Abstract
Efficacy of R. fruticosus leaves extract in stabilizing sunflower oil during accelerated storage has been studied. Extracts of R. fruticosus were prepared in different solvents which methanolic extract yield with 15.43 % was higher than water and acetone ones (11.87 and 6.62 %, respectively). Methanolic extract was chosen to evaluate its thermal stability at 70 °C in sunflower oil, due to the highest yield, antioxidant and antiradical potential and also high content of phenolic compounds campared to other solvents. So, different concentrations of methanolic extract (200, 400, 600, 800 and 1,000 ppm) were added to sunflower oil. BHA and BHT at 200 ppm served as standards besides the control. Peroxide value (PV) and thiobarbituric acid (TBA) were taken as parameters for evaluation of effectiveness of R. fruticosus leaves extract in stabilization of sunflower oil. Moreover, antioxidant activity index (AAI) of the extract at 120 °C at rancimat were conducted. Results from different parameters were in agreement with each other, suggesting the highest efficiency of 1,000 ppm of the extract followed by BHT, BHA and other concentrations of the extract. Results reveal the R. fruticosus leaves extract to be a potent antioxidant for stabilization of sunflower oil.
Keywords: Antioxidant activity, R. fruticosus leaves extract, Sunflower oil, Thermal stability
Introduction
Vegetable oils are recognized as important compounds of our diet. They provide essential fatty acids, which control many physiological factors such as cholesterol level, blood pressure and the reproductive system (Eshghi et al. 2014). Lipid oxidation is one of the most important quality deteriorating processes in oil industry. It results in the losses of nutritional value of oils as well as changes in color, flavor and other physiological properties (Fennema 1996). Autoxidation of lipids is a natural process that occurs between molecular oxygen and unsaturated fatty acids through a free-radical chain mechanism that involves the formation of fat free radicals, peroxide free radicals and hydroperoxides. The hydroperoxides are very unstable and decompose to secondary products including aldehydes, ketones, alcohols and acids, which cause off-odors and off-flavors (Marinova and Yanishlieva 1992). The oils with higher contents of unsaturated fatty acids, especially polyunsaturated fatty acid, are more susceptible to oxidation. Addition of synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and tertiary butyl hydro- quinone (TBHQ) can inhibit lipid oxidation of edible oils (Suja et al. 2004). However, the use of synthetic antioxidants such as TBHQ has been restricted because of their health side effects and toxicity. The most powerful synthetic antioxidant (TBHQ) is not allowed for food application in Japan, Canada and Europe. Similarly, BHT has also been removed from the generally recognized as safe (GRAS) list of compounds (Asnaashari et al. 2014). Therefore, there is a strong interest in replacing synthetic antioxidants with natural ones which can prevent formation of oxidation products. The effectiveness of different natural sources in stabilizing edible oils has been previously studied. Fruits, vegetables, nuts, seeds and barks are being investigated for their antioxidant potential (Iqbal et al. 2005; Iqbal and Bhanger 2007).
The genus Rubus (raspberry, blackberry) comprises around 700 species, naturally occurring in temperate climates. Some are cultivated in numerous varieties as industrial plants for the quality of nutritious and tasty fruits (Patel et al. 2004). One of the varieties of Rubus is fruticosus which was introduced to official register and has been used in traditional medicine for their many medicinal properties (Alice and Campbell 1999). The leaves of Rubus fruticosus also has medicinal functions and has been used for their astringent, antidiarrhoeic, hypoglycemic activities and as an anti- inflammatory agent for the mucous membrane of the oral cavity and throat. Moreover, its leaves extract has been reported to have relaxant effect, particularly on uterine muscles and beneficial effects on pregnancy period (Simpson et al. 2001; Rojas-Vera et al. 2002). Previous phytochemical investigations, which were carried out on different plant parts taken from R. fruticosus, have proved the presence of structurally and biogenetically diverse secondary metabolites. R. fruticosus leaves are especially rich in tannins, flavonoid compounds, quercetin, phenolic acids, triterpenes, mineral salts as well as vitamin C. Katalinic et al. (2006) determined the antioxidant capacity (ferric reducing antioxidant capacity (FRAP)) and phenolic content of 70 medicinal plants. The leaves of raspberry, blackberry, and strawberry were among eleven of the most effective plants. In another study, the antioxidant capacity (oxygen radical absorbance capacity (ORAC)) of leaves and berries of strawberry, blackberry, and raspberry were compared. The results indicated the leaves were a richer source of antioxidants and had a greater content of phenolic substances than berries (Wang and Lin 2000).
There are several studies which indicated the stabilization of sunflower oil with different synthetic and natural antioxidants (Iqbal and Bhanger 2007; Sharif et al. 2009). But the antioxidant activity of R. fruticosus leaves extract has not been studied. So, the aim of this study was to clarify the total phenolic content, total antioxidant activity, reducing power and DPPH radical scavenging activity of various solvent extract of R. fruticosus leaves extract using methanol, water and acetone. Moreover, the antioxidant behavior of the extract added to sunflower oil and oxidative stability were determined using primary and secondary oxidation products.
Materials and methods
Materials
Refined, bleached and deodorized sunflower oil (with no added antioxidants) was obtained from Behgol Company, Nieshaboor, Iran. The leaves of R. fruticosus were collected from the fields of Mazandaran, Iran in June. All the chemicals and reagents used were of analytical reagent grade and were purchased from Sigma (St. Louis, MO, USA) and Merck (Darmstadt, Germany) Company. BHA and BHT were purchased from Sigma Chemical Co (St. Louis, MO, USA).
Extraction
After drying in the shade, the leaves of R. fruticosus were grindered using Cuisinart SG-10 Electric Spice-and-Nut Grinder . The powder of dried leaves were extracted separately into methanol, water and acetone (1 : 50 wt/vol) by agitation in a dark place at room temperature for 24 h. Then, the extracts were filtered using Whatman No. 42 filter paper and residue was again extracted. The procedure was repeated twice to ensure the complete extraction. Then, the filtrate was subjected to rotary evaporator at 40 °C under pressure for the removal of solvent. The extracts were weighted to calculate the yield and were stored under nitrogen prior to further analyses (Chang et al. 2002).
Total phenolic content
The total phenolic content was determined spectrophotometrically using Folin-Ciocalteau’s reagent according to the method described by Iqbal et al. (2005). The 0.5 ml of extract was added to 2.5 ml of a 10-fold diluted Folin-Ciocalteau reagent and 2 ml of 7.5 % Na2CO3, in a volumetric flask reaching the final volume (50 ml) with purified water. The samples were stored overnight and the spectrophotometric analysis was performed at 765 nm. Results were expressed in microgram of gallic acid per milliliter of extract.
Reducing power
The reducing power of R. fruticosus leaves extract was determined by the method of Yao and Qing (2007). The extracts (50–200 mg) which were diluted in 1.0 ml distilled water with different concentrations, were mixed with sodium phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1 %). The mixture was incubated at 50 °C for 20 min. At the end of the incubation, trichloroacetic acid (2.5 ml, 10 %) was added to the mixtures, followed by centrifuging (HERMEL Z * 200A, Germany) at 116.272 g for 10 min. The upper layer (2.5 ml) was mixed with distilled water (2.5 ml) and ferric chloride (0.5 ml, 0.1 %), and the absorbance was measured at 700 nm. As the absorbance of the reaction mixture increased, the reducing power also enhanced.
Determination of radical scavenging activity
Radical scavenging capacity was measured using DPPH (1,1-Diphenyl-2-picrylhydrazyl) method. This method described previously by Lima et al. (2006). The samples were reacted with the stable DPPH radical in a methanol solution. After a 30 min incubation period at room temperature under dark condition, the absorbance of the resulting solution was measured at 517 nm using a spectrophotometer (Shimadzu UV mini 1240, Duisburg, Germany). Inhibition of free radical DPPH in percent (I%) was calculated as following equation:
where A blank is the absorbance of the control reaction (containing all reagent except the test compound), and A sample is the absorbance of the test compound. The concentration of sample required to scavenge DPPH radical by 50 % value) was obtained by linear regression analysis of dose–response curve plotting between percent (%) inhibition and concentrations. BHA and BHT were used as control antioxidants.
Evaluation of total antioxidant capacity
An aliquot of 0.1 ml of R. fruticosus leaves extract sample solution containing a reducing species (in water, methanol or acetone) was combined in an Eppendorf tube with 1 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The tubes were capped and incubated in a thermal block at 95 °C for 90 min. After the samples had cooled to room temperature, the absorbance of the aqueous solution of each sample was measured at 695 nm against a blank (Prieto et al. 1999). Lipid-soluble and water-soluble antioxidant capacities were expressed as equivalents of α-tocopherol and ascorbic acid, respectively.
Fatty acid composition of sunflower oil
Fatty acid composition of the oil samples was determined by gas–liquid chromatography and was reported in relative area percentages. Fatty acid composition of the sunflower oil was transesterified into their corresponding FAME by vigorous shaking of a solution of oil in hexane (0.3 g in 7 mL) with 2 mL 7 N methanolic potassium hydroxide at 50–55 °C for 10 min.. The FAME were identified using an HP-5890 chromatograph (Hewlett-Packard, CA, USA) equipped with a CP-SIL 88 (Supelco, Bellefonte, PA, USA) capillary column of fused silica, 60 m in length 0.22 mm i.d., 0.2 mm film thickness, and a flame ionization detector (FID). Helium was used as carrier gas with a flow rate of 1 ml/min. The oven temperature was maintained at 195 °C, and that of the injector and the detector at 250 °C (Metcalf et al. 1996).
Calculated oxidizability value
The calculated oxidizability (Cox) value of the oils was calculated by the percentage of unsaturated C18 fatty acids, applying the formula proposed by Fatemi and Hammond (1980):
Preparation of oil samples
Refined, bleached and deodorized sunflower oil (5 g) containing different concentrations of R. fruticosus leaves extract (0, 200, 400, 600, 800 and 1,000 ppm) and 200 ppm of BHA and BHT as control antioxidants were stored in a 1-mm layer in a Petri dish with a diameter of 9 cm at 70 °C. Progress of oxidation was monitored by the determination of peroxide value and thiobarbituric acid (TBA) method.
Peroxide value(PV)
The PV of sunflower oil samples containing different concentration of R. fruticosus leaves extract and BHA and BHT were measured spectrophotometrically at 500 nm by UV–VIS instrument (model 160A Shimadzu, Tokyo, Japan). The oil samples were mixed in with 9.8 ml chloroform–methanol (7:3 v/v) on a vortex mixer for 2–4 s. Ammonium thiocyanate solution (50 ml, 30 % w/v) and 50 ml of iron (II) chloride solution ([0.4 g barium chloride dihydrate dissolved in 50 ml H2O] + [0.5 g FeSO4.7H2O dissolved in 50 ml H2O] + 2 ml 10 M HCl, with the precipitate, barium sulphate, filtered off to produce a clear solution]) were added, respectively and after adding each of them, the sample was mixed on a vortex mixer for 2–4 s. Then, the absorbance of the sample was read, after 5 min incubation at room temperature. Results were expressed in milliequivalents of oxygen per kilogram of oil (Shantha and Decker 1994).
Tiobarbituric acid test
Measurement of 2-tiobarbituric acid value was done by heating a 5 mL aliquot of a solution of sample (50–200 mg) in 25 mL 1-butanol with 5 mL TBA reagent at 95 °C for 120 min and reading the absorbance at 530 nm using distilled water As a the reference cuvette (AOCS 1998).
Rancimat test
Sunflower oil (3 g), was mixed separately with different concentrations of R. fruticosus leaves extract (0, 200, 400, 600, 800 and 1,000 ppm) and 200 ppm of BHA and BHT as control antioxidants and then exposed to the rancimat test by Metrohm Rancimat model 734 at 120 °C at an airflow of 20 L/h. Measuring vessels, electrodes, connecting tubes and glassware were cleaned several times before the experiments (Farhoosh and Tavassoli-Kafrani 2011).
Statistical analysis
All determinations were carried out in duplicate, and data were subjected to analysis of variance (ANOVA). ANOVA analyses were performed according to SAS software. Significant differences between means were determined by Duncan’s multiple range tests; p values less than 0.05 were considered statistically significant.
Results and discussion
Evolution of antioxidant activity of R. fruticosus leaves extracts
The yields and antioxidative activities of R. fruticosus leaves using different organic solvent are shown in Table 1. The results indicated that efficiency of the solvents for the extraction of R. fruticosus leaves was in the order of methanol (15.43 %) > water (11.87 %) > acetone (6.52 %). This is in agreement with the report of Economou et al. (1991) that methonal is a widely used and effective solvent for the extraction of antioxidants. It is considered that the phenolic compounds contribute to overall antioxidant activities of R. fruticosus leaves extract. Total phenolic content of R. fruticosus leaves extracts from different solvents were presented in Table 1. Among the three organic solvents, methonalic extracts exhibited the highest phenolic content (108.64 μg gallic acid/ ml extract) followed by aqueous (86.88(μg gallic acid/ ml extract) and acetonic extract (57.55(μg gallic acid/ ml extract). The results indicated that the methanolic extract was more selective to the phenolic compounds present in R. fruticosus leaves extract than other two solvents.
Table 1.
Yield and phenolic content of R. fruticosus leaves extract with various solvents
| Solvent | Yield (%) | Phenolic content (μg gallic acid/ ml extract) | Total antioxidant activity (nmol of α-tocopherol/g) | Reducing power (μg/ml) | IC50 (μg/ml) |
|---|---|---|---|---|---|
| Methanol | 15.43 ± 0.01a | 108.64 ± 0.33a | 508.61 ± 1.2a | 157.32 ± 0.13d | 65.043 ± 0.42d |
| Water | 11.87 ± 0.03b | 86.88 ± 0.24b | 352.88 ± 0.16c | 333.34 ± 0.17b | 120.064 ± 0.56b |
| Acetone | 6.52 ± 0.06c | 57.55 ± 0.65c | 192.53 ± 0.18d | 615.45 ± 1.2a | 230.217 ± 0.04a |
| BHA | – | – | 425.4 ± 0.15b | 214.3 ± 0.196c | 88.99 ± 0.16c |
| BHT | – | – | 177.4 ± 0.06e | 176.3 ± 0.2e | 41.61 ± 0.1e |
Means within a column with the same lowercase letters are not significantly different at p > 0.05
Therefore, we focused on the use of methanolic extracts from R. fruticosus leaves extract for the stabilization of sunflower oil. Total antioxidant activity of three solvents used for extraction of R. fruticosus leaves extract also indicated the methanol with 508.61 (nmol of α-tocopherol/g) act better than two other solvents with 352.016 (nmol of α-tocopherol/g) and 192.53 (nmol of α-tocopherol/g) for aqueous and acetone, respectively.
The reducting capacity of a compound may serve as a significant indicator of its potential antioxidant activity. The effect of different solvents on the reducing power of R. fruticosus leaves extract was compared to that BHA and BHT. In Table 1, it was shown that methanolic extract with 157.32 μg/ml was higher than aqueous (333.34 μg/ml) and acetonic (615.45 μg/ml). Also, the methanolic extract of R. fruticosus is higher than BHA with 214.303 μg/ml and BHT with 176.1 μg/ml. Indeed, the reducing properties are generally associated with the presence of reductones. It is reported that the antioxidant action of reductones is based on the breaking of the free radical chain by donating a hydrogen atom, or reacting with certain precursors of peroxide or prevent peroxide formation (Meir et al. 1995). The data shown in Table 1 indicate that the marked reducing power of R. fruticosus leaves extracts seem to be the result of their antioxidant activity. It is presumed that the phenolic compounds may act in a similar fashion as reductones by donating electrons and reacting with free radicals to convert them to more stable products and terminating the free radical chain reaction. The free-radical scavenging ability is a known mechanism for determining the antioxidant activity of antioxidants. When DPPH, encounters proton radical scavengers its purple color fades rapidly as a measurement of absorption at 517 nm. The half-inhibition concentration (IC50) can be calculated as the antioxidant concentration required for providing 50 % of DPPH scavenging ability (Gorden et al. 2001). In the present study, methanolic extract was found to be the most active antiradical agent (IC50 = 65.043 μg/ml), followed by aqueous extract (IC50 = 120.064 μg/ml) and acetone extract (IC50 = 230.217 μg/ml) (Table 1). So, the lower IC50 values indicated higher antioxidant activity in the mathanolic extract. However, the IC50 of BHA and BHT was 89.054 and 41.06 μg/ml, respectively.
