in the atria than in the ventricles. Nagy et al (Nagy et al., 2009) showed that apamin did not alter APD in either normal atrial or ventricular cardiomyocytes of the dog, rat and human. Patch clamp study also showed that apamin had no effect on K+ currents in the rat and dog ventricular cells, although both the rat and dog ventricular tissues abundantly expressed SK2 channel protein. The reason SK channel blockers neither inhibited K+ currents nor altered APD in normal ventricular cardiomyocytes in spite of an abundant presence of the SK channel proteins is still a mystery (Nagy et al., 2009; Tuteja et al., 2005; Xu et al., 2003). To investigate the importance of SK channels in normal and failing ventricles, Chua et al (Chua et al., 2011) performed optical mapping studies in Langendorff perfused rabbit ventricles and showed that apamin had little effects on APD in normal ventricles. However, significant APD prolongation occurred in failing ventricles. In addition, apamin prolonged APD and eliminated post-shock recurrent spontaneous VF in failing rabbit hearts. The increased Ca2+ sensitivity of SK currents was proposed to be one of the mechanisms. Chang et al (Chang et al., 2013a) further showed upregulation of SK currents in failing human ventricles. Both enhanced Ca2+ sensitivity and increased SK2 channel protein contributed to the upregulation of IKAS. The authors also showed heterogeneous upregulation of IKAS: the epicardial and the endocardial myocytes expressed greater current density than the mid-myocardial cells. The heterogeneity might also contribute to the arrhythmogenecity in failing hearts. More recently, Bonilla et al and Ni et al confirmed these observations by showing that apamin significantly prolonged APD in failing human and canine ventricular cardiomyocytes, along with increased expression of SK channel protein in failing ventricles (Bonilla et al., 2014; Ni et al., 2013). The authors further demonstrated that there was a trend for more ventricular SK protein expression and greater APD prolongation in dogs with 4 months than with 1 month of HF. However, in contrast to APD prolongation in normal atrial myocytes, SK blockade did not affect APD in either human or canine failing atrial cells. The results raised a concern of pharmacological SK blockade for the treatment of atrial arrhythmias. SK channel inhibition for atrial arrhythmias could be ineffective in patients with HF; moreover, it has potential risk of pro-arrhythmic effects in the failing ventricles.
Besides HF, chronic myocardial infarction (MI) also upregulates SK currents (Lee et al., 2013). In that study, the authors showed that apamin prolonged APD more in chronic MI rabbit ventricles than in controls, and that the effects of APD prolongation were magnified by rapid heart rates. Heterogeneous IKAS upregulation contributed to the greater APD prolongation in the peri-infarct zone. In addition to chronic MI, Stowe et al (Stowe et al., 2013) showed that activation of SK channels protected hearts against acute ischemia-reperfusion injury and SK channel blockers antagonized the protection. However, the activation of SK channels might also contribute to development of ventricular tachyarrhythmias during acute myocardial infarction (AMI). Gui et al (Gui et al., 2013) showed that pretreatment of SK channel blockers significantly prolonged APD and prevented ventricular tachyarrhythmias in AMI animals.
In addition to cardiomyocytes, coronary arteries and cardiac stellate ganglia also express SK channels, and regulation of SK channels may also play significant roles in arrhythmogenesis. Endothelial SK channel activation leads to hyperpolarization, and contributes to the conducted dilatation of coronary arteries. Dysfunction of SK channels in endothelial cells, such as that occurs during aging, may contribute to impaired myocardial flow reserve (Feher et al., 2014). The SK channels in cardiac stellate ganglia are associated with sympathetic outflow. Shen et al (Shen et al., 2011) demonstrated that low-level vagus nerve stimulation reduced cardiac stellate ganglion activity and paroxysmal AF. The mechanism of the reduced stellate ganglion activity might be attributable in part to the upregulation of SK2 channels in the stellate ganglion (Shen et al., 2013).
The effects of SK channels activation and inhibition on arrhythmogenesis in failing hearts
SK channel activation may have both antiarrhythmic and proarrhythmic effects, depending on the underlying clinical situations. Apamin reduces APD heterogeneity and prevented post-shock spontaneous VF (Chua et al., 2011; Hsieh et al., 2013); on the other hand, apamin also prolongs APD, increases incidence of early afterdepolarizations (EADs) and induces torsades de pointes (TdP) ventricular tachyarrhythmia (Figure 2) in failing hearts (Chang et al., 2013b). Baseline heart rate of the animal models is probably an important factor whether apamin is anti-arrhythmic and pro-arrhythmic in failing ventricles (table 1): at rapid heart rate (sinus or paced rhythm in intact hearts), apamin prevents acute shortening of APD and recurrent spontaneous VF; at slow heart rate (AV block in intact hearts or paced rhythm in isolated cardiomyocytes), apamin further prolongs APD and induces EADs. The phenomenon can be explained by the U curve effect of apamin on APD: apamin prolongs APD more prominently at either very short or at very long pacing cycle lengths in failing ventricles (Figure 3) (Hsieh et al., 2013). At very short pacing cycle lengths, SK channels are activated by elevated Cai while at very long pacing cycle lengths, long Cai transient duration with persistent trans-sarcolemmal Ca2+ influx through L-type Ca2+ channels may also facilitate activation of SK channels. The effects are compatible with many clinical anti-arrhythmic agents that prolong atrial and ventricular effective refractory period and reduce tachyarrhythmias, but patients pay the price of prolonged QT interval and increased risk of TdP. The phenomenon may also explain previous conflicting reports about SK channel blockers in managing atrial tachyarrhythmias. The inhibition of SK channels in ex vivo or in vivo models with normal sinus rate appeared to be anti-arrhythmic; however, SK channel inhibition was proarrhythmic in AV block, SK knock-out models, isolated left atrial models or isolated cardiomyocytes paced at slow rates.
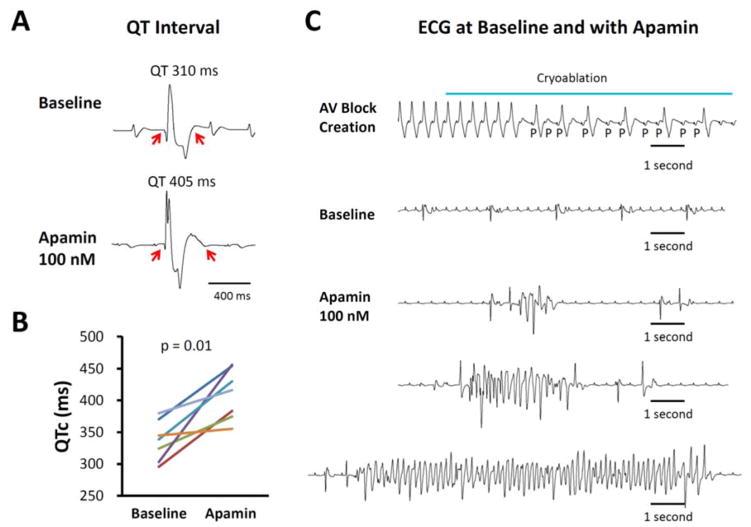
Figure 2.
Apamin effect on QT interval and arrhythmias in failing rabbit hearts. A. Representative pseudoECG (pECG) traces of QT interval in a failing heart with complete atrioventricular (AV) block before and after 100 nmol/l apamin. B. Paired dot plot shows QTc at baseline and in the presence of apamin 100 nmol/L. There was significant prolongation of QTc. C. Representative traces at baseline and in the presence of apamin. Top panel, complete AV block developed during AV node cryoablation. Second panel, no polymorphic ventricular tachycardia was recorded at baseline. However, several episodes of spontaneous TdP polymorphic ventricular arrhythmia developed in the presence of apamin (bottom panels). From Chang et al (Chang et al., 2013b), with permission.
Table 1.
The effects of SK channel blockade in the ventricle
| Model | Medication | Baseline rhythm | Result | Reference |
|---|---|---|---|---|
| Normal mouse cardiomyocytes | Apamin | Paced rhythm | Little effect | Xu et al., 2003 |
| Normal human cardiomyocytes | Apamin | Paced rhythm | No effect | Nagy et al., 2009 |
| Rabbit HF | Apamin | Sinus rhythm | Prolonged APD and prevents acute APD shortening, Anti-arrhythmic: Prevent spontaneous VF | Chua et al., 2011 |
| Human HF cardiomyocytes | Apamin | Paced rhythm | Prolonged APD | Chang et al., 2013 |
| Rabbit HF | Apamin | Sinus rhythm | Prolonged APD Anti-arrhythmic: Reduced APD heterogeneity |
Hsieh et al., 2013 |
| Rabbit HF | Apamin | AV block | Prolonged APD Pro-arrhythmic: Increased EADs and TdP |
Chang et al., 2013b |
| Rabbit Chronic MI | Apamin | Sinus rhythm | Prolonged APD and prevents acute APD shortening | Lee et al., 2013 |
| Rat Acute MI | Apamin, UCL1684 | Sinus rhythm | Anti-arrhythmic: APD prolongation | Gui et al., 2013 |
| Canine HF cardiomyocytes; Human HF cardiomyocytes | Apamin | Paced rhythm | Prolonged APD Pro-arrhythmic: Increased EADs |
Bonilla et al., 2014 |
APD, action potential duration; AV, atrioventricular; EAD, early afterdepolarization; HF, heart failure; MI, myocardial infarction; TdP, torsades de pointes; VF, ventricular fibrillation.
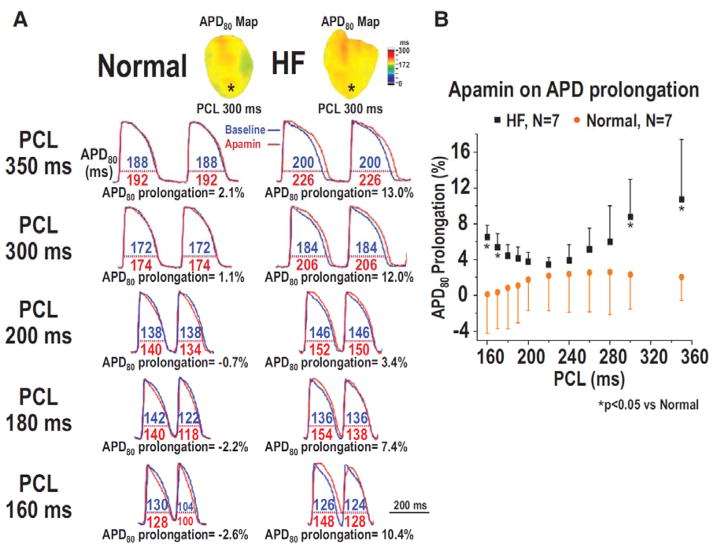
Figure 3.
Effect of apamin on the percentage of action potential duration (APD) prolongation in normal and failing rabbit ventricles. A. APD80 before (blue line) and after (red line) apamin infusion, and the percentage of APD80 prolongation at pacing cycle length (PCL) 350, 300, 200, 180, and 160 ms in a normal and a HF ventricle. B. PCL and the percentage of APD80 prolongation by apamin in all normal and HF ventricles. Note that the differences between HF and normal ventricles were significant only at very long (350–300 ms) and short (170–160 ms). PCLs (asterisks), but not with intermediate (280–180 ms) PCLs. From Hsieh et al (Hsieh et al., 2013), with permission.
Pharmacologic therapy for ventricular arrhythmias with SK channel modulation
Little information is available on the efficacy and safety of SK channel modulation in the treatment of ventricular arrhythmias in humans. Studies in a rabbit model of HF showed that SK channel blockade by apamin can lengthen the postshock APD and prevent recurrent VF (Chua et al., 2011). SK channel blockade can also be used to suppress ventricular tachyarrhythmias in a rat model of acute myocardial ischemia (Gui et al., 2013). Amiodarone is a commonly used antiarrhythmic drug in suppressing recurrent ventricular arrhythmias in humans (Kowey et al., 1995). Because amiodarone is an effective SK channel blocker (Turker et al., 2013), it is possible that the SK channel blocking action has contributed to the acute antiarrhythmic effects of amiodarone therapy. However, chronic therapy with SK channel blockers has significant proarrhythmic potential in patients with impaired ventricular function, ischemic heart diseases or bradycardia. In addition to safety concerns, the SK channel blockers may not be effective in treating atrial arrhythmias associated with HF. A recent study showed that the atrial myocyte action potentials were unchanged by SK current blockade in a canine model of HF (Bonilla et al., 2014). Induction of bradycardia is another concern of chronic SK channel therapy. Bradycardia is known to facilitate the development of EADs and TdP in failing rabbit ventricles with atrioventricular block (Chang et al., 2013b). Sinus node and AV node express SK channels (Chandler et al., 2009; Zhang et al., 2008). Therefore, inhibition of SK channels may lead to sinus bradycardia and AV nodal block (Li et al., 2009) and further facilitate the development of EADs and TdP arrhythmias. SK channel therapy also has potential neuromuscular toxic effects. Because apamin can cross the blood brain barrier and leads to convulsion (Habermann, 1984), it is not a candidate for anti-arrhythmic therapy in humans. A cardiac specific SK channel blocker is needed to test the antiarrhythmic efficacy and safety of SK channel blockers. An alternative approach is indirect modulation of the SK channels rather than targeting SK channels themselves. Ni et al (Ni et al., 2013) showed that treating HF rats with bisoprolol downregulated the expression of SK1 and SK3 channels. Bisoprolol also effectively downregulated IKAS density as well as the sensitivity of IKAS to Cai. Marshall et al (Marshall et al., 2012) showed that chronic β-blocker treatment reduces atrial Ito and IK1 without reducing the expression of associated ion channel subunits. It is possible that observed changes in SK channel expression seen with bisoprolol are part of a class response to β-blocker therapy. The off-target effects of bisoprolol on the SK channels may play a role in regulating ventricular repolarization in HF.
Prospects of SK channel research in ventricular arrhythmias
SK channel modulation is potentially useful in treating electrical storm or ventricular tachyarrhythmia induced by acute myocardial ischemia. Cardioselective SK channel activators or blockers are needed to test the efficacy and safety of the SK channel blockade. The subcellular mechanisms of SK channel regulation in ventricular cardiomyocytes in diseased hearts are still unclear and further investigation is required. SK channel research may also have significant implication in drug safety. Apamin is proarrhythmic in failing rabbit ventricles by prolonging the APD, which in turn promotes EAD, triggered activity and TdP ventricular arrhythmia. Drugs that inhibit SK channels may reduce the repolarization reserve in patients with HF or MI, resulting in reduced safety. Because drug safety is a major public health concern (Pollard et al., 2010), better understanding of the SK current blocking effects of commonly used drugs should be an important field of research.
Acknowledgments
Sources of Funding: This work is funded in part by Taiwan National Science Council Grant (NSC 102-2314-B-182A-038-MY2), Chang Gung Memorial Hospital Medical Research Programs (CMRPG3D0341), United States NIH Grants P01 HL78931, R01 HL71140, R41HL124741, a Medtronic-Zipes Endowment of the Indiana University and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Footnotes
Disclosures: Peng-Sheng Chen has equity interest in Arrhythmotech, LLC. Our laboratory receives equipment donations from Medtronic, St Jude and Cyberonics Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelman JP, Maylie J, Sah P. Small-Conductance Ca(2+)-Activated K(+) Channels: Form and Function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Current opinion in cardiology. 2010;25:29–36. doi: 10.1097/HCO.0b013e328333d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla IM, Long VP, 3rd, Vargas-Pinto P, et al. Calcium-activated potassium current modulates ventricular repolarization in chronic heart failure. PloS one. 2014;9:e108824. doi: 10.1371/journal.pone.0108824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- Chandler NJ, Greener ID, Tellez JO, et al. Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation. 2009;119:1562–1575. doi: 10.1161/CIRCULATIONAHA.108.804369. [DOI] [PubMed] [Google Scholar]
- Chang P-C, Turker I, Lopshire JC, et al. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. JAHA. 2013a;1:e004713. doi: 10.1161/JAHA.112.004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Hsieh YC, Hsueh CH, Weiss JN, Lin SF, Chen PS. Apamin Induces Early Afterdepolarizations and Torsades de Pointes Ventricular Arrhythmia From Failing Rabbit Ventricles Exhibiting Secondary Rises in Intracellular Calcium. Heart rhythm: the official journal of the Heart Rhythm Society. 2013b;10:1516–1524. doi: 10.1016/j.hrthm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SK, Chang PC, Maruyama M, et al. Small-Conductance Calcium-Activated Potassium Channel and Recurrent Ventricular Fibrillation in Failing Rabbit Ventricles. Circ Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diness JG, Sorensen US, Nissen JD, et al. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:380–390. doi: 10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nature genetics. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nature genetics. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger CM, Rauer H, Neben AL, et al. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J Biol Chem. 2001;276:12249–12256. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- Feher A, Broskova Z, Bagi Z. Age-related impairment of conducted dilation in human coronary arterioles. Am J Physiol Heart Circ Physiol. 2014;306:H1595–1601. doi: 10.1152/ajpheart.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L, Bao Z, Jia Y, et al. Ventricular tachyarrhythmias in rats with acute myocardial infarction involves activation of small-conductance Ca2+-activated K+ channels. Am J Physiol Heart Circ Physiol. 2013;304:H118–130. doi: 10.1152/ajpheart.00820.2011. [DOI] [PubMed] [Google Scholar]
- Habermann E. Apamin. Pharmacol Ther. 1984;25:255–270. doi: 10.1016/0163-7258(84)90046-9. [DOI] [PubMed] [Google Scholar]
- Hougaard C, Fraser MO, Chien C, et al. A positive modulator of K Ca 2 and K Ca 3 channels, 4,5-dichloro-1,3-diethyl-1,3-dihydro-benzoimidazol-2-one (NS4591), inhibits bladder afferent firing in vitro and bladder overactivity in vivo. J Pharmacol Exp Ther. 2009;328:28–39. doi: 10.1124/jpet.108.143123. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Chang PC, Hsueh CH, et al. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol. 2013;6:410–418. doi: 10.1161/CIRCEP.111.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh CH, Chang PC, Hsieh YC, Reher T, Chen PS, Lin SF. Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart rhythm: the official journal of the Heart Rhythm Society. 2013;10:891–898. doi: 10.1016/j.hrthm.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Kowey PR, Levine JH, Herre JM, et al. Randomized, double-blind comparison of intravenous amiodarone and bretylium in the treatment of patients with recurrent, hemodynamically destabilizing ventricular tachycardia or fibrillation. Circulation. 1995;92:3255–3263. doi: 10.1161/01.cir.92.11.3255. [DOI] [PubMed] [Google Scholar]
- Lee YS, Chang PC, Hsueh CH, et al. Apamin-Sensitive Calcium-Activated Potassium Currents in Rabbit Ventricles with Chronic Myocardial Infarction. J Cardiovasc Electrophysiol. 2013;24:1144–1153. doi: 10.1111/jce.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Timofeyev V, Tuteja D, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zhang Q, Timofeyev V, et al. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res. 2007;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- Mahida S, Mills RW, Tucker NR, et al. Overexpression of KCNN3 results in sudden cardiac death. Cardiovasc Res. 2014;101:326–334. doi: 10.1093/cvr/cvt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Coste B, Hao J, et al. Neurotransmitter modulation of small-conductance Ca2+-activated K+ channels by regulation of Ca2+ gating. Neuron. 2008;59:439–449. doi: 10.1016/j.neuron.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GE, Russell JA, Tellez JO, et al. Remodelling of human atrial K+ currents but not ion channel expression by chronic beta-blockade. Pflugers Archiv: European journal of physiology. 2012;463:537–548. doi: 10.1007/s00424-011-1061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, Szuts V, Horvath Z, et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J Mol Cell Cardiol. 2009;47:656–663. doi: 10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Ni Y, Wang T, Zhuo X, et al. Bisoprolol reversed small conductance calcium-activated potassium channel (SK) remodeling in a volume-overload rat model. Molecular and cellular biochemistry. 2013;384:95–103. doi: 10.1007/s11010-013-1785-5. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Morita N, Tang L, et al. Mechanisms of recurrent ventricular fibrillation in a rabbit model of pacing-induced heart failure. Heart Rhythm. 2009;6:784–792. doi: 10.1016/j.hrthm.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgen N, Dun W, Sosunov EA, et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res. 2007;75:758–769. doi: 10.1016/j.cardiores.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, D’Hoedt D, Doorty KB, et al. Tamapin, a venom peptide from the Indian red scorpion (Mesobuthus tamulus) that targets small conductance Ca2+-activated K+ channels and afterhyperpolarization currents in central neurons. J Biol Chem. 2002;277:46101–46109. doi: 10.1074/jbc.M206465200. [DOI] [PubMed] [Google Scholar]
- Pollard CE, Abi Gerges N, Bridgland-Taylor MH, Easter A, Hammond TG, Valentin JP. An introduction to QT interval prolongation and non-clinical approaches to assessing and reducing risk. British journal of pharmacology. 2010;159:12–21. doi: 10.1111/j.1476-5381.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S, Hatton WJ, Koh SD, Horowitz B. Molecular properties of small-conductance Ca2+-activated K+ channels expressed in murine colonic smooth muscle. American journal of physiology Gastrointestinal and liver physiology. 2001;281:G964–973. doi: 10.1152/ajpgi.2001.281.4.G964. [DOI] [PubMed] [Google Scholar]
- Shen MJ, Hao-Che Chang X, Park HW, et al. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart rhythm: the official journal of the Heart Rhythm Society. 2013;10:910–915. doi: 10.1016/j.hrthm.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe DF, Gadicherla AK, Zhou Y, et al. Protection against cardiac injury by small Ca(2+)-sensitive K(+) channels identified in guinea pig cardiac inner mitochondrial membrane. Biochimica et biophysica acta. 2013;1828:427–442. doi: 10.1016/j.bbamem.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Rochira JA, Terentyeva R, Roder K, Koren G, Li W. Sarcoplasmic reticulum Ca2+ release is both necessary and sufficient for SK channel activation in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2013 doi: 10.1152/ajpheart.00621.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- Turker I, Yu C-C, Chang P, et al. Amiodarone Inhibits Apamin-Sensitive Potassium Currents. PloS one. 2013;8:e70450. doi: 10.1371/journal.pone.0070450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja D, Xu D, Timofeyev V, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- Weatherall KL, Goodchild SJ, Jane DE, Marrion NV. Small conductance calcium-activated potassium channels: from structure to function. Prog Neurobiol. 2010;91:242–255. doi: 10.1016/j.pneurobio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tuteja D, Zhang Z, et al. Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- Yu CC, Ai T, Weiss JN, Chen PS. Apamin Does Not Inhibit Human Cardiac Na+ Current, L-type Ca2+ Current or Other Major K+ Currents. PloS one. 2014;9:e96691. doi: 10.1371/journal.pone.0096691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Timofeyev V, Lu L, et al. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res. 2008;102:465–471. doi: 10.1161/CIRCRESAHA.107.161778. [DOI] [PMC free article] [PubMed] [Google Scholar]