Abstract
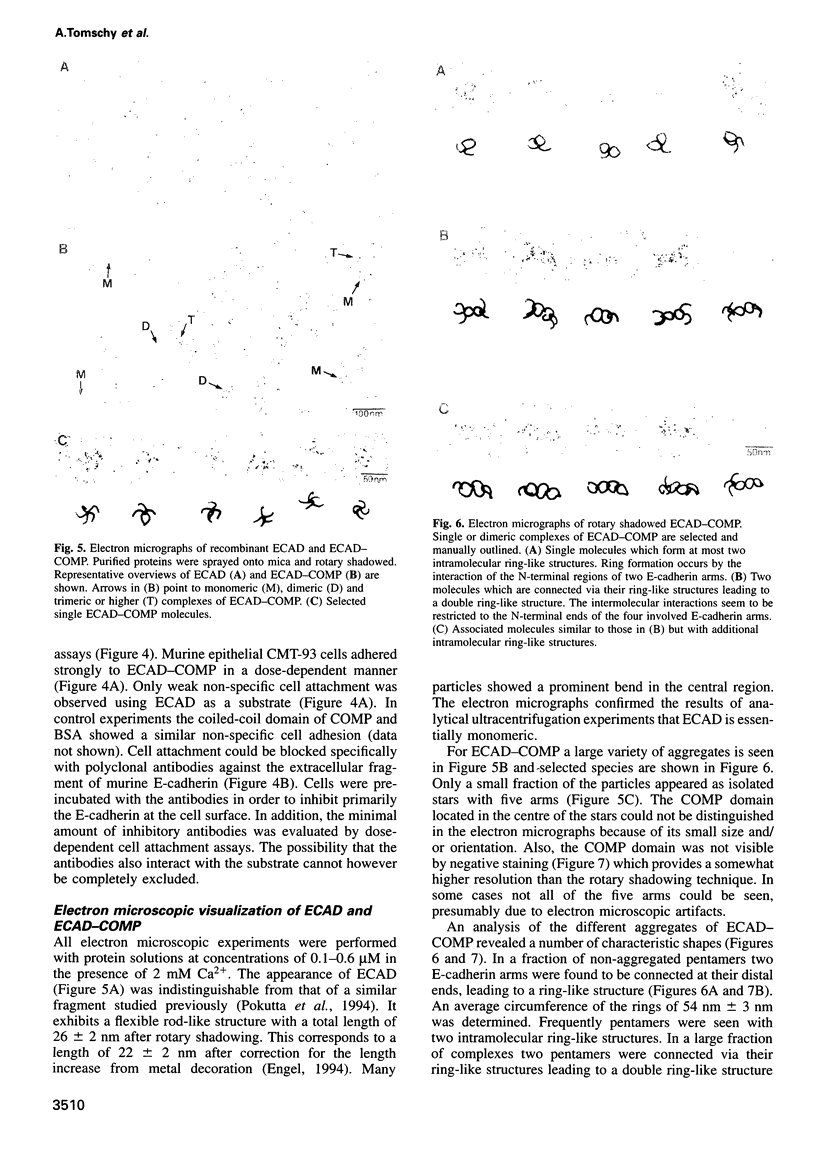
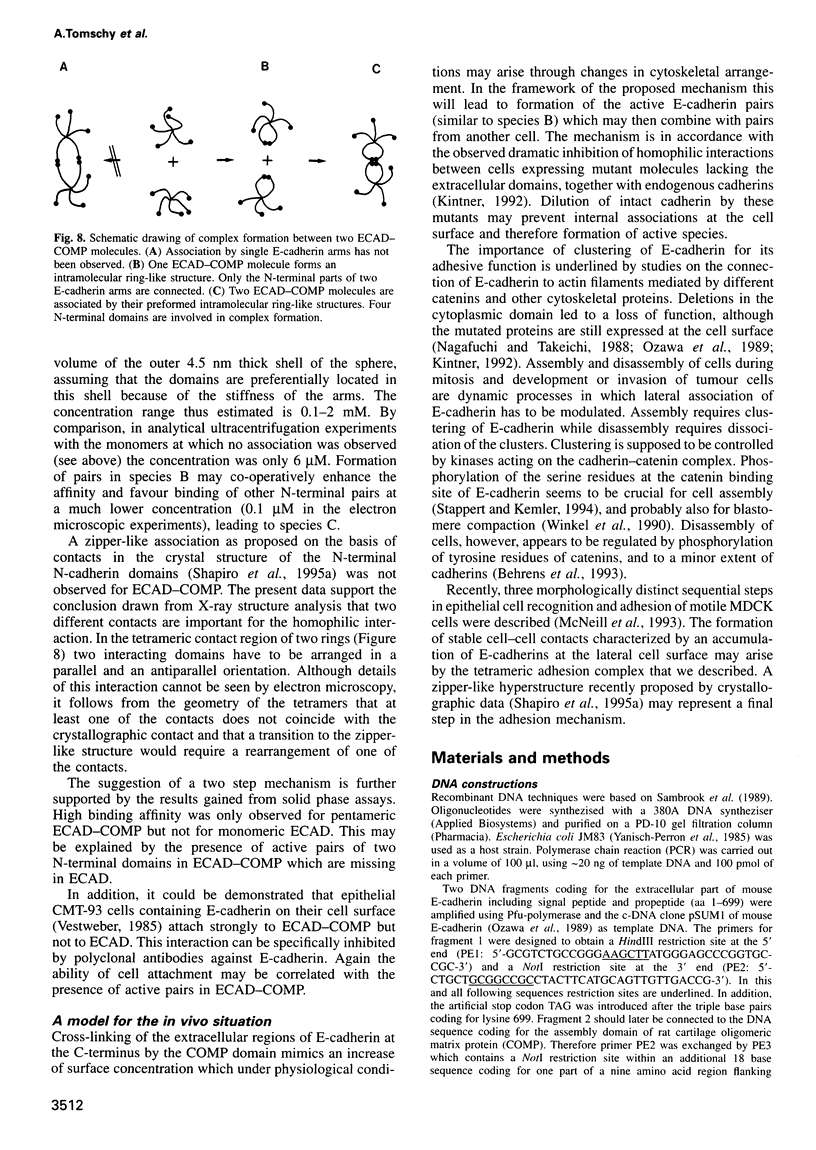
Cluster formation of E-cadherin on the cell surface is believed to be of major importance for cell-cell adhesion. To mimic this process the extracellular part of mouse E-cