Abstract
The effect of UV-C treatments (0.32, 0.97, 2.56, 4.16 and 4.83 kJ.m−2 at 254 nm) on the physical-chemical properties [colour, texture, total phenolic content (TPC), weight loss (WL)], and mesophylic counts of whole tomato, was evaluated during 15 days at 10 °C. During storage, the Ctr samples acquired faster red colour than all UV-C samples (higher a* and lower °h values). Comparing texture of Ctr and UV-C samples at 15th storage day, an increase of 9 and 8 % on firmness of treated samples at low UV-C intensities (0.32 and 0.97 kJ.m−2, respectively) was observed. At the end of the storage, Ctr samples showed ca. 4 Log10 of mesophylic load, and the samples treated at 0.97 and 4.83 kJ.m−2 revealed the lowest microbial load (1.9 and 3.2 Log10, respectively). These results indicate that UV-C radiation, at an appropriate dose, combined with low storage temperature (10 °C) are an effective method to preserve the postharvest life of tomato, without adversely affecting quality parameters.
Keywords: UV-C radiation, Tomato, Quality, Storage, Microbial load
Introduction
Developing newer techniques and technologies in order to improve postharvest longevity of horticultural crops has always been a challenge to researchers. With the application of adequate technology to prevent deterioration after harvest, and considering the biochemical characteristics of the produce, postharvest losses can be reduced significantly. A treatment that could activate the mechanisms of the plant against the senescence can be a useful method in the preservation of fresh crops. One example of such technology is UV-radiation.
UV-radiation has been classified as UV-C (200–280 nm), UV-B (280–320 nm) and UV-A (320–400 nm). UV-A and B are present in the ultraviolet light of the atmosphere, while UV-C is blocked from reaching the earth’s surface (Gómez-López 2012). Each band can induce significantly different biological effects on crops (Bintsis et al. 2000; Shama 2007). Most of the information on the biological effects of UV-radiation is derived from experiments using artificial UV-C, particularly 254 nm radiations (Bintsis et al. 2000).
Ultraviolet-C radiation is widely used as an alternative strategy to control microorganism in food products (Nigro et al. 1998; Stevens et al. 1999; D’hallewin et al. 2000; Djenane et al. 2001; Shama and Alderson 2005). Microorganisms that are exposed to UV light are affected at the DNA (deoxyribonucleic acid) level (Terry and Joyce 2004; Perkins-Veazie et al. 2008). Thus, the injured reproduction systems of cells lead to their death (Guerrero-Beltrán and Barbosa-Cánovas 2004).
Low levels of this stressor may also stimulate other beneficial responses in horticultural crops, a phenomenon known as hormesis (Gómez-López 2012). The use of UV-C radiation as a postharvest elicitor of beneficial responses in these products has become a key area of interest. However, the mechanisms by which such changes take place have not been fully described. The potential of UV-C was also used to extend the shelf-life of various fruit and vegetables, such as strawberry and blueberry (Allende et al. 2007; Perkins-Veazie et al. 2008; Pombo et al. 2009; Cote et al. 2013), fresh-cut pineapples (Pan and Zu 2012), fresh-cut melon (Manzocco et al. 2011), broccoli (Costa et al. 2006; Lemoine et al. 2010; Martínez-Hernández et al. 2011), mushrooms (Jiang et al. 2010), fresh-cut carrot (Alegria et al. 2012) and spinach (Escalona et al. 2010; Gómez-López et al. 2013; Karaca and Velioglu 2014). Moreover, it has been reported that these abiotic stresses may enhance the nutraceutical content of fresh fruit and vegetables (Cisneros-Zevallos 2003). However, a reduction of vitamin C content immediately after UV-C treatment in fresh-cut pineapple, banana and guava was reported by Alothman et al. (2009). Total dosage (energy per unit area) is a main factor determining fruit and vegetable responses to UV-C (Civello et al. 2006), but the intensity of the radiation (dose per unit time) may also determine treatment outcome.
Other advantages when applying UV-C radiation are that the equipment is relatively inexpensive, the technique is easy to use, lack of residual compounds and avoids the use of chemicals that can cause ecological problems and/or are potentially harmful to humans (Allende and Artés 2003; López-Rubira et al. 2005).
To our knowledge, for the tomato fruit Charles et al. (2008 and 2009) in a comprehensive study showed that susceptibility of tomato fruit to Botrytis cinerea increased immediately after UV-C treatments, and Liu et al. (2011) studied gene expression of tomato fruit in response to postharvest UV-C irradiation. They concluded that this treatment can induce the expression of a number of defence response genes, and suppress the expression of major genes involved in cell wall disassembly, lipid metabolism and photosynthesis. These gene changes underline the biochemical and physiological changes induced by UV-C, such as increased defence ability, delayed softening, better maintenance of nutritional and sensory qualities and extension of shelf-life in tomato fruit. Other studies on radiated tomato fruit were performed to improve lycopene content (Liu et al. 2009), to investigate the possibility of delaying the fruits senescence (Maharaj et al. 2010), and to evaluate the impact on enzymic and non-enzymic phytochemicals (Maharaj et al. 2014). Nevertheless, there has been little research conducted about the effect of UV-C postharvest treatments on tomato (Solanum lycopersicum, cv. Zinac), ripening process and corresponding physical-chemical and microbiological parameters related to this fruit quality. Tomato cv. Zinac, is an important cultivar widely grown in Portugal for the fresh market (Anuário Vegetal 2003). Fruits of this cultivar exhibit attractive characteristics to the consumer, such as bright colour and intense flavour, and the texture changes during postharvest result in more juicy and farinaceous fruits (Reis et al. 2005).
The aims of this work were to select an appropriate UV-C dose for whole tomato, and to evaluate the effect on physical-chemical and microbiological quality under low-temperature storage.
Materials and methods
Plant material
Tomato (Solanum lycopersicum, cv. Zinac) fruits at green maturity stage, according to the USDA standard tomato colour classification (USDA 1991), with uniform size and without bruises or signs of infection were obtained from a commercial greenhouse Carmo & Silvério in centre west of Portugal. An initial physical-chemical properties and microbial characterization of tomato fruits without any treatment (untreated, Ctr samples) was done, to provide a baseline comparison to the UV-C treatments effects, and results are shown in Table 1.
Table 1.
Quality attributes of untreated tomato (average ± standard deviation)
| Quality attributes | |
|---|---|
| Colour parameters | |
| L* | 44.7 ± 2.2 |
| a* | −9.4 ± 1.9 |
| b* | 21.4 ± 1.6 |
| °h | 113.5 ± 3.5 |
| Texture | |
| Firmness (N) | 11.4 ± 2.1 |
| Total phenolic content | |
| TPC (mg GAE.100 g−1) | 24.7 ± 0.8 |
| Microbial load | |
| Mesophylic count (Log10 cfu.g−1) | 2.2 ± 1.0 |
UV-C treatments and storage conditions
UV- C treatments were conducted in a closed box (43 cm (w) × 50 cm (l) × 24 cm (h)) equipped with two germicidal lamps (TUV 15 W/G15 T8, Philips, Holland), emitting at 254 nm and placed 10 cm above tomato fruits. The box was covered with aluminium foil to promote a homogeneous light distribution. Prior to use, UV-C lamps were stabilized by turning them on for 15 min. Whole tomatoes were placed in a single layer on the illumination area at the fixed distance and rotated manually (180°) in order to ensure total UV exposure. The tested UV-C intensity was measured by a photo-radiometer (DELTA OHM LP9021 UVC), giving corresponding doses of 0.32, 0.97, 2.56, 4.16 and 4.83 kJ.m−2, after exposure times of 1, 3, 8, 13 and 15 min, respectively. After treatments, both samples (UV-C treated and Ctr samples) were evaluated during storage at 10 ± 0.5 °C (FITOCLIMA S 600 Pharma, Lisboa, Portugal) at 0, 9 and 15 days (Pinheiro et al. 2013).
Colour
Tomato colour was evaluated using a tristimulus colorimeter (Minolta chroma Meter, CR-300, Osaka, Japan), measuring the CIEL*a*b* parameters. The instrument was calibrated using a white standard tile (L*= 97.10, a* = 0.19, b* = 1.95), and the illuminate C (10° observer). L* values represent the luminosity of samples (0-black to 100-white), and a* and b* values indicate the variation of greenness to redness (−60 to +60) and blueness to yellowness (−60 to +60), respectively. From the CIELab coordinates, hue angle (°h = arctg (b*/a*)) was calculated. Four determinations for each fruit were performed in the equatorial zone. Sixteen measurements were determined per treatment condition.
Texture
Tomato texture was determined by a penetration test with a Texture Analyzer (TA.HDi, Stable Microsystem Ltd, Godalming, UK), using a 50 N load cell and a stainless steel cylinder probe with a 2 mm diameter. The penetration test was performed at a speed of 3 mm.s−1 and 7.5 mm of penetration distance in the equatorial zone of the fruits. Force-distance curves were recorded and firmness (maximum peak force (N)) was used as indicator of texture. Firmness was measured after holding tomato at room temperature for 2 h, to avoid interference from storage temperature on the determination. Sixteen measurements were taken for each treatment conditions.
Weight loss
Tomato weight loss was measured according to Dijk et al. (2006). A batch constituted by five fruits per day of analysis was weighted. After weighting, tomatoes were put back to original storage conditions. The weight loss was calculated relative to the weight at day zero (t = 0).
| 1 |
where W0 is the average weight of the first batch (three replicates) at day 0 and Wt is the average weight of the same batch at day t.
Total phenolic content
Tomato total phenolic content (TPC) was determined using the Folin-Ciocalteau reagent (Singleton and Rossi 1965). Samples (10 g) were homogenized in 70 % aqueous methanol (10 ml), using a Yellow line DI 25 basic polytron (IKA-Labortechnik, Stauten, Germany), and centrifuged (Sorvall RC-5, rotor SS34, DuPont, Wilmington, United States) at 19,000 rpm for 20 min at 4 °C and the supernatant collected. One hundred microliter of supernatant was mixed with 5 ml of Folin-Ciocalteau (1/10, v/v) and 4 ml of Na2CO3 (7.5 %, w/v). The mixture was placed in a water-bath (45 °C for 15 min) and the absorbance measured at 765 nm in an ATI Unicam UV/VIS UV4 spectrophotometer (Unicom Limited, Cambridge, United Kingdom), using gallic acid as a standard. Results (six replicates) were expressed as milligram gallic acid equivalents (mg GAE.100 g−1) of fresh weight.
Microbial load
Total mesophylic count was performed according to ISO 4833 2003. Ten g of sample was mixed with 90 ml peptone saline solution in a sterile stomacher bag and homogenized for 1 min using a Stomacher. Dilutions were made in peptone water as needed for plating. Plate Count Agar was used as the media for total mesophylic counts pour plate, incubated at 30 °C for 3 days.
Statistical analysis
Analysis of variance (ANOVA), followed by Tukey test (P ≤ 0.05), was performed on the data using Statistica™v.7.0 Software from Stasoft (StatSoft Inc. 2004).
Results and discussion
Colour
The a* value of a typical tomato fruit will increase as the ripening process progresses and the tomato becomes redder (Jagadeesh et al. 2011). Tomato colour (a* and °h values) were influenced by UV-C treatments as shown in Fig. 1. At day 0, all samples were described as green colour, due to low a* and corresponding high °h values. Of all UV-C treated samples, the intensities of 0.97 and 4.16 kJ.m−2 led to a no significant (P > 0.05) reduction of a* value, −10.6 and −10.9, respectively, compared to Ctr sample value (−9.4). After 9 days at 10 °C, a significant (P < 0.05) increase was observed on untreated samples (Ctr) reaching to the highest a* value of 9.7, which represents the redder colour on tomato surface. On the other hand, all UV-C treated samples denoted lower a* and higher °h values. At the end of storage (15 days), Ctr sample and UV-C treated at 2.56 kJ.m−2 showed an increase of red colour development. Noting the evolution of colour parameters of UV-C treated samples, the delay on red colour development was achieved on tomatoes treated with the lowest intensity (0.32 kJ.m−2).
Fig. 1.