Fatty acids composition of sunflower oil
Chemical characteristics of the sunflower oil used in this study are shown in Table 2. The sunflower oil was mainly constituted linoleic (55.1 %), oleic (27.4 %), palmitic (8.03 %), stearic (4.32 %), linolenic (2.79 %) and Arachidic acids (0.96 %). Among the fatty acids, the highest percentage of the saturated, monounsaturated and polyunsaturated fatty acids were palmitic acid, oleic acid and linoleic acid, respectively The fatty acid composition of sunflower oil indicated that the amount of Gadoleic acid (0.48 %) and myristic acid (0.13 %) are so low, as we expected from sunflower standard oil. The fatty acid composition of the sunflower oil use in this study was in agreement with data reported by Sharif et al. (2009). The PUFA/ SFA ratio of sunflower oil was 4.307 which shows the high nutrition value of sunflower oil in comparison with other edible oils. Moreover, the MUFA/PUFA ratio of sunflower oil was 0.481. This factor was a measure of oil tendency to oxidation. The higher amount of this factor show the more oxidative stablility of oil especially in frying process. Sunflower oil was perhaps the edible vegetable oil that was nutritionally well-balanced, based on a low content of SFA, a high content of MUFA, and a ratio of W6 and W3 PUFA. The content of linoleic acid in sunflower oil (55.1 %) was almost the same as soybean oil (57–60 %) and is higher than canola oil (22–25 %) (Akhman 1990). The high amounts of unsaturated fatty acids especially linoleic acid make sunflower oil as an important functional food because of its lower oxidation stability, antioxidants need to be added. Also, Cox value of sunflower oil was 6.55. Indeed, the PUFA/SFA ratio and COX value were usually taken as measures of tendency of oils to undergo autoxidation. Fresh sunflower oil contained trace amount of 16:1 and 18:3 fatty acids and moderate amount of oleic acid and its cox value was in the range of 6.2–7.25. These values reveal low resistant of sunflower during oxidation. So, mizing sunflower oil with different levels of antioxidant is led to an increase in its stability during oxidation (Sharif et al. 2009).
Table 2.
The fatty acid composition of sunflower oil
| Fatty acids (%) | Sunflower oil |
|---|---|
| Myristic acid (C14:0) | 0.13 ± 0.12 |
| Palmitic acid (C16:0) | 8.03 ± 0.83 |
| Stearic acid (C18:0) | 4.32 ± 0.04 |
| Oleic acid (C18:1) | 27.4 ± 0.62 |
| Linoleic acid (C18:2) | 55.1 ± 0.01 |
| α-Linolenic acid (C18:3) | 2.79 ± 0.01 |
| Gadoleic acid (C20:1) | 0.48 ± 0.02 |
| Arachidic acid (C22:0) | 0.96 ± 0.48 |
| Others | 0.79 ± 0.52 |
| Saturated fatty acids (SFA) | 13.44 ± 1.03 |
| Monounsaturated fatty acids (MUFA) | 27.88 ± 0.53 |
| Polyunsaturated fatty acids (PUFA) | 57.89 ± 0.01 |
| PUFA / SFA | 4.307 ± 0.07 |
| MUFA / PUFA | 0.481 ± 0.05 |
| USFA / SFA | 6.38 ± 0.01 |
| C18:2 / C18:3 | 19.75 ± 0.1 |
| Calculated oxidizability value (Cox value) | 6.55 ± 0.01 |
Oxidative stability of sunflower oil samples including R. fruticosus leaves extract
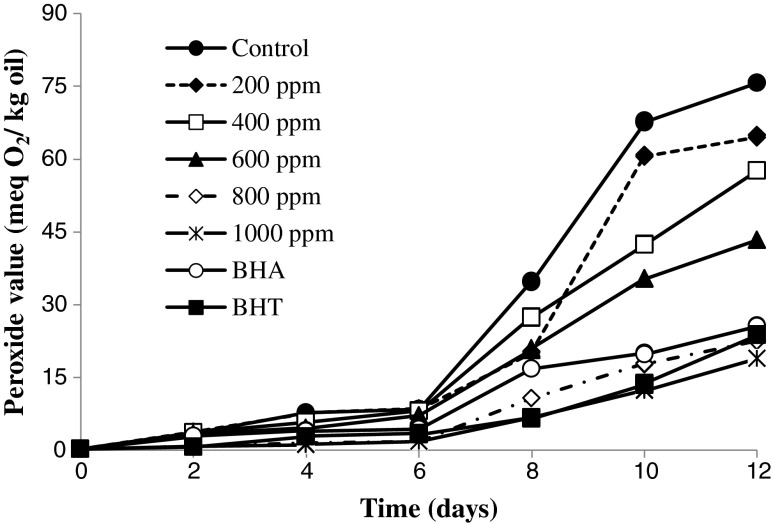
Figure 1 shows the PV changes of the sunflower oil samples including different concentrations of R. fruticosus leaves extract during heating process at 70 °C. Hydroperoxides are the primary products of lipid oxidation. It is well known that hydroperoxides have no undesirable flavour, whereas their decomposed products are mostly responsible for rancid off-flavour. PVs of sunflower oil samples increased from the beginning of the storage period to the last day, showing the progression of oxidation. For example the PV of control sample during 12 days changed from 0.231 meqO2/kg oil to 75.741 meqO2/kg oil. R. fruticosus leaves extract at all concentrations, controlled peroxide value appreciably, revealing good antioxidant efficacy in stabilization of sunflower oil. Moreover, as the concentration of the R. fruticosus leaves extract increased, the oxidation inhibition of the extract also enhanced. So that, The PVs of sunflower oil samples added 200 ppm R. fruticosus leaves extract which stored at 70 °C after 12 days, changed from 0.244 meqO2/kg oil to 64.885 meqO2/kg oil. However, the PVs of sunflower oil samples including 1,000 ppm of the extract reached 18.95 meqO2/kg oil. A regular increase in PV as a function of storage time was observed for all the samples at all intervals. Initially, the difference in peroxide content of control and stabilized sunflower oil samples was not noticeable. It became significant just after heating up to 6 days. After the 6th day, there was a tremendous rise in PV of control sample from 8.35 meqO2/kg oil to 75.74 meqO2/kg oil. However, PVs of sunflower oil samples including 1,000 ppm of the extract changed from 1.8 meqO2/kg oil at 6th day to 18.8 meqO2/kg oil at the end of 12 days. PV of sunflower oil samples including BHA and BHT were became almost equal to the sunflower oil including 1,000 ppm of the extract initially, but increase sharply to 25.54 meq O2/kg oil and 23.76 meqO2/kg oil at the end of 12 days, respectively suggesting greater stability of R. fruticosus extract than BHA and BHT. Moreover, the rate of hyrdoperoxide formation at sunflower oil samples including 1,000 ppm of extract was lower than the other concentration (200, 400, 600 and 800 ppm). The results calculated from the linear relationship between the peroxide value (PV) with the heating time for sunflower oil samples represented in Table 3. As can be seen in Table 3, the slope of the linear equations (a values) which were considered to be a measure to the rate of PV increase during heating process, was significantly different for the sunflower oil samples including different concentrations of R. fruticosus extract. Sunflower oil samples with no added R. fruticosus leaves extractshowed the lowest heating stability (a = 6.81), whereas, 200, 400, 600, 800 and 1,000 ppm of the extractshowed higher heating stability 5.707, 4.844, 3.74, 1.963 and 1.515, respectively. However, the slopes of oxidation curve in BHA and BHT were 2.194 and 1.792, respectively. This indicates that 1,000 ppm of the extract due to high stability and long term effectiveness can be act as same as synthetic antioxidants in retarding the sunflower oil oxidation. Indeed, all antioxidants remain effective during specific period and as the time passed, their effectiveness reduced and they finally become ineffective. Such antioxidants interrupt oil deterioration in the early stages and thus delay onset of the reaction and are found to be efficient only up to a specific period. It may be hypothesized that phenolic antioxidants inhibit lipid peroxidation at the cost of their own life and thus decompose and deteriorate themselves with the course of time. With these perspectives only those antioxidants are be preferred which have good effectiveness over longer periods and drastic conditions (Gertz et al. 2000)
Fig. 1.
Peroxide value of the sunflower oil as affected by the different concentrations of R. fruticosus leaves extract (0, 200, 400, 600, 800 and 1,000 ppm) and 200 ppm of BHT and BHA as control antioxidant during the storage period (12 days) at 70 °C
Table 3.
The results calculated from the linear relationship between the peroxide value (PV) and Thiobarbituric acid value (TBA) the storage time of sunflower oil (12 days) at 70 °C as affected by different concentrations of R. fruticosus leaves extract and 200 ppm of BHA and BHT as control antioxidants
| Antioxidant | PV = a (time) + b | TBA = a (time) + b | ||||
|---|---|---|---|---|---|---|
| aa | b | ΔPV | a | b | ΔTBA | |
| Control | 6.81 ± 0.004a | −12.62 ± 0.038h | 81.784 ± 0.05a | 0.014 ± 0.001a | −0.035 ± 0.004h | 0.5 ± 0.014a |
| 200 | 5.707 ± 0.009b | −10.56 ± 0.025g | 68.48 ± 0.11b | 0.035 ± 0.006b | −0.024 ± 0.001f | 0.364 ± 0.007b |
| 400 | 4.844 ± 0.001c | −8.33 ± 0.02f | 58.12 ± 0.018c | 0.03 ± 0.006c | −0.019 ± 0.001e | 0.32 ± 0.007c |
| 600 | 3.74 ± 0.0006d | −6.048 ± 0.01e | 44.87 ± 0.007d | 0.024 ± 0.001d | −0.014 ± 0.006d | 0.288 ± 0.001d |
| 800 | 1.963 ± 0.007f | −3.9 ± 0.022d | 23.556 ± 0.09f | 0.0129 ± 0.00f | −0.0003 ± 0.00b | 0.168 ± 0.001g |
| 1,000 | 1.515 ± 0.001h | −3.092 ± 0.002b | 18.17 ± 0.018h | 0.01 ± 0.0006h | 0.003 ± 0.002a | 0.128 ± 0.007h |
| BHA | 2.194 ± 0.005e | −2.66 ± 0.014a | 26.328 ± 0.06e | 0.023 ± 0.00e | −0.025 ± 0.00g | 0.276 ± 0.001e |
| BHT | 1.792 ± 0.008g | −3.45 ± 0.036c | 21.5 ± 0.1g | 0.017 ± 0.005g | −0.009 ± 0.001c | 0.208 ± 0.007f |
aMeans within a column with the same lowercase letters are not significantly different at p > 0.05. value of oxidation rate constant (a), intercept (b)
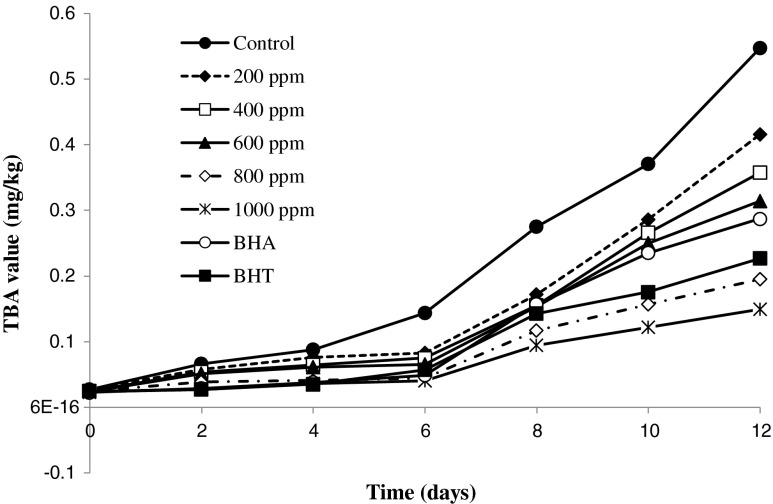
Although hyrdoperoxides are more stable than radical species, leading to secondary oxidation products including aldehydes, ketones, acids, alcohols, and lactones. The secondary products are responsible for impaired taste and flavor of oils. The activity of an antioxidant can be estimated by quantitatively determining primary or secondary products of oxidation (Sun et al. 2011). Generally, the delay in the hydroperoxides formation or reproduction of secondary products of autoxidation by chemical or sensory methods can be used to evaluate the efficacy of antioxidants. The 2-thiobarbituric acid (TBA) method is an old and most frequently used test for assessing the extent of lipid oxidation in oils. The basis of this method is reaction of two molecular of TBAs with unsaturated fatty acids result in red color which can be measured using a spectrophotometer. Other products of lipid oxidation, such as 2-alkenals and 2,4-alkadienals, also react with the TBA reagent (Ulu 2004). Indeed, when hydroperoxides break down, they produce aldehydes and carbonyls which may contribute to off-flavor of oxidized oils which measured by TBA test. TBA for all sunflower oil samples including R. fruticosus leaves extract were determined up to 12 days of storage under heating condition at 70 °C (Fig. 2). In general, TBA value went to increasing with the increase in storage time for all samples, but no regular pattern of increase could be observed. Control exhibited the highest TBA, while BHT and sunflower oil samples including 1,000 ppm of the extract exhibited the least. Initially, there was no increase in TBA value of stabilized samples but, after 6 days of storage period an increase was observed. The TBA value of sunflower oil samples including 1,000 ppm of R. fruticosus leaves extract changes from 0.023 to 0.149 mg/kg after 12 days of storage. However, the TBA value of control changed from 0.026 to 0.54 mg/ kg at the end of storage period. So, as we expected, the formation of secondary oxidation products at higher concentrations of R. fruticosus leaves extract reduced in comparison with control samples. So that the TBA values of sunflower oil samples including 1,000 ppm of the extract increases to540.4 %. However, for sunflower oil samples including 800, 600,400 and 200 ppm of the extract showed increases to 695.9, 1,220.59, 1,358.86 and 1,449.8 %, respectively. The changes of TBA value of BHA and BHT were 1,177.3 and 835.67 %, respectively. So, the higher concentrations of R. fruticosus leaves extract act better than synthetic antioxidants such as BHA and BHT in prevention of oil oxidation. The results calculated from the linear relationship between the TBA values and heating time for sunflower oil samples including R. fruticosus leaves extract are shown in Table 3. The a value of the samples including R. fruticosus leaves extract decreased at higher concentrations. So that in the sunflower oil samples including 1,000 to 200 ppm of the extract, the a value of TBA changed from 0.01 to 0.035, whereas the a value of BHA and BHT were 0.023 and 0.017, respectively. So the rate of secondary products in the sunflower oil samples including 800 and 1,000 ppm of the extract was lower than these two synthetic antioxidants. This indicates slightly higher antioxidant potential for R. fruticosus leaves extract even at high temperature condition. In general, based on the formation of primary and secondary lipid oxidation products, the oxidative stability of the sunflower oil samples including R. fruticosus is remarkable. Regard to the high oxidative stability of R. fruticosus leaves extract and also its high phenolic contents and nutritional value, it was expected that the addition of this extract to other vegetable oils would improve the oxidative stability.
Fig. 2.
TBA value of the sunflower oil as affected by the different concentrations of R. fruticosus leaves extract (0, 200, 400, 600, 800 and 1,000 ppm) and 200 ppm of BHT and BHA as control antioxidant during the storage period (12 days) at 70 °C
Oxidative stability index of sunflower oil samples with R. fruticosus leaves extract added
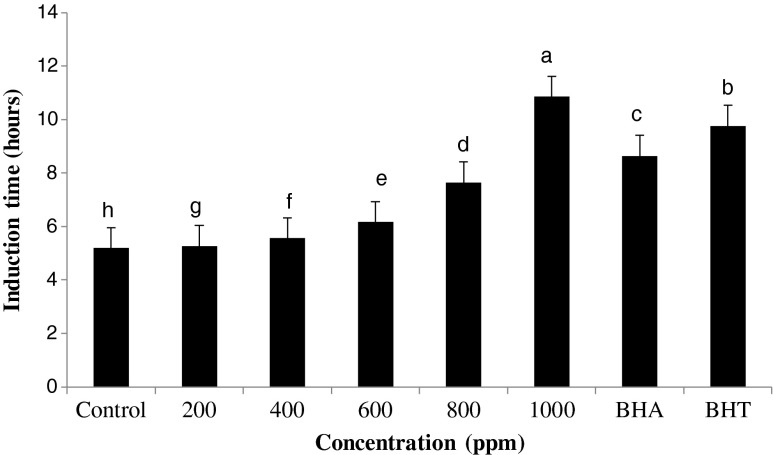
The main cause of deterioration of oils and fats is lipid oxidation. The degree of lipid oxidation can be measured by chemical and physical methods as well as stability tests, which measure the stability of oil under conditions that try to accelerate the normal oxidation. Rancimat test is an accelerated technique that has been designed to speed up the oxidation process by exposing oil samples to elevated temperatures in the presence of excess amounts of air or oxygen. Oxidative stability of edible oils as affected by the antioxidative additives has widely been measured by this method (Sun et al. 2011). It is obvious that those antioxidative compounds will effectively be able to prevent oxidative reactions under harsh conditions of Rancimat test that have good carry-through properties (resistance to be destroyed by heat and/or lost through volatilization) (Fennema 1996). Figure 3 shows the oxidative stability index (OSI) of the sunflower oil samples as affected by the different concentrations of R. fruticosus leaves extract added at 120 °C. The control sample of sunflower oil with no added R. fruticosus extract had the lowest oxidative stability (OSI 5.19 h), which was significantly promoted by adding the extract. All concentrations of the R. fruticosus leaves extract significantly improved the OSI of the sunflower oil samples. The highest stabilizing effect belonged to the 1,000 ppm of the extract (10.85 h), BHT (9.76 h) and BHA (8.63 h), followed by 800, 600, 400 and 200 of the extract (7.64, 6.162, 5.557 and 5.258 h, respectively). Indeed, Induction period (IP) provides direct evidence for trends in resistance to oxidative rancidity of edible oils. Antioxidant activity index (AAI) is a recommended criterion for evaluation of effectiveness of antioxidant and is determined as the ratio of IP of stabilized sample to that of control. Indeed, AAI indicates the possibility of blocking the chain radical process in interaction with radicals, which is responsible for the duration of induction period (Anwar et al. 2006). The AAI value of samples were 1.013, 1.07, 1.187, 1.47 and 2.09 for 200, 400, 600, 800 and 1,000 ppm of R. fruticosus extract, respectively suggesting an appreciable effectiveness of R. fruticousus extracts, at all concentrations, on oxidative stability of sunflower oil. And, as the concentration of R. fruticosus extract increased, the AAI value also enhanced. The best concentration of R. fruticocus extract showed higher stabilization factor to both BHA (1.66) and BHT (1.88). This can be indicated the high potent of natural of R. fruticosus extract as natural antioxidant for stabilization of various lipid system.
Fig. 3.
oxidative stability index (OSI, Rancimat test) of sunflower oil as affected by different concentrations of R. fruticosus leaves extract (200, 400,600, 800 and 1,000 ppm), BHA (200 ppm) and BHT (200 ppm) at 120 °C and airflow rate of 15 l/h. Means ± SD(standard deviation) with the same lowercase letters are not significantly different at P > 0.05
Conclusion
From the present study, it is concluded that R. fruticosus leaves extract can stabilized sunflower oil up to a greater extent than commonly employed by synthetic antioxidants. It inhibits thermal deterioration of oil oxidation and losses of polyunsaturated fatty acids of sunflower oil due to carry through property and high content of phenolic compounds. R. fruticosus leaves extract not only is good source of natural antioxidant due to remarkably high scavenging activity toward chemically generated superoxide radicals that can be used as alternative of synthetic antioxidant, but also high thermal stability of the extract shows an added advantage at high processing temperatures, contrary to synthetic antioxidants.
Contributor Information
Maryam Asnaashari, Phone: +989151081224, Email: ma.asnaashari@stu.um.ac.ir.
Raheleh Tajik, Email: rtajik.8@gmail.com.
Mohammad Hossein Haddad Khodaparast, Email: dr.m.haddad@gmail.com.
References
- Akhman RC. Canola fatty acids -an ideal mixture for health, nutrition and food use. In: Shahidi F, editor. Canola and rapeseed production, chemistry, nutrition and processing technology. New York: Van Nostrand reinhold; 1990. pp. 81–98. [Google Scholar]
- Alice LA, Campbell CS. Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Am J Bot. 1999;86:81–97. doi: 10.2307/2656957. [DOI] [PubMed] [Google Scholar]
- Anwar F, Jamil A, Iqbal S, Sheikh MA. Antioxidant activity of some plant extracts for edible oils at ambiet and accelerated conditions. Grasasy Aceties. 2006;57:189–197. [Google Scholar]
- AOCS . 5th ed Methods Cd 8–53, Cd 18–90, Official methods and recommended practices. IL: S Champaign; 1998. [Google Scholar]
- Asnaashari M, Farhoosh R, Sharif A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem. 2014;159:439–444. doi: 10.1016/j.foodchem.2014.03.038. [DOI] [PubMed] [Google Scholar]
- Chang LW, Yen WJ, Hung SC, Duh PD. Antioxidant activity of sesame coat. Food Chem. 2002;78:347–354. doi: 10.1016/S0308-8146(02)00119-X. [DOI] [Google Scholar]
- Economou KD, Oreopoulou V, Thomopoulos J. Antioxidant activity of some plant extracts of the family Labiatae. J AOCS. 1991;68:109–113. [Google Scholar]
- Eshghi N, Asnaashari M, Haddad Khodaparast MH, Hosseini F. Evaluating of potential of natural curcumin for oxidative stability of soybean oil. J Nat Prod Res. 2014 doi: 10.1080/14786419.2014.901319. [DOI] [PubMed] [Google Scholar]
- Farhoosh R, Tavassoli-Kafrani MH. Simulataneous monitoring of the conventional qualitative indicators during frying of sunflower oil. Food Chem. 2011;125:209–213. doi: 10.1016/j.foodchem.2010.08.064. [DOI] [Google Scholar]
- Fatemi SH, Hammond EG. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids. 1980;15:379–385. doi: 10.1007/BF02533555. [DOI] [Google Scholar]
- Fennema OR. Food chemistry. New York: Marcel Dekker Inc; 1996. [Google Scholar]
- Gertz C, Klostermann S, Kochhar SP. Testing and comparing oxidative stability of vegetable oil and fats at frying temperature. Eur J Lipid Sci Technol. 2000;102:543–551. doi: 10.1002/1438-9312(200009)102:8/9<543::AID-EJLT543>3.0.CO;2-V. [DOI] [Google Scholar]
- Gorden MH, Paivia-Martins F, Almeida M. Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J Agric Food Chem. 2001;49:2480–2485. doi: 10.1021/jf000537w. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Bhanger MI. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007;100:246–254. doi: 10.1016/j.foodchem.2005.09.049. [DOI] [Google Scholar]
- Iqbal S, Bhanger MI, Anwar F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005;93:265–272. doi: 10.1016/j.foodchem.2004.09.024. [DOI] [Google Scholar]
- Katalinic V, Milos M, Kulisic T, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. doi: 10.1016/j.foodchem.2004.12.004. [DOI] [Google Scholar]
- Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Phenolic compounds protect HepG2 cells from oxidative damage: relevance of glutathione levels. Life Sci. 2006;79:2056–2068. doi: 10.1016/j.lfs.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Marinova EM, Yanishlieva NV. Inhibited oxidation of lipids. II. Comparison of the antioxidative properties of some hydroxy derivatives of benzoic and cinnamic acids. Fat Sci Technol. 1992;94:428–432. [Google Scholar]
- Meir S, Kenner J, Akiri B, Hadas SP. Deterrmination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem. 1995;43:1813–1815. doi: 10.1021/jf00055a012. [DOI] [Google Scholar]
- Metcalf LC, Schmitz AF, Pcllca JR. Rapid prepration of methyl esters from lipid for chromatography analysis. Anal Chem. 1996;38:514–415. doi: 10.1021/ac60235a044. [DOI] [Google Scholar]
- Patel AV, Rojas-Vera J, Dacke CG. Therapeutic constituents and actions of Rubus species. Curr Med Chem. 2004;11:1501–1512. doi: 10.2174/0929867043365143. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Rojas-Vera J, Patel AV, Dake CG. Relaxant activity of raspberry (Rubus ideaus) leaf extract in guinea-pig ileum in vitro. Phytother Res. 2002;16:665–668. doi: 10.1002/ptr.1040. [DOI] [PubMed] [Google Scholar]
- Shantha NC, Decker EA. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J AOAC Int. 1994;77:421–424. [PubMed] [Google Scholar]
- Sharif A, Farhoosh R, Tavassoli Kafrani MH. Antioxidant activity of bene hull oil compared with sesame and rice bran oils during the frying process of sunflower oil. J Food Lipids. 2009;16:394–406. doi: 10.1111/j.1745-4522.2009.01154.x. [DOI] [Google Scholar]
- Simpson M, Parsons M, Greenwood J, Wade K. Raspberry leaf in pregnancy: its safety and efficacy in labor. J Mildwifery Womens Health. 2001;46:51–59. doi: 10.1016/S1526-9523(01)00095-2. [DOI] [PubMed] [Google Scholar]
- Suja KP, Abraham JT, Thamizh SN, Jayalekshmy A, Arumughan C. Antioxidant efficacy of sesame cake extract in vegetable oil protection. Food Chem. 2004;84:393–400. doi: 10.1016/S0308-8146(03)00248-6. [DOI] [Google Scholar]
- Sun Y, Wang W, Chen H, Li C. Autioxidation of unsaturated lipids in food emulsion. Food Sci Nutr. 2011;51:453–466. doi: 10.1080/10408391003672086. [DOI] [PubMed] [Google Scholar]
- Ulu H. Evaluation of three 2-thiobarbituric acid methods for the measurement of lipid oxidation in various meats and meat products. Meat Sci. 2004;67:683–687. doi: 10.1016/j.meatsci.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, Raspberry, and Strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000;48:140–146. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- Yao H, Qing L. Antioxident activites of barley seeds extracts. Food Chem. 2007;102:732–737. doi: 10.1016/j.foodchem.2006.06.051. [DOI] [Google Scholar]