Abstract
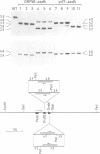
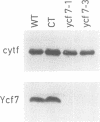
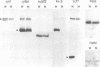
The small chloroplast open reading frame ORF43 (ycf7) of the green unicellular alga Chlamydomonas reinhardtii is cotranscribed with the psaC gene and ORF58. While ORF58 has been found only in the chloroplast genome of C.reinhardtii, ycf7 has been conserved in land plants and its sequence suggests that its product is a hydrophobic protein with a single transmembrane alpha helix. We have disrupted ORF58 and ycf7 with the aadA expression cassette by particle-gun mediated chloroplast transformation. While the ORF58::aadA transformants are indistinguishable from wild type, photoautotrophic growth of the ycf7::aadA transformants is considerably impaired. In these mutant cells, the amount of cytochrome b6f complex is reduced to 25-50% of wild-type level in mid-exponential phase, and the rate of transmembrane electron transfer per b6f complex measured in vivo under saturating light is three to four times slower than in wild type. Under subsaturating light conditions, the rate of the electron transfer reactions within the b6f complex is reduced more strongly in the mutant than in the wild type by the proton electrochemical gradient. The ycf7 product (Ycf7) is absent in mutants deficient in cytochrome b6f complex and present in highly purified b6f complex from the wild-type strain. Ycf7-less complexes appear more fragile than wild-type complexes and selectively lose the Rieske iron-sulfur protein during purification. These observations indicate that Ycf7 is an authentic subunit of the cytochrome b6f complex, which is required for its stability, accumulation and optimal efficiency. We therefore propose to rename the ycf7 gene petL.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthold D. A., Schmidt C. L., Malkin R. The deletion of petG in Chlamydomonas reinhardtii disrupts the cytochrome bf complex. J Biol Chem. 1995 Dec 8;270(49):29293–29298. doi: 10.1074/jbc.270.49.29293. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Factors regulating the slow electrogenic phase in green algae and higher plants. Biochim Biophys Acta. 1981 Apr 13;635(2):327–340. doi: 10.1016/0005-2728(81)90031-1. [DOI] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Breyton C., de Vitry C., Popot J. L. Membrane association of cytochrome b6f subunits. The Rieske iron-sulfur protein from Chlamydomonas reinhardtii is an extrinsic protein. J Biol Chem. 1994 Mar 11;269(10):7597–7602. [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Li L. B., Yu L., Yu C. A. Identification of a Mr = 17,000 protein as the plastoquinone-binding protein in the cytochrome b6-f complex from spinach chloroplasts. J Biol Chem. 1989 Jan 25;264(3):1387–1392. [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Gavel Y., Steppuhn J., Herrmann R., von Heijne G. The 'positive-inside rule' applies to thylakoid membrane proteins. FEBS Lett. 1991 Apr 22;282(1):41–46. doi: 10.1016/0014-5793(91)80440-e. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker of site-directed transformation of chlamydomonas. Nucleic Acids Res. 1991 Aug 11;19(15):4083–4089. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J., Bogorad L. A 4-kDa maize chloroplast polypeptide associated with the cytochrome b6-f complex: subunit 5, encoded by the chloroplast petE gene. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1534–1538. doi: 10.1073/pnas.86.5.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauska G., Hurt E., Gabellini N., Lockau W. Comparative aspects of quinol-cytochrome c/plastocyanin oxidoreductases. Biochim Biophys Acta. 1983 Jul 15;726(2):97–133. doi: 10.1016/0304-4173(83)90002-2. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hope A. B. The chloroplast cytochrome bf complex: a critical focus on function. Biochim Biophys Acta. 1993 Jun 10;1143(1):1–22. doi: 10.1016/0005-2728(93)90210-7. [DOI] [PubMed] [Google Scholar]
- Joliot P., Delosme R. Flash-induced 519 nm absorption change in green algae. Biochim Biophys Acta. 1974 Aug 23;357(2):267–284. doi: 10.1016/0005-2728(74)90066-8. [DOI] [PubMed] [Google Scholar]
- Joliot P., Joliot A. Mechanism of electron transfer in the cytochrome b/f complex of algae: evidence for a semiquinone cycle. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1034–1038. doi: 10.1073/pnas.91.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W., Witt H. T. On the ion transport system of photosynthesis--investigations on a molecular level. Z Naturforsch B. 1968 Feb;23(2):244–254. doi: 10.1515/znb-1968-0222. [DOI] [PubMed] [Google Scholar]
- Kanno A., Hirai A. A transcription map of the chloroplast genome from rice (Oryza sativa). Curr Genet. 1993 Feb;23(2):166–174. doi: 10.1007/BF00352017. [DOI] [PubMed] [Google Scholar]
- Kuras R., Wollman F. A. The assembly of cytochrome b6/f complexes: an approach using genetic transformation of the green alga Chlamydomonas reinhardtii. EMBO J. 1994 Mar 1;13(5):1019–1027. doi: 10.1002/j.1460-2075.1994.tb06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Piccioni R. G., Bennoun P., Chua N. H. A nuclear mutant of Chlamydomonas reinhardtii defective in photosynthetic photophosphorylation. Characterization of the algal coupling factor ATPase. Eur J Biochem. 1981 Jun;117(1):93–102. doi: 10.1111/j.1432-1033.1981.tb06307.x. [DOI] [PubMed] [Google Scholar]
- Pierre Y., Breyton C., Kramer D., Popot J. L. Purification and characterization of the cytochrome b6 f complex from Chlamydomonas reinhardtii. J Biol Chem. 1995 Dec 8;270(49):29342–29349. doi: 10.1074/jbc.270.49.29342. [DOI] [PubMed] [Google Scholar]
- Pierre Y., Popot J. L. Identification of two 4-kDa miniproteins in the cytochrome b6f complex from Chlamydomonas reinhardtii. C R Acad Sci III. 1993 Dec;316(12):1404–1409. [PubMed] [Google Scholar]
- Popot J. L., de Vitry C. On the microassembly of integral membrane proteins. Annu Rev Biophys Biophys Chem. 1990;19:369–403. doi: 10.1146/annurev.bb.19.060190.002101. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Goldschmidt-Clermont M., Soen S. Y., Franzén L. G., Rochaix J. D. Directed chloroplast transformation in Chlamydomonas reinhardtii: insertional inactivation of the psaC gene encoding the iron sulfur protein destabilizes photosystem I. EMBO J. 1991 Aug;10(8):2033–2040. doi: 10.1002/j.1460-2075.1991.tb07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Matsumoto H., Goldschmidt-Clermont M., Rochaix J. D. Directed disruption of the Chlamydomonas chloroplast psbK gene destabilizes the photosystem II reaction center complex. Plant Mol Biol. 1994 Mar;24(5):779–788. doi: 10.1007/BF00029859. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Beerman N., Griffith O. M. A small-scale five-hour procedure for isolating multiple samples of CsCl-purified DNA: application to isolations from mammalian, insect, higher plant, algal, yeast, and bacterial sources. Anal Biochem. 1986 Feb 1;152(2):376–385. doi: 10.1016/0003-2697(86)90423-9. [DOI] [PubMed] [Google Scholar]
- de Vitry C., Breyton C., Pierre Y., Popot J. L. The 4-kDa nuclear-encoded PetM polypeptide of the chloroplast cytochrome b6f complex. Nucleic acid and protein sequences, targeting signals, transmembrane topology. J Biol Chem. 1996 May 3;271(18):10667–10671. doi: 10.1074/jbc.271.18.10667. [DOI] [PubMed] [Google Scholar]