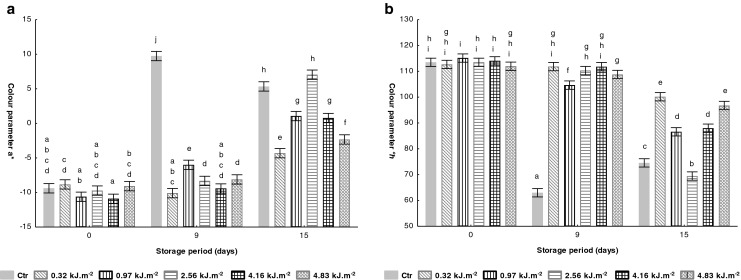
Effect of UV-C treatments on tomato colour parameters (a: a* and b: °h) during storage at 10 °C. Vertical bars denoted 95 % of confidence intervals. Means with same letter are not significantly different (P > 0.05)
Tiecher et al. (2013) investigated the effects of UV-C treatment (3.7 kJ.m−2) and 1-methylcyclopropene (1-MCP; 2 μL.L−1), both separately and in combination, on colour of tomato fruits at breaker ripening stage at room temperature. Results showed that UV-C treated samples had higher °h values (ca. 70°) after 7 days of storage compared to untreated fruit (ca. 60°). These changes are in agreement with our results, meaning that UV-C treatment lead to greenness and less red fruits compared to the non-treated. Also, Liu et al. (2009) showed a beneficial effect in retarding tomato senescence, after UV-C treatment at a higher dose (13.7 kJ.m−2), where a decrease on tomato a* colour was obtained on UV-C treated fruits along 21 storage days (14 °C). Song et al. (2011) evaluated the effect of aqueous chlorine dioxide (10 mg.L−1) and UV-C treatment (5 kJ.m−2) on colour of cherry tomatoes. They concluded that tomato colour was not significantly different (P > 0.05) among treatments during storage, and for this reason the combined treatment could be useful in quality preservation. Vicente et al. (2005) also found higher °h values on UV-C treated peppers than those of control fruits during storage at 10 °C.
Texture
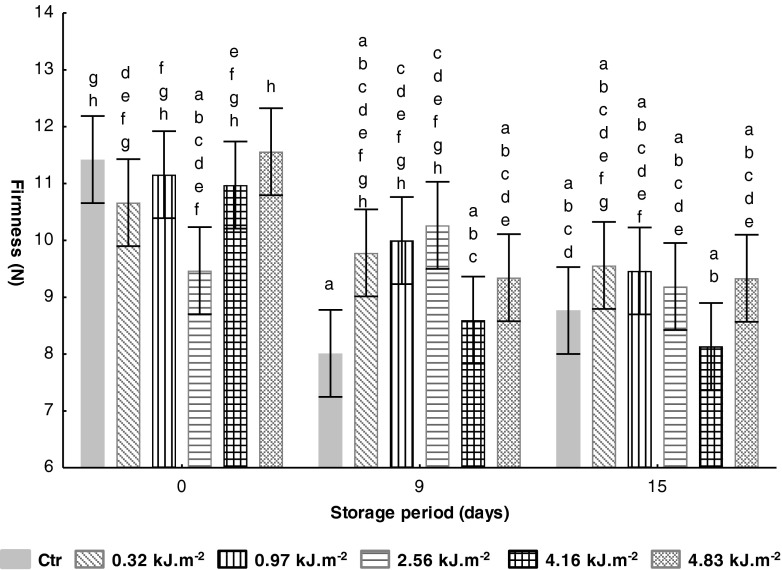
Exposing tomato fruits to UV-C treatments delays fruit softening, one of the main factors determining fruit postharvest life (Ribeiro et al. 2012). Changes in tomato firmness of untreated and UV-C treated along 15 days of storage at 10 °C are presented in Fig. 2. After UV-C treatment a non significant (P > 0.05) decrease on firmness of all samples was observed, being the highest reduction (17 %) on samples treated with 2.56 kJ.m−2, compared with the value encountered on untreated sample (11.4 N). At the end of storage, untreated samples revealed a slightly higher firmness (ca. 10 %) compared with the value by 9th storage day. Comparing the firmness of all samples at 15th storage day, the sample treated with 4.16 kJ.m−2 presents the highest firmness lost regarding the initial value. Moreover, by day 15 of storage and comparing Ctr and UV-C treated samples at lower intensities (0.32 and 0.97 kJ.m−2), a slightly higher firmness of 9 and 8 % (P > 0.05), respectively, was observed. The increased firmness of UV-C treated fruits during storage could be associated with activation of pectinmethylesterase (PME) and subsequent formation of calcium pectates (Barka et al. 2000; Hemmaty et al. 2007), and by reduction in activities of cell wall degrading enzymes (Khademi et al. 2013).
Fig. 2.
Effect of UV-C treatments on tomato texture (firmness, N) during storage at 10 °C. Vertical bars denoted 95 % of confidence intervals. Means with same letter are not significantly different (P > 0.05)
This behaviour is consistent with the study developed by Pombo et al. (2009) while studying the effect of UV-C treatment (4.1 kJ.m−2) on firmness of strawberry stored at 20 °C during 4 days. In this study, firmness of UV-C treated samples was not affected immediately after radiation. During storage, the firmness of all samples decreased but the UV-C treated sample remained firmer than control fruits. Charles et al. (2009) revealed a softening delay in UV-C treated tomato fruits with UV-C intensity of 3.7 kJ.m−2 compared with untreated samples, during 30 storage days at 13 °C.
Additionally, other works have been reporting the benefits of UV-C treatment to maintain postharvest firmness, such as in Kent and Seascape cultivars of strawberry treated with UV-C doses of 1 and 4.1 kJm−2, respectively (Pan et al. 2004). Ribeiro et al. (2012) suggested that softening delay would be due to changes in the enzymatic activities and proteins involved in cell wall disassembly.
Weight loss
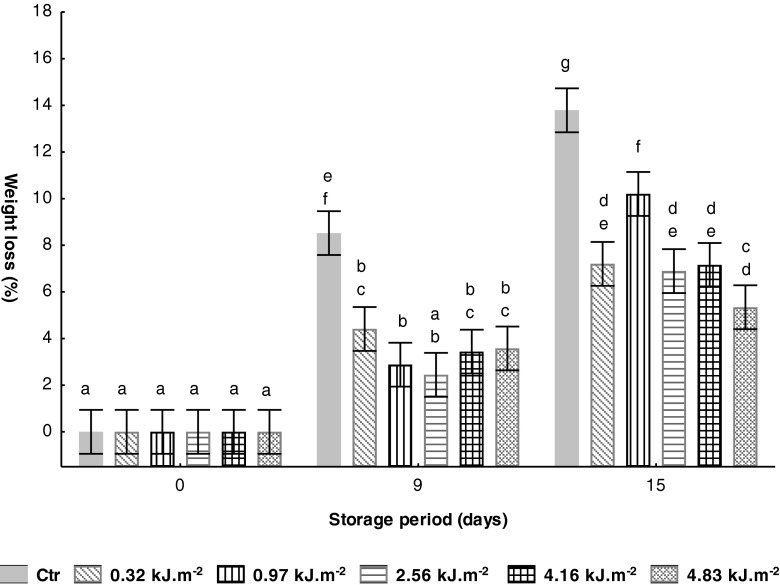
In the course of the present study, an increase on weight loss of all samples (untreated and UV-C treated) was observed, as presented in Fig. 3. The Ctr samples showed a significantly (P < 0.05) increase of weight loss, reaching values of ca. 9 and 14 %, after 9 and 15 storage days, respectively, at 10 °C. Untreated fruits reached the shelf-life limit defined by Getinet et al. (2008) (WL = 10 %) on 9th storage day. In contrast, this value was only reached by the UV-C treated with 0.97 kJ.m−2 at the end of storage, after 6 days in comparison with Ctr samples, showing an extension of shelf-life fruits. However, comparing the weight losses of UV-C treated samples at the end of storage, the UV-C intensity of 0.97 kJ.m−2 presents the highest value. The increase of fruits weight losses results from physiological phenomena like transpiration and respiration rates. Daiuto et al. (2013) reported that higher intensity of UV-C radiation could be effective in delaying respiration rate, which retards the increase of fruits weight losses. The lower UV-C intensity of 0.97 kJ.m−2 is not sufficient for delaying the metabolic degradation processes and for this reason the UV-C treated samples at this intensity presented the highest weight loss at the end of storage. The lowest value of weight loss verified on all UV-C treated samples during storage period had beneficial effects mainly on fruits appearance visual evaluation (data not shown).
Fig. 3.
Effect of UV-C treatments on tomato weight loss (%) during storage at 10 °C. Vertical bars denoted 95 % of confidence intervals. Means with same letter are not significantly different (P > 0.05)
However, in a study developed by Cote et al. (2013), a weight lost of 6 and 11 %, after 3 and 5 days, respectively, was observed on untreated strawberries during storage at 10 °C, and similar values were encountered in UV-C treated samples with low intensity (dose of 4 kJ.m−2 with radiation intensity of 3 W.m−2).
Total phenolic content
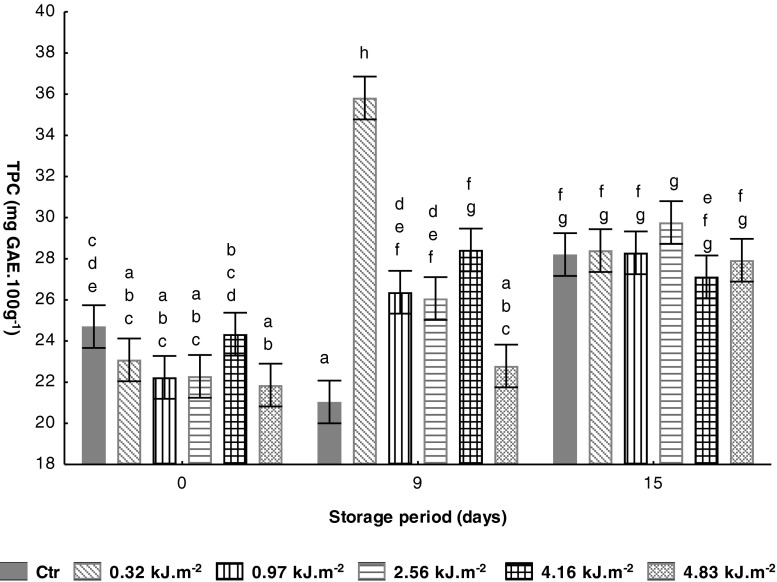
Crude extracts of fruits and vegetables are rich in phenolics and they retard oxidative degradation of lipids and thereby improve the quality and nutritional value of products (Aberoumand and Deokule 2010). Initial value of fresh tomato (cv. Zinac) at mature-green of maturity stage was 24.7 ± 0.8 mg GAE.100 g−1 (Table 1). Similar value was reported by Brat et al. (2006) on fresh tomato (<23.0 mg GAE.100 g−1). After UV-C treatment a no significant (P > 0.05) reduction on total phenolic content (TPC) was found in all tomato samples (Fig. 4). The highest UV-C intensity (4.83 kJ.m−2) was found to significantly (P < 0.05) reduce the TPC, as compared to untreated fruits. At the 9th day of storage, all UV-C treated samples showed an increase of TPC compared to content determined on the first storage day. Also, the phenolic content of UV-C treated tomato at 0.32 kJ.m−2 was 55 % higher than observed on Ctr samples (21.0 mg GAE.100 g−1). However, by the last day of storage, the TPC of all UV-C samples reached a similar value (P > 0.05) to Ctr samples (28.2 mg GAE.100 g−1).
Fig. 4.
Effect of UV-C treatments on tomato total phenolic content (TPC) (mg GAE.100 g−1) during storage at 10 °C. Vertical bars denoted 95 % of confidence intervals. Means with same letter are not significantly different (P > 0.05)
UV-C treatment has been associated with enhancement of bioactive compounds, such as vitamins, carotenoids and phenolic compounds, in several fruits and vegetables, e.g. tomatoes (Jagadeesh et al. 2011; Maharaj et al. 2014), strawberries (Pombo et al. 2009; Tsormpatsidis et al. 2011), carrots (Heredia and Cisneros-Zevallos 2009; Alegria et al. 2012), and broccoli (Lemoine et al. 2010; Martínez-Hernández et al. 2011). There is strong evidence that the DNA-damaging effect of UV light induces the accumulation of UV-light-absorbing flavonoids and other phenolics, predominantly in the epidermal tissues of the plant body (Bravo et al. 2013). An important flavonoid phenolic compound in tomato is the flavonols, which are restricted almost entirely in the skin. The penetrating ability of UV-C radiation is limited to the outer pericarp and an increase in phenolic compounds, mainly in the skin, is to be expected with less impact on more inner tissue (Jagadeesh et al. 2011). Moreover, the increase of total phenolic content after UV-C treatments could be considered an adaptation mechanism of tomato due to UV-stress, which promotes the enzymatic activity of phenylalanine ammonia-lyase, a key enzyme in the production of phenylpropanoids, leading to an increase of phenols, phytoalexins, and lignins (Ryalls et al. 1996). On the other hand, the reduction on phenolic compounds can be the result of UV-C radiation action on phenylpropanoid pathway responsible for phenolics synthesis (Ke and Saltveit 1989), or due to polyphenoloxidase enzymatic activity that oxidizes phenolics into quinones polymers (Kim and Jung 2011).
Microbial load
One of the many advantages of UV-C treatment application was the inactivation/elimination of microbial load and this has been attributed to photochemical lesion induced on microorganisms DNA and RNA, resulting in the inability to perform regular transcription and replication of nucleic acids and normally the cell dies (Bolton and Linden 2003; Unluturk et al. 2008).
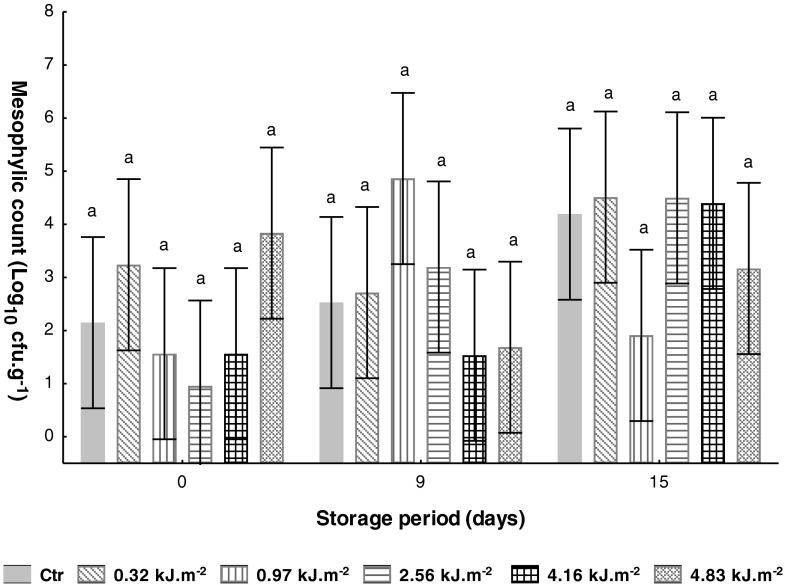
Before UV-C treatment, the microbial load for total mesophyles was 2.15 Log10 cfu.g−1 (Table 1). Immediately after treatments at 0.97 and 4.16 kJ.m−2 the achieved microbial reduction between samples was similar (0.6 Log10), and at 2.56 kJ.m−2 a 1.2 Log10 reduction was attained (Fig. 5). Nine days after UV-C treatment, the microbial population of UV-C-treated samples with the highest intensity (4.16 and 4.83 kJ.m−2) was ca. 1 Log10 lower than the control fruits (2.53 Log10). Similar reduction was attained by Kim et al. (2008) in fresh-cut tomato (cv. Durinta) treated with UV-C intensity of 9.6 and 19.2 kJ.m−2 after 3 days of storage at 10 °C.
Fig. 5.
Effect of UV-C treatments on tomato mesophylic count (Log10 cfu.g−1) during storage at 10 °C. Vertical bars denoted 95 % of confidence intervals. Means with same letter are not significantly different (P > 0.05)
At the end of storage, untreated samples showed the highest mesophylic load (ca. 4 Log10), and UV-C treated samples at 0.97 and 4.83 kJ.m−2 revealed the lowest microbial load, 1.9 and 3.2 Log10, respectively. The inferior microbial development found in UV-C treated samples at lower dose of 0.97 kJ.m−2 support that UV-C treatment could be an effective decontamination alternative to microbial control in fresh tomato. UV-C treatment results on the synthesis of a number of anti-fungal compounds (phytoalexins, flavonoids, phenolic acids, lignin, catalase, peroxidase, ascorbate peroxidase and phenylalanine ammonia-lyase) which retard fungal growth and decrease disease incidence severity (Khademi et al. 2013). Martínez-Hernández et al. (2011) found that low intensity of UV-C (1.5, 4.5 and 9.0 kJ.m−2) induced a higher reduction on microbial development in fresh-cut broccoli, compared to UV-C treatment of 15 kJ.m−2. It seems that highest UV-C intensities can be responsible for damage increase of superficial tissues, allowing the release of nutrients for microbial growth (Artés et al. 2009). However, our results do not always coincide with this statement.
Conclusion
The results show that UV-C treatment must be considered as postharvest treatment of fresh whole tomato fruits (Cv. Zinac). It seems that low intensity of UV-C treatment (0.97 kJ.m−2) retained the two main important quality attributes of fresh tomato (colour and texture) and retarded the natural microbial growth, during storage at 10 °C. It should be useful to revaluate this singular condition or to integrate it with other effective postharvest treatments to ensure tomato quality for longer periods. Moreover, our results suggest that UV-C treatment could be used as a pre-treatment, as a tool to enhance health promoting bioactive compounds such as phenolic content. However, further studies are needed to optimize UV-C treatment conditions for tomato (Cv. Zinac), for improving fruit quality and at the same time enhance phenolic content. This may greatly benefit human health and can extend the shelf-life of tomato fruits.
Acknowledgments
The author Joaquina Pinheiro gratefully acknowledges her Ph.D grant (SFRH/BD/24913/2005) to Fundação para a Ciência e a Tecnologia (FCT) from Ministério da Ciência e do Ensino Superior (Portugal). The authors greatly acknowledge the technical assistance of Maria do Carmo and Ana Magalhães for helping in performing the microbial analysis. This work was supported by National Funds from FCT through project PEst-OE/EQB/LA0016/2011.
References
- Aberoumand A, Deokule SS. Total phenolic contents of some plant foods as an antioxidant compound. J Food Technol. 2010;8:131–133. doi: 10.3923/jftech.2010.131.133. [DOI] [Google Scholar]
- Alegria C, Pinheiro J, Duthoit M, Gonçalves EM, Moldão-Martins M, Abreu M. Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. LWT Food Sci Technol. 2012;48:197–203. doi: 10.1016/j.lwt.2012.03.013. [DOI] [Google Scholar]
- Allende A, Artés F. UV-C radiation as a novel technique for keeping quality of fresh processed ‘Lollo Rosso’ lettuce. Food Res Int. 2003;36:739–746. doi: 10.1016/S0963-9969(03)00054-1. [DOI] [Google Scholar]
- Allende A, Marín A, Buendía B, Tomás-Barberán F, Gil MI. Impact of combined postharvest treatments (UV-C light, gaseous O3, superatmospheric O2 and high CO2) on health promoting compounds and shelf life of strawberries. Postharvest Biol Technol. 2007;46:201–211. doi: 10.1016/j.postharvbio.2007.05.007. [DOI] [Google Scholar]
- Alothman M, Bhat R, Karim AA. UV radiation-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov Food Sci Emerg. 2009;10:512–516. doi: 10.1016/j.ifset.2009.03.004. [DOI] [Google Scholar]
- Artés F, Gómez P, Aguayo A, Escalona V, Artés-Hernández F. Sustainable sanitation techniques for keeping quality and safety of fresh-cut plant commodities. Postharvest Biol Technol. 2009;51:287–296. doi: 10.1016/j.postharvbio.2008.10.003. [DOI] [Google Scholar]
- Barka EA, Kalantari S, Makhlouf J, Arul J. Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J Agric Food Chem. 2000;48:667–671. doi: 10.1021/jf9906174. [DOI] [PubMed] [Google Scholar]
- Bintsis T, Litopoulou-Tzanetaki E, Robinson RK. Existing and potential application of ultraviolet light in the food industry–a critical review. J Sci Food Agric. 2000;90:637–645. doi: 10.1002/(SICI)1097-0010(20000501)80:6<637::AID-JSFA603>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Bolton JR, Linden KG. Standarization of methods for fluence (UV dose) determination in bench-scale UV experiments. J Environ Eng. 2003;129:209–215. doi: 10.1061/(ASCE)0733-9372(2003)129:3(209). [DOI] [Google Scholar]
- Brat P, Georgé S, Bellamy A, Chaffaut LD, Scalbert A Mennen L, Arnault N, Amiot MJ (2006) Daily polyphenol intake in France from fruit and vegetables. J Nutr Epidemiol 2368–2373 [DOI] [PubMed]
- Bravo S, García-Alonso J, Martín-Pozuelo G, Gómez V, García-Valverde N-GI, Periago MJ. Effects of postharvest UV-C treatment on carotenoids and phenolic compounds of vine-ripe tomatoes. Int J Food Sci Technol. 2013;48:1744–1749. doi: 10.1111/ijfs.12146. [DOI] [Google Scholar]
- Charles MT, Benhamou N, Arul J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. IV. Biochemical modification of structural barriers. Postharvest Biol Technol. 2008;47:41–53. doi: 10.1016/j.postharvbio.2007.05.019. [DOI] [Google Scholar]
- Charles MT, Tano K, Asselin A, Arul J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. V. Constitutive defence enzymes and inducible pathogenesis-related proteins. Postharvest Biol Technol. 2009;51:414–424. doi: 10.1016/j.postharvbio.2008.08.016. [DOI] [Google Scholar]
- Cisneros-Zevallos L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J Food Sci. 2003;68:1560–65. doi: 10.1111/j.1365-2621.2003.tb12291.x. [DOI] [Google Scholar]
- Civello P, Vicente A, Martínez G (2006) UV-C technology to control postharvest diseases of fruits and vegetables. Recent advances in alternative postharvest technologies to control fungal diseases in fruits and vegetables. Transworld research network, 37/661 (2), India, pp. 71–102
- Costa L, Vicente AR, Civello PM, Chaves AR, Martínez GA. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol Technol. 2006;39:204–210. doi: 10.1016/j.postharvbio.2005.10.012. [DOI] [Google Scholar]
- Cote S, Rodoni L, Miceli E, Concellón A, Civello PM, Vicente AR. Effect of radiation intensity on the outcome of postharvest UV-C treatments. Postharvest Biol Technol. 2013;83:83–89. doi: 10.1016/j.postharvbio.2013.03.009. [DOI] [Google Scholar]
- D’hallewin G, Schirra M, Pala M, Ben-Yehoshua S. Ultraviolet C irradiation at 0.5 kJ. m−2 reduces decay without causing damage or affecting postharvest quality of Star Ruby grapefruit (C. paradisi Macf.) J Agric Food Chem. 2000;48:4571–4575. doi: 10.1021/jf000559i. [DOI] [PubMed] [Google Scholar]
- Daiuto ER, Vieites RL, Remocoldi MA, Carvalho LR, Fumes JGF. Postharvest of ‘Hass’ avocados submitted to UV-C radiation. Rev Colomb Mag Hortíc. 2013;7(2):149–160. doi: 10.17584/rcch.2013v7i2.2231. [DOI] [Google Scholar]
- Dijk CV, Boeriu C, Peter F, Stolle-Smits T, Tijsken LMM. The firmness of stored tomatoes (cv. Tradiro). 1. Kinetic and near infrared models to describe firmness and moisture loss. J Food Eng. 2006;77:575–584. doi: 10.1016/j.jfoodeng.2005.07.029. [DOI] [Google Scholar]
- Djenane D, Sánchez-Escalante A, Beltrán JA, Roncalés P. Extension of the retail display life of fresh beef packaging in modified atmosphere by varying lighting conditions. J Food Sci. 2001;66:181–186. doi: 10.1111/j.1365-2621.2001.tb15603.x. [DOI] [Google Scholar]
- Escalona VH, Aguayo E, Martínez-Hernández GB, Artés F. UV-C doses to reduce pathogen and spoilage bacterial growth in vitro and in baby spinach. Postharvest Biol Technol. 2010;56:223–231. doi: 10.1016/j.postharvbio.2010.01.008. [DOI] [Google Scholar]
- Getinet H, Seyoum T, Woldetsadik K. The effect of cultivar, maturity stage and storage environment on quality of tomatoes. J Food Eng. 2008;87:467–78. doi: 10.1016/j.jfoodeng.2007.12.031. [DOI] [Google Scholar]
- Gómez-López VM (2012) Decontamination of fresh and minimally processed produce- continuous UV-C light. First edition, John Wiley & Sons, Inc, Chapter 21
- Gómez-López VM, Marín A, Medina-Martínez MS, Gil MI, Allende A. Generation of trihalomethanes with chlorine-based sanitizers and impact on microbial, nutritional and sensory quality of baby spinach. Postharvest Biol Technol. 2013;85:210–17. doi: 10.1016/j.postharvbio.2013.05.012. [DOI] [Google Scholar]
- Guerrero-Beltrán JA, Barbosa-Cánovas GV. Advantages and limitations on processing foods by UV light. Food Sci Technol Int. 2004;10:137–147. doi: 10.1177/1082013204044359. [DOI] [Google Scholar]
- Hemmaty S, Moallemi N, Naseri L. Effect of UV-C radiation and hot water on the calcium content and postharvest quality of apples. Span J Agric Res. 2007;5(4):559–568. doi: 10.5424/sjar/2007054-277. [DOI] [Google Scholar]
- Heredia JB, Cisneros-Zevallos L. The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biol Technol. 2009;51:242–249. doi: 10.1016/j.postharvbio.2008.07.001. [DOI] [Google Scholar]
- ISO 4833 (2003) Microbiology of food and animal feeding stuffs - horizontal method for the enumeration of microorganisms - colony-count technique at 30° C
- Jagadeesh SL, Charles MT, Gariepy Y, Goyette B, Raghavan GSV, Vigneault C. Influence of postharvest UV-C hormesis on the bioactive components of tomato during post-treatment handling. Food Bioprocess Technol. 2011;4:1463–72. doi: 10.1007/s11947-009-0259-y. [DOI] [Google Scholar]
- Jiang T, Jahangir MM, Jiang Z, Lu X, Ying T. Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biol Technol. 2010;56:209–215. doi: 10.1016/j.postharvbio.2010.01.011. [DOI] [Google Scholar]
- Karaca H, Velioglu YS. Effects of ozone treatments on microbial quality and some chemical properties of lettuce, spinach, and parsley. Postharvest Biol Technol. 2014;88:46–53. doi: 10.1016/j.postharvbio.2013.09.003. [DOI] [Google Scholar]
- Ke D, Saltveit ME. Wound-induced ethylene production, phenolic metabolism and susceptibility to russet spotting in iceberg lettuce. Physiol Plant. 1989;76:412–418. doi: 10.1111/j.1399-3054.1989.tb06212.x. [DOI] [Google Scholar]
- Khademi O, Zamani Z, Poor AE, Kalantari S. Effect of UV-C radiation on postharvest physiology of persimmon fruit (Diospyros kaki Thunb.) cv. ‘Karaj’ during storage at cold temperature. Food Res Int. 2013;20(1):247–253. [Google Scholar]
- Kim SJ, Jung KM. Effects of the PPO (Polyphenol oxidase) activity and total phenolic contents on browning and quality of dried-persimmon according to maturity degree of astringent persimmon (Diospyros kaki Thunb.) Int Conf Biotechnol Food Sci. 2011;7:115–118. [Google Scholar]
- Kim HJ, Fonseca MJ, Kubota C, Kroggel M, Choi J. Quality of fresh-cut tomatoes as affected by salt content in irrigation water and post-processing ultraviolet-C treatment. J Sci Food Agr. 2008;88:1969–74. doi: 10.1002/jsfa.3305. [DOI] [Google Scholar]
- Lemoine ML, Chaves AR, Martínez GA. Influence of combined hot air and UV-C treatment on the antioxidant system of minimally processed broccoli (Brassica oleracea L. var. Italica) LWT Food Sci Technol. 2010;43:1313–1319. doi: 10.1016/j.lwt.2010.05.011. [DOI] [Google Scholar]
- Liu LH, Zabaras D, Bennett LE, Aguas P, Woonton BW. Effects of UV-C, red light and sun light on the carotenoid content and physical qualities of tomatoes during post-harvest storage. Food Chem. 2009;115:495–500. doi: 10.1016/j.foodchem.2008.12.042. [DOI] [Google Scholar]
- Liu CH, Cai LY, Han XX, Ying TJ. Temporary effect of postharvest UV-C irradiation on gene expression profile in tomato fruit. Gene. 2011;486:56–64. doi: 10.1016/j.gene.2011.07.001. [DOI] [PubMed] [Google Scholar]
- López-Rubira F, Conesa A, Allende A, Artés F. Shelf life and overall quality of minimally processed pomegranate arils modified atmosphere packaged and treated with UV-C. Postharvest Biol Technol. 2005;37:174–185. doi: 10.1016/j.postharvbio.2005.04.003. [DOI] [Google Scholar]
- Maharaj R, Arul J, Nadeau P. UV-C Irradiation of tomato and its effects on colour and pigments. Adv Environ Biol. 2010;4:308–315. [Google Scholar]
- Maharaj R, Arul J, Nadeau P. UV-C irradiation effects on levels of enzymic and non-enzymic phytochemicals in tomato. Innov Food Sci Emerg Technol. 2014;21:99–106. doi: 10.1016/j.ifset.2013.10.001. [DOI] [Google Scholar]
- Manzocco L, Pieve SD, Maifreni M. Impact of UV-C light on safety and quality of fresh-cut melon. Innov Food Sci Emerg Technol. 2011;12:13–17. doi: 10.1016/j.ifset.2010.11.006. [DOI] [Google Scholar]
- Martínez-Hernández GB, Gómez PA, Pradas I, Artés F, Artés-Hernández F. Moderate UV-C pretreatment as a quality enhancement tool in fresh-cut Bimi® broccoli. Postharvest Biol Technol. 2011;62:327–37. doi: 10.1016/j.postharvbio.2011.06.015. [DOI] [Google Scholar]
- Nigro F, Ippolito A, Lima G. Use of UV-C light to reduce Botrytis storage rot of table grapes. Postharvest Biol Technol. 1998;13:171–181. doi: 10.1016/S0925-5214(98)00009-X. [DOI] [Google Scholar]
- Pan YG, Zu H. Effect of UV-C radiation on the quality of fresh-cut pineapples. Procedia Eng. 2012;37:113–119. doi: 10.1016/j.proeng.2012.04.212. [DOI] [Google Scholar]
- Pan J, Vicente AR, Martinez GA, Chavez AR, Civello PM. Combined use of UV-C irradiation and heat treatment to improve postharvest life of strawberry fruit. J Sci Food Agric. 2004;84:1831–1838. doi: 10.1002/jsfa.1894. [DOI] [Google Scholar]
- Perkins-Veazie P, Collins JK, Howard L. Blueberry fruit response to postharvest application of ultraviolet radiation. Postharvest Biol Technol. 2008;47:280–285. doi: 10.1016/j.postharvbio.2007.08.002. [DOI] [Google Scholar]
- Pinheiro J, Alegria C, Abreu M, Gonçalves EM, Silva CLM. Kinetics of changes in the physical quality parameters of fresh tomato fruits (Solanum lycopersicum, cv. ‘Zinac’) during storage. J Food Eng. 2013;114:338–345. doi: 10.1016/j.jfoodeng.2012.08.024. [DOI] [Google Scholar]
- Pombo MA, Dotto MC, Martínez GA, Civello PM. UV-C irradiation delays strawberry fruit softening and modifies the expression of genes involved in cell wall degradation. Postharvest Biol Technol. 2009;51:141–148. doi: 10.1016/j.postharvbio.2008.07.007. [DOI] [Google Scholar]
- Reis M, Silva R, Gomes C, Rosa A, Coelho L, Marreiros A, Fernandes M, Caço J, Monteiro A. Comparação da qualidade do tomate obtido segundo o modo de produção biológico, por métodos convencionais no solo e em cultura em lã de rocha. Porto: V Congresso Ibérico de Ciências Hortícolas / IV Congresso Iberoamericano de Ciências Hortícolas; 2005. [Google Scholar]
- Ribeiro C, Canada J, Alvarenga B. Prospects of UV radiation for application in postharvest technology. Emir J Food Agric. 2012;24:586–597. doi: 10.9755/ejfa.v24i6.586597. [DOI] [Google Scholar]
- Ryalls J, Neuenschwander U, Willits M, Molina A, Steiner HY, Hunt M. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shama G. Process challenges in applying low doses of ultra violet light to fresh produce for eliciting beneficial hermetic responses. Postharvest Biol Technol. 2007;44:1–8. doi: 10.1016/j.postharvbio.2006.11.004. [DOI] [Google Scholar]
- Shama G, Alderson P. UV hormesis in fruits: a concept ripe for commercialization. Trends Food Sci Technol. 2005;16:128–136. doi: 10.1016/j.tifs.2004.10.001. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Song HJ, Choi DW, Song KB. Effect of aqueous chlorine dioxide and UV-C treatment on the microbial reduction and color of cherry tomatoes. J Hortic Sci Biotechnol. 2011;52:488–493. doi: 10.1007/s13580-011-0043-6. [DOI] [Google Scholar]
- StatSoft Inc (2004) STATISTICA (data analysis software system), version 7. www.statsoft.com
- Stevens C, Khan VA, Lu JY, Chalutz E, Droby S, Kabwe MK, Haung Z, Adeyeye O, Pusey LP, Tang AYA. Induced resistance of sweet potato to Fusarium root rot by UV-C hormesis. Crop Prot. 1999;18:463–470. doi: 10.1016/S0261-2194(99)00045-9. [DOI] [Google Scholar]
- Terry LA, Joyce DC. Elicitors of induced disease resistance in postharvest. horticultural crops: a brief review. Postharvest Biol Technol. 2004;32:1–13. doi: 10.1016/j.postharvbio.2003.09.016. [DOI] [Google Scholar]
- Tiecher A, Paula LA, Chaves FC, Rombaldi CV. UV-C effect on ethylene, polyamines and the regulation of tomato fruit ripening. Postharvest Biol Technol. 2013;86:230–239. doi: 10.1016/j.postharvbio.2013.07.016. [DOI] [Google Scholar]
- Tsormpatsidis E, Ordidge M, Henbest RGC, Wagstaffe A, Battey NH, Hadley P. Harvesting fruit of equivalent chronological age and fruit position shows individual effects of UV radiation on aspects of the strawberry ripening process. Environ Exp Bot. 2011;74:178–185. doi: 10.1016/j.envexpbot.2011.05.017. [DOI] [Google Scholar]
- Unluturk S, AtIlgan MR, Handan BA, Tari C. Use of UV-C radiation as a non-thermal process for liquid egg products (LEP) J Food Eng. 2008;85:561–568. doi: 10.1016/j.jfoodeng.2007.08.017. [DOI] [Google Scholar]
- USDA . United States Standard for grades of fresh tomatoes. United States department of agriculture, agricultural marketing service. Washington DC: USDA; 1991. [Google Scholar]
- Vegetal A. Gabinete de planeamento e política agro-alimentar. Lisboa: Castel-Publicação e Edição S.A; 2003. [Google Scholar]
- Vicente AR, Peneda C, Lemoine L, Civello PM, Martinez GA, Chaves AR. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol Technol. 2005;35:69–78. doi: 10.1016/j.postharvbio.2004.06.001. [DOI] [Google Scholar]