Abstract
A method involving hydration, tempering and heating steps is presented to process rough rice as alternative to traditional parboiling with pressure steam. The effects of temperature (66–84 °C), tempering time (60–420 min) and heating time (30–180 min) on gelatinization degree and milling yield were analyzed by response surface method (RSM). A maximum value of gelatinization degree (37.0 %) and milling yield of 67.7 % were reached with a process temperature of 84 °C using tempering and heating times of 178 and 104 min respectively. A slight reduction of crystallinity (14 %) and a significant improvement of nutritional value with increments of 150 and 60 % in riboflavin and calcium contents were obtained in comparison with control (untreated rice). Hardness and adhesiveness of processed rice were intermediate between those of control and completely gelatinized rice. The proposed method, with lower temperature requirements than traditional parboiling, is presented to obtain an alternative product, expanding consumer choices.
Keywords: Gelatinization, Crystallinity, Nutritional value, Texture, Thermal properties
Introduction
Different cooking methods have been used to process rough rice. Usually, these methods have two process steps: hydration or soaking; followed by cooking in hot water (Pillaiyar et al. 1996), steam (Iyengar et al. 1980; Fellers and Deissinger 1983; Mahanta and Bhattacharya 2010), or other heating methods (Khan et al. 1974; Wang et al. 2011). During soaking, rice grains reach either moisture saturation or a partial hydration, depending on process conditions. Total or partial gelatinization occurs in the cooking step. It can be said that gelatinization level, as well as physical and organoleptic characteristics of cooked rice depend on process variables such as soaking time, water temperature, steam pressure and heating time.
Arai et al. (1975) analyzed the effect of steam pressure and heating time on rice yield and textural attributes of whole grain. Ong and Blanshard (1995) observed the relation between hardness of rice kernels and cooking conditions. Lai (2001) found that gelatinization level in rice kernels varies with heating process as well as with rice variety. In order to investigate the effect of heat treatment severity on cooked rice characteristics, Kaddus Miah et al. (2002a) evaluated the gelatinization degree using differential scanning calorimetry. They concluded that gelatinization has a marked influence on the physical and chemical characteristics of cooked rice. Kaddus Miah et al. (2002b) also investigated the effect of soaking time on color attributes of milled rice. Manful et al. (2008) studied the effect of cooking conditions on thermal properties and crystallinity characteristics of parboiled rice.
Although high levels of saturation and high cooking temperatures are appropriate to reach starch gelatinization, there may appear unfavorable changes in grain structure such as rupture of rice hull and solids loss. In addition, the use of high temperatures to obtain a complete gelatinization of rice starch increases the cost of cooking equipment and downgrades the quality of cooked rice (Goyal et al. 2012; Fellers and Deissinger 1983; Pillaiyar 1984). Parboiling procedures by grain soaking in hot water at atmospheric pressure were also developed due to the fact that gelatinization can be achieved with lower temperatures than 100 °C. (Unnikrishnan et al. 1982; Pillaiyar et al. 1996). However, there is very limited information about rice parboiling by soaking in hot water. Consequently, the present work has the following aims: a) to analyze the influence of hydrothermal process variables: temperature, tempering time and heating time on milling yield and gelatinization degree of processed rice; b) to optimize the process conditions in order to maximize process responses; c) to characterize the optimum processed rice by means of milling yield, thermal properties, crystallinity, contents of nutrients (Ca, P and thiamine and riboflavin) and textural characteristics of cooked rice grain.
Materials and methods
Material
A local variety of long-grain rice with amylose content of 23.7 g /100 g (dry basis) provided by Molinos Río de La Plata S.A. (Argentina) was used in this study. The rough rice was stored in sealed containers at -20 °C. Before hydrothermal test was performed, stored rice was taken from the hermetic container and it was equilibrated at 20 °C for 60 min.
Amylose content was determined by Morrison and Laignelet method (1983). Moisture content of rice grains determined by AOAC 943.01 method (AOAC 2000) was 0.115 g /g (dry basis). Fat content (0.5 g/100 g) was determined by AOAC 920.39 method (AOAC 2000).
Rice flour was obtained by grinding in a Butt mill (Decalab Fbr ®, Buenos Aires, Argentina) for 30 s and flour fraction of 177–74 μm was selected to perform different analysis.
A commercial parboiled rice (Molinos Río de La Plata S.A, Buenos Aires, Argentine) was adopted as reference exclusively for texture analysis.
Hydrothermal processing
Hydrothermal process was performed in three steps: hydration, tempering and heating. An experimental equipment was constructed to realize the experimental tests. The main component of the system was the isolated vessel (4 L) where the hydrothermal process take place. The process water was pumped to the vessel at constant rate (11.5 L/min) with a centrifugal pump (IWAKI, model MD10, IWAKI Co. Ltd, Japan). Water temperature was controlled by means of a thermostatic bath with agitation (Julabo, Labortechnik GMBH, West Germany). A basket with rough rice (100 g) was introduced into the vessel and plunged into the water to complete the hydration step. Hydration step was performed following the method of Bello et al. (2007) to reach grain saturation avoiding hull splitting. Five levels of temperature: 66.3 °C (180 min), 70 °C (140 min), 75 °C (110 min), 80 °C (75 min) and 83.7 °C (50 min) were used. Saturated grain was transferred to an isolated container to perform tempering step at 45 °C. Tempered rice grain was then located above the water level into the vessel to carry out the heating step at the same temperature of hydration step (at constant atmospheric pressure). Processed rice was air dried at 35.0 ± 0.5 °C to a safe moisture content (12 g water/ 100 g dry solid) and it was stored at 25 °C for a week before rice milling.
Milling yields
Milling of processed rice (100 g of rough rice) was performed in a laboratory rice mill Suzuki (MT-95, Suzuki Co., Saõ Paulo, Brazil) to obtain milled rice (7–8 % degree of milling) and bran. Finally, whole milled rice fraction was separated from broken rice grains. Considering that brown rice is rough rice without hull, the mean values of degree of milling (DOM), milling yield (MY) and head rice yield (HRY) were calculated from triplicate using the following equations:
| 1 |
| 2 |
| 3 |
Thermal properties
Thermal analysis was performed in a Differential Scanning Calorimeter (DSC 822 Mettler Toledo, Switzerland). Approximately 4.0 mg of flour sample was weighed in an aluminum pan after which distilled water was added to obtain a flour-to-water ratio of 1:3 (g/g). The sealed pan was equilibrated for 24 h before analysis. Scans were run by triplicate at a heating rate of 10 °C/min from 30 °C to 120 °C using an empty pan as reference. Gelatinization temperatures, onset (To), peak (Tp) and endset (Te), together with gelatinization enthalpy (ΔHg) were recorded in triplicate. The gelatinization degree (GD) was calculated as percentage using:
| 4 |
where ∆Hg,sample, is the gelatinization enthalpy of hydrothermal processed rice and ∆Hg,control, is the gelatinization enthalpy of untreated rice.
Experimental design
Table 1 shows the experimental design of Box and Hunter (1957) which included 14 experimental points with five levels of each variable in terms of real and codified factors. The following linear expressions were used to relate the experimental variables: process temperature (T), tempering time (tt) and heating time (t) with coded temperature (x1), coded tempering time (x2) and coded heating time (x3), respectively:
| 5 |
| 6 |
| 7 |
Table 1.
Experimental design, milling yields and thermal properties
| N° | T 1 (°C) (x1) | tt2 (min) (x2) | t3 (min) (x3) | MY5 (%) | HRY6 (%) | GD7 (%) | TP 8 (°C) |
|---|---|---|---|---|---|---|---|
| 1 | 80 (1) | 344 (1) | 62 (−1) | 69.0 ± 1.2bc | 88.3 ± 1.6bc | 14.4 ± 1.0ef | 69.3 ± 0.2de |
| 2 | 70 (−1) | 344 (1) | 62 (−1) | 70.6 ± 1.2bc | 90.2 ± 1.7bc | 0.0 ± 1.7a | 65.7 ± 0.4a |
| 3 | 70 (−1) | 344 (1) | 148 (1) | 69.0 ± 1.2bc | 88.1 ± 1.6bc | 9.1 ± 0.2cd | 68.7 ± 0.3cd |
| 4 | 80 (1) | 344 (1) | 148 (1) | 69.4 ± 1.2bc | 88.9 ± 1.7bc | 19.4 ± 0.2g | 71.8 ± 0.1h |
| 5 | 80 (1) | 136 (−1) | 62 (−1) | 70.6 ± 1,2bc | 90.6 ± 1.7c | 25.4 ± 0.9h | 71.2 ± 0.2h |
| 6 | 70 (−1) | 136 (−1) | 62 (−1) | 70.4 ± 1.2bc | 90.0 ± 1.7bc | 10.2 ± 0.1d | 67.4 ± 0.4b |
| 7 | 80 (1) | 136 (−1) | 148 (1) | 70.7 ± 1.2c | 90.7 ± 1.7c | 25.8 ± 0.4h | 71.1 ± 0.3gh |
| 8 | 70 (−1) | 136 (−1) | 148 (1) | 68.6 ± 1.2bc | 87.4 ± 1.6bc | 4.4 ± 0.9b | 68.7 ± 0.3cd |
| 9 | 75 (0) | 240 (0) | 30 (−Ѵ3) | 70.8 ± 1.1c | 90.6 ± 1.7c | 11.7 ± 1.6de | 68.0 ± 0.7bc |
| 10 | 75 (0) | 60 (−Ѵ3) | 105 (0) | 70.7 ± 1.0c | 90.7 ± 1.7c | 16.3 ± 0.5fg | 70.0 ± 0.1ef |
| 11 | 75 (0) | 420 (Ѵ3) | 105 (0) | 68.6 ± 1.2bc | 87.6 ± 1.6bc | 6.7 ± 0.2bc | 69.6 ± 0.1e |
| 12 | 75 (0) | 240 (0) | 180 (Ѵ3) | 69.4 ± 1.2bc | 89.0 ± 1.7bc | 13.8 ± 1.0ef | 70.4 ± 0.7fg |
| 13 | 83.7 (Ѵ3) | 240 (0) | 105 (0) | 69.2 ± 1.2bc | 88.7 ± 1.7bc | 38.2 ± 0.9i | 73.2 ± 0.3i |
| 14 | 66.3 (−Ѵ3) | 240 (0) | 105 (0) | 68.0 ± 1.0b | 86.9 ± 1.6b | 12.2 ± 0.5de | 67.3 ± 0.3b |
| C4 | 64.5 ± 1.9a | 82.1 ± 1.5a | –a | 65.5 ± 0.3a |
1 Temperature ; 2 tempering time, 3 heating time, 4 Control, 5 milling yield, 6 head rice yield, 7 gelatinization degree, 8 peak temperature of gelatinization. Means and standard deviation followed by different superscript letters in a column are significantly different at p < 0.05
Experimental range of tempering and heating times were set based on previous tests (Bello et al. 2007). The temperature range was extended from gelatinization temperature (65.2 °C ~66 °C) to 20 °C over this value.
Characterization of final product
Final rice product was obtained by setting optimal conditions of hydrothermal process. Untreated rice (control) and completely gelatinized rice (commercial parboiled rice) were adopted as references samples. Milling yield (HRY) and thermal properties were determined by triplicate according to previously described procedures.
The X-ray diffraction patterns of rice flour samples (177–74 μm) were measured in duplicate using a Philips PW1730/10 diffractometer (Netherlands) under the following conditions: Copper target tube, settled at 40 kV and 37 mA, scanning regions of the diffraction angle 2θ = 6–32°, and scanning velocity 4.0 °/min. Crystallinity degree (expressed as percentage) was calculated as a ratio between crystalline and total (crystalline and amorphous) areas which were obtained from XRD spectra (Manful et al. 2008).
Phosphorous content was determined by spectrophotometric analysis following the procedure of Fiske-Subarrow (1925).
Calcium content was obtained by calcination of rice flour at 500 °C followed by atomic spectrometry analysis of ash according to ASTM 19.1 method (ASTM 2000). A Perkin Elmer spectrometer model AAnalyst 100 with a graphite furnace was used.
Thiamine and riboflavin contents were determined by duplicate using HPLC chromatography (ThermoQuest Corporation, San Jose, CA, USA) with a fluorescence detector (thiamine: λexc: 370 nm; λem: 435 nm; riboflavin: λexc: 370 nm; λem: 520 nm) following the procedure of Sims and Shoemaker (1993). The mobile phase was ammonium acetate 0.005 M: Methanol (72:8), with a flow of 1.5 ml/min using a column C18 Kromasil (150 mm × 4.6 mm × 5 μm).
A texture analyzer, TA-XT2 (Texture Technologies Corp., Scardale, NY) was used to obtain the texture profile of cooked rice samples (Bello et al. 2006). Milled rice (200 g, 13 % dry basis) was cooked with 600 ML of boiling water. Cooking times were 14, 15.5 and 20 min for control sample, final product and parboiled rice respectively. Textural parameters, determined by means of Texture Export for Window (Stable Micro Systems, Godalming, UK), were hardness (H), adhesiveness (A), springiness (S), cohesiveness (C), gumminess (G), chewiness (Chew) and resilience (R).
Statistical analysis
Results are expressed as mean values and standard deviation of their mean. Data were analyzed using one-way analysis of variance (ANOVA). The significance of differences between experimental values (duplicates) was determined by Tukey’s test. Statistical analysis was performed by using the Statgraphics® Package (Statistical Graphics Corporation, Virginia, Washington, USA). A response surface methodology (RSM) was applied to analyze the effect of hydrothermal process variables on rice properties. The studied responses (YK, K = 1,…, p) were matched to the coded factors (xi, i = 1,…, n) by the following polynomial model associated to experimental design (Khuri and Cornell 1987):
| 8 |
The coefficients a0, ai and aii represent the constant, linear and quadratic effects, respectively, and aij represents the interaction effects of coded factors xi and xj. A linear codification of factors was used based on coded levels of experimental design (Eqns. 5, 6 and 7). The Statgraphics® software Packaged (Statistical Graphics Corporation, Virginia, Washington, USA) was used to perform the statistical analysis.
Results and discussion
Milling yields
Samples of processed rice were obtained following the previously described hydrothermal procedure, setting the operative variables according to experimental design (Table 1). Processed rice was dried and milling up to 8 % of DOM (Eq. 1). Equations 2 and 3 were used to calculate milling yield (MY) and head rice yield (HRY), respectively. Values of milling yields of control (unprocessed rice) and processed rice samples are also shown in Table 1. It can be appreciated the ability of hydrothermal process to increase rice yields in comparison with control sample.
Others parboiling methods are also useful to enhance milling yields values. Marshall et al. (1993) found an increase in HRY by microwave rice cooking. The authors have attributed such effect to greater degree of gelatinization reached during grain cooking. More recently, Patindol et al. (2008) have also observed that pressure parboiling of rice significantly improves the performance of milling yields.
Thermal parameters
Values of peak temperature and gelatinization degree (Eq. 4) are presented in Table 1 as function of hydrothermal conditions. It can be observed that gelatinization degree varies from low values (<10 %) where the gelatinization was incipient to values close to 40 %. A moderate level of gelatinization was obtained by means of the proposed treatment in comparison with traditional rice parboiling where 100 % of gelatinization degree is reached.
It must be noted that literature information about partially gelatinized rice is very scarce. Manful et al. (2008), who investigated rice parboiling, reported partial gelatinization with GD of 68 %. Patindol et al (2008) produced partially gelatinized rice with gelatinization percentages in the range of 13 to 42 %. These authors, in contrast to the present work, have controlled the gelatinization degree by setting process time in conventional rice parboiling.
A linear relationship between peak temperature and gelatinization degree was found (Tp (°C) = 0.17 + 66.87 GD (%), r2 = 0.73).A displacement of peak temperature to higher values as gelatinization degree increases was observed. The gelatinization endotherms presented a narrowing of the endothermic peak as gelatinization increases. This behavior known as “annealing” (Lund 1987) is produced by starch heating in water excess at sub-gelatinization temperatures or by insufficient process time at gelatinization temperatures. This phenomenon is associated to partial melting of starch crystallites and the rearranging of amorphous regions of starch structure which lead to higher temperature of melting (Zobel 1992).
Others authors also observed the effect of process conditions on thermal properties. Kaddus Miah et al. (2002b) analyzed the effect of hydration time on gelatinization degree in traditional parboiling. Lai (2001) determined the effect of hydrothermal treatments on different physicochemical properties of rice flour, and Manful et al. (2008) also associated the increase of gelatinization temperature with increasing severity of parboiling.
RSM analysis
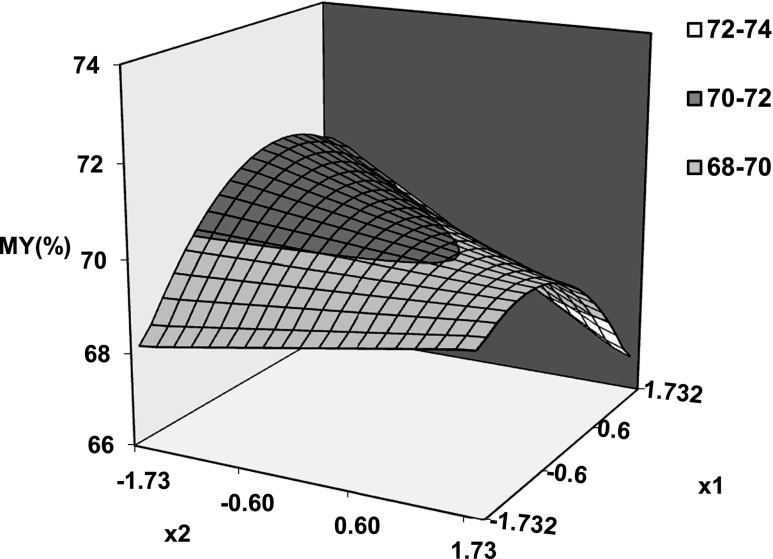
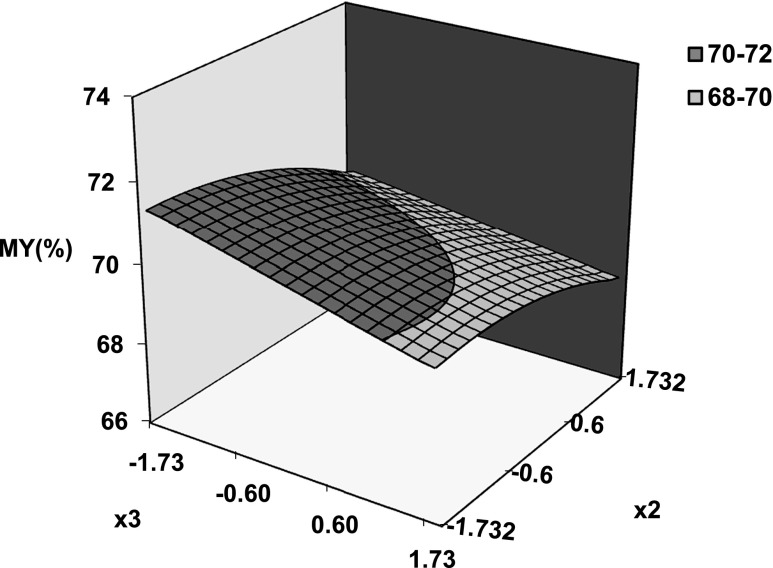
Equation 8 was used to simulate the effect of hydrothermal conditions in terms of codified variables (x1, x2, x3) on milling yields and gelatinization degree. The coefficients of Eq. 8 are shown in Table 2 together with statistical estimators of goodness of fit and significance levels of each coefficient at 95 % confidence interval. It is clear from the Table that Eq. 8 provides an efficient simulation of experimental data. All investigated factors had a significant influence on studied responses being significant the interaction effects temperature-heating time and temperature- tempering time on milling yields. Figure 1 shows predicted surface of milling yield as function of codified temperature and tempering time. Linear and quadratic effect of tempering time and process temperature respectively can be clearly appreciated. A similar behavior is found when HRY is plotted as a function of temperature and heating time (not shown here). Figure 2 evidences the slight effect of heating time and tempering time on milling yields at selected temperature (75 °C). However, these effects become more marked as process temperature increases (Fig. 1).
Table 2.
Effects of process conditions on milling yields and gelatinization degree. Coefficients of Eq. 8 and statistical parameters
| Coefficient | MY1 | HRY2 | GD3 |
|---|---|---|---|
| A0 | 70.29 | 90.00 | 23.55 |
| A1 | 0.23 | 0.42** | 7.60*** |
| A2 | −0.42** | −0.61*** | −2.82** |
| A3 | −0.38** | −0.48** | 0.88 |
| A11 | −0.50** | −0.67** | – |
| A22 | −0.15 | −0.22 | −4.57*** |
| A33 | – | – | −4.15*** |
| A12 | −0.43** | −0.63** | −1.49 |
| A13 | 0.48** | 0.68** | 0.26 |
| A23 | 0.06 | 0.13 | 2.44 |
| R2 | 92.5 | 94.1 | 96.6 |
| MAE | 0.21 | 0.25 | 1.49 |
| Durbin-watson | 1.24 (P = 0.13) | 1.43 (P = 0.23) | 1.33 (P = 0.18) |
**: significant coefficient to 95 % confidence interval; ***: significant coefficient to 99 % confidence interval; R2: correlation coefficient; MAE: mean absolute error. 1 milling yield, 2 head rice yield, 3 gelatinization degree
Fig. 1.
Predicted surface plot of milling yield as function of coded temperature (x 1) and tempering time (x 2) at constant heating time of 105 min
Fig. 2.
Predicted surface plot of milling yield as function of coded tempering time (x 2) and heating time (x 3) at constant temperature of 75 °C
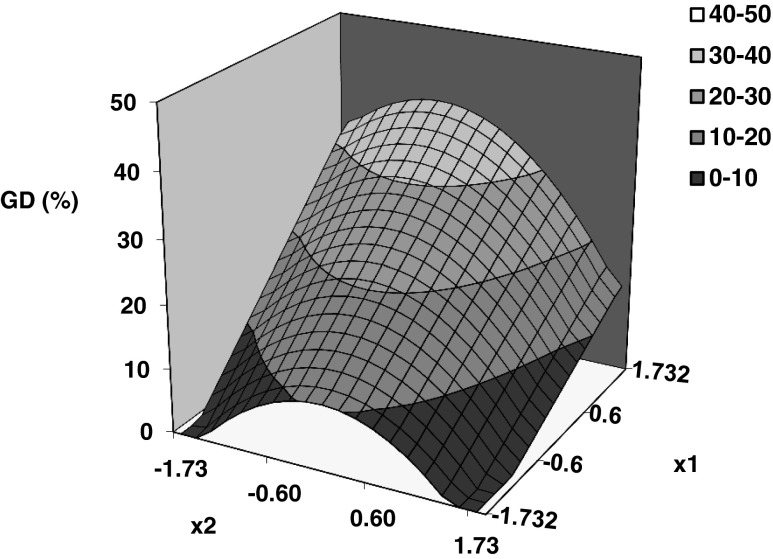
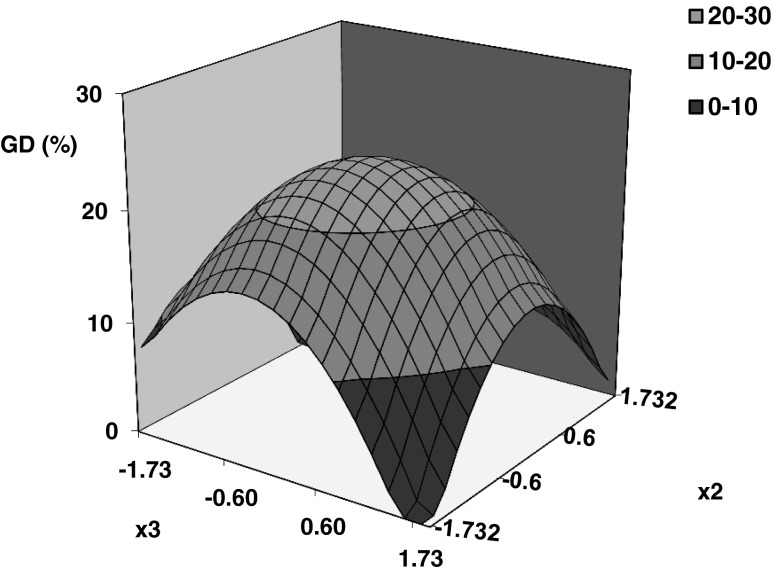
Coefficients of Eq. 8 for gelatinization degree (Table 2) evidenced that this response was significantly affected by hydrothermal conditions. Linear effect of process temperature and quadratic effect of tempering and heating times have resulted highly significant (99 % confidence interval). Non significant effects of interactions among studied factors were determined. In Figs. 3 and 4 can be appreciated the linear effect (positive) of temperature (x1) as well as the quadratic effect of tempering time (x2) and heating time (x3) on gelatinization degree. Process temperature exerts the main effect, an increase in gelatinization degree from zero to 38 % can be observed by increasing temperature from 66 to 84 °C.
Fig. 3.
Predicted surface plot of gelatinization degree as function of coded temperature (x 1) and tempering time (x 2) at constant heating time of 105 min
Fig. 4.
Predicted surface plot of gelatinization degree as function of coded tempering time (x 2) and heating time (x 3) at constant temperature of 75 °C
The optimization goal was to maximize simultaneously gelatinization degree and milling yields. Predicted optimum values were 38.3 % (GD), 68 % (MY) and 86.5 % (HRY) which were calculated (Eq. 8) with 83.7 °C of process temperature, 178.1 min of tempering time and 104.4 min of heating time. An experimental test was performed by setting optimum processing conditions in order to check the predicted responses. The following experimental values were obtained: 37.0 ± 0.5 % (GD), 67.7 ± 2.0 % (MY) and 86.7 ± 2.6 % (HRY), in accordance with predicted values from optimization analysis.
Product characterization
Quality attributes of optimum processed rice are shown in Table 3 together with the corresponding values of control sample (unprocessed rice grain). MY and HRY values of processed rice increased 3.8 and 4.3 % respectively in comparison with those of control sample as a consequence of hydrothermal process. This result is relevant from economics point of view and considering that similar values of MY and HRY were reported for traditional parboiling with a major energy requirement (Fellers and Deissinger 1983; Patindol et al. 2008).
Table 3.
Properties of optimum processed rice (OPR) in comparison with untreated rice (control)
| Measured parameter | Control | OPR |
|---|---|---|
| HRY1 (%) | 82.1 ± 1.5a | 86.7 ± 2.6b |
| MY2 (%) | 64.5 ± 1.9a | 67.7 ± 2.0a |
| TP 3 (°C) | 65.5 ± 0.3a | 74.3 ± 0.4b |
| ΔH4 (J/g b.s.) | 8.17 ± 0.06a | 5.15 ± 0.04b |
| GD5 (%) | – | 37.0 ± 0.5 |
| CD6 (%) | 36a | 31b |
| Calcium (mg/kg) | 25.3 ± 0.6a | 40.7 ± 0.6b |
| Phosphorus (mg/kg) | 470 ± 14a | 560 ± 14b |
| Thiamine (mg/kg) | 2.1 ± 0.1a | 2.5 ± 0.1b |
| Riboflavin (μg/kg) | 20 ± 2a | 50 ± 2b |
Means and standard deviation followed by different superscript letters in a file are significantly different at p < 0.05. 1 head rice yield, 2 milling yield, 3 peak temperature of gelatinization, 4 gelatinization enthalpy, 5 gelatinization degree, 6crystallinity degree
Regarding thermal properties of processed rice, it can be observed a shift of peak temperature and gelatinization degree to higher values in comparison with those of control sample. The proposed hydrothermal treatment led to partially gelatinized rice (GD = 37.0 %) in contrast with traditional parboiling (GD = 100 %).
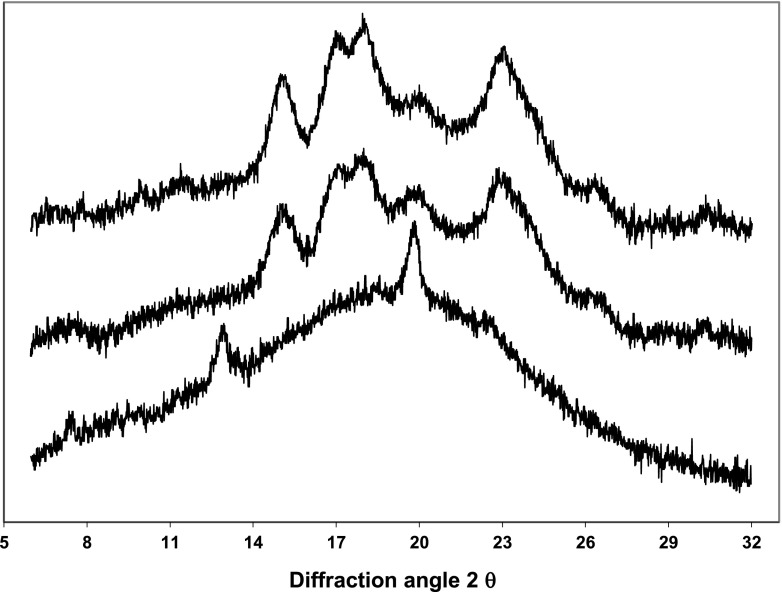
Figure 5 shows XRD spectral data of processed rice, control sample (GD = 0 %) and completely gelatinized rice (GD = 100 %). A-type XRD characteristic patterns of unprocessed rice (control) and processed rice can be observed (Ong and Blanshard 1995; Manful et al. 2008). However, processed rice also exhibits an incipient peak at 20° and a decrease of peak areas respect to control due to starch gelatinization. In contrast, fully gelatinized rice (parboiled rice) presents a V-type XRD pattern of with diffraction peaks at 20° and 13° which was associated to amylose-lipid complex formation during rice parboiling (Ong and Blanshard 1995). Crystallinity degree of optimum processed rice (31 %) was between values of control (36 %) and completely gelatinized rice (5 %). Maximum crystallinity loss (95 %) is achieved during traditional parboiling (Ong and Blanshard 1995) while lower reduction of crystallinity degree (~20 %) was found for partially gelatinized rice (GD = 68 %) as in the case reported by Manful et al. (2008). Crystalline loss and endothermic peak reduction (DSC analysis) evidence the modification of rice grain during starch gelatinization.
Fig. 5.
X-ray diffraction patterns. a) untreated rice (control); b) optimum processed rice (OPR); c) completely gelatinized rice or parboiled rice (PR)
In the same way of traditional parboiling, hydrothermal processing also enhanced the nutritional value of processed rice respect to the control (Subba Rao and Bhattacharya 1966). Table 3 shows significant increase in calcium content (60 %), riboflavin content (150 %), phosphorous and thiamine contents (~20 %).
Regarding textural properties of cooked rice (see Table 4) a significant reduction of rice grain adhesiveness (21 %) was achieved by applying the hydrothermal process in comparison with control sample. In addition, hardness, chewiness and gumminess of processed rice were higher than control values. Textural parameters of processed rice such as springiness (S), cohesiveness (C) and resilience (R) were similar to those of control. Considering the textural attributes of parboiled rice included in the Table 4, it can be concluded that processed rice shows intermediate characteristics between control and completely gelatinized rice grain.
Table 4.
Textural attributes of optimum processed rice (OPR) in comparison with untreated rice (control) and parboiled rice (PR)
| Textural parameter | Control | OPR | PRa |
|---|---|---|---|
| H1 (g) | 1,056 ± 86a | 1,355 ± 148b | 1,743 ± 283b |
| A2 (g.s) | −3.7 ± 1.4a | −2.9 ± 1.3ab | −1.2 ± 0.4b |
| S3 | 0.55 ± 0.04a | 0.66 ± 0.08a | 0.80 ± 0.13b |
| C4 | 0.55 ± 0.03a | 0.57 ± 0.03a | 0.66 ± 0.02b |
| G5 | 584 ± 72a | 771 ± 97b | 1,138 ± 165c |
| Chew6 | 321 ± 33a | 509 ± 92b | 925 ± 226c |
| R7 | 0.340 ± 0.024a | 0.348 ± 0.021a | 0.46 ± 0.02b |
a completely gelatinized rice. Means and standard deviation followed by different superscript letters in a file are significantly different at p < 0.05. 1 hardness, 2 adhesiveness, 3 springiness, 4 cohesiveness, 5 gumminess, 6 chewiness and 7 resilience
Conclusions
The potential of non conventional treatment for rough rice, using lower temperature requirements than traditional parboiling, was analyzed in order to obtain a partially gelatinized grain. The proposed method included a tempering between hydration and heating steps in contrast with conventional parboiling. Experimental tests, based on three factors design (temperature, tempering time and heating time) produced enhanced values of milling yields, in comparison with control, and limited gelatinization degree (up to 39 %). Structural changes of rice starch were evidenced by gelatinization and annealing phenomena from DSC analysis of hydrothermal processed rice. Effects of process conditions on milling yields and gelatinization degree were satisfactorily modeled using a second order polynomial equation by means of RSM method. Tempering induces moisture equilibration within rice kernel reducing grain fragility. As a consequence very satisfactory milling yields were obtained although a complete gelatinization was not reached. Hydro-processed rice shows significant increments of several nutrients, a reduced crystallinity level and textural properties between control and traditional parboiled rice. A particular rice product, with differential characteristics in terms of physical, nutritional and textural attributes, is presented as a new option to consumers.
Acknowledgments
The authors acknowledge the financial support from UBACYT (Project UBACyT 20020110200357). MAL is fellowship of CONICET.
References
- AOAC . Official methods of analysis. 17. Washington: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Arai K, Rao SN, Desikachar HS. Studies of the effect of parboiling on Japonica and Indica rice. Jpn J Trop Agric. 1975;19:7–14. [Google Scholar]
- ASTM . Standard test methods for calcium and magnesium in water. West Conshohocken: American society for testing and materials; 2000. [Google Scholar]
- Bello M, Baeza R, Tolaba M. Quality characteristics of milled and cooked rice affected by hydrotermal treatment. J Food Eng. 2006;72:124–133. doi: 10.1016/j.jfoodeng.2004.11.026. [DOI] [Google Scholar]
- Bello M, Tolaba M, Suarez C. Water absorption and starch gelatinization in whole rice grain during soaking. LWT. 2007;40:313–318. doi: 10.1016/j.lwt.2005.09.017. [DOI] [Google Scholar]
- Box G, Hunter J. Multi-factor experimental designs for exploring response surfaces. Ann Math Stat. 1957;28:195–241. doi: 10.1214/aoms/1177707047. [DOI] [Google Scholar]
- Fellers DA, Deissinger AE. Preliminary study on the effect of steam treatment of paddy on milling properties and rice stickiness. J Cereal Sci. 1983;1:147–157. doi: 10.1016/S0733-5210(83)80032-0. [DOI] [Google Scholar]
- Fiske CH, Subarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Goyal SK, Jogdand SV, Agrawal AK. Energy use pattern in ricemilling industries-a critical appraisal. J Food Sci Technol. 2012 doi: 10.1007/s13197-012-0747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar NG, Rajendran G, Yusuff KM, Ganesan G. Pressure parboiling of paddy without the use of boiler. J Food Sci Technol. 1980;17:139–140. [Google Scholar]
- Kaddus Miah MA, Haque A, Douglass MP, Clarke B. a) Parboiling of rice. Part I: Effect of hot soaking time on quality of milled rice. Int J Food Sci Technol. 2002;37:527–537. doi: 10.1046/j.1365-2621.2002.00610.x. [DOI] [Google Scholar]
- Kaddus Miah MA, Haque A, Douglass MP, Clarke B. b) Parboiling of rice. Part II: Effect of hot soaking time on the degree of starch gelatinization. Int J Food Sci Technol. 2002;37:539–545. doi: 10.1046/j.1365-2621.2002.00611.x. [DOI] [Google Scholar]
- Khan AV, Amilhussin A, Arboleda JR, Manalo AS, Chancellor WJ. Accelerated drying of rice using heat-conduction media. Trans ASAE. 1974;17:949–955. doi: 10.13031/2013.37005. [DOI] [Google Scholar]
- Khuri AI, Cornell JA (1987) Response surfaces. Designs and analyses. Marcel Dekker Inc, American Society for Quality Control, Quality Press
- Lai HM. Effects of hydrothermal treatment on the physicochemical properties of pregelatinized rice flour. Food Chem. 2001;72:455–463. doi: 10.1016/S0308-8146(00)00261-2. [DOI] [Google Scholar]
- Lund D. Influence of time, temperature, moisture, ingredients and processing conditions on Starch gelatinization. Crit Rev Food Sci Nutr. 1987;20:249–252. doi: 10.1080/10408398409527391. [DOI] [PubMed] [Google Scholar]
- Mahanta CL, Bhattacharya KR. Relationship of starch changes to puffing expansion of parboiled rice. J Food Sci Technol. 2010;47(2):182–187. doi: 10.1007/s13197-010-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manful JT, Grimm CC, Gayin J, Coker RD. Effect of variable parboiling on cristallinity of rice samples. Cereal Chem. 2008;85(1):92–95. doi: 10.1094/CCHEM-85-1-0092. [DOI] [Google Scholar]
- Marshall WE, Wadsworth JI, Verma LR, Velupillai L. Determining the degree of gelatinization in parboiled rice: comparison of a subjective and an objective method. Cereal Chem. 1993;70(2):226–230. [Google Scholar]
- Morrison WR, Laignelet B. An improved colorimetric procedure for determining apparent and total amylose in cereal and other starches. J Cereal Sci. 1983;1:19–35. doi: 10.1016/S0733-5210(83)80004-6. [DOI] [Google Scholar]
- Ong MH, Blanshard MV. The significance of starch polymorphism in commercially produced parboiled rice. Starch. 1995;47:7–13. doi: 10.1002/star.19950470104. [DOI] [Google Scholar]
- Patindol J, Newton J, Wang YJ. Functional properties as affected by laboratory-scale parboiling of rough rice and brown rice. J Food Sci. 2008;73(8):370–377. doi: 10.1111/j.1750-3841.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- Pillaiyar P. Applicability of the rapid gel test for indicating the texture of commercial parboiled rices. Cereal Chem. 1984;61:255–256. [Google Scholar]
- Pillaiyar P, Sabarathinam PL, Subramaniyan V, Sulochana S. Parboiling of paddy using thermic fluid. J Food Eng. 1996;27:267–278. doi: 10.1016/0260-8774(95)00009-7. [DOI] [Google Scholar]
- Sims A, Shoemaker D. Simultaneous liquid chromatographic determination of thiamine and riboflavin in selected foods. J AOAC. 1993;76(5):1156–1160. [PubMed] [Google Scholar]
- Subba Rao PV, Bhattacharya KR. Effect of parboiling on thimine content of rice. J Agric Food Chem. 1966;14(5):479–482. doi: 10.1021/jf60147a010. [DOI] [Google Scholar]
- Unnikrishnan KR, Viraktamath CS, Krishnamurthy H, Bhattacharya KR. Parboiling of paddy by simple soaking in hot water. J Food Technol. 1982;17:499–506. doi: 10.1111/j.1365-2621.1982.tb00205.x. [DOI] [Google Scholar]
- Wang JP, An HZ, Jin ZY, Xie ZJ, Zhuang HN. Emulsifiers and thickeners on extrusion-cooked instant rice product. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel HF (1992) Starch granules structure. In: Alexander RJ, Zobel H (ed) Developments in carbohydrate chemistry, AACC, St Paul MN, pp 1–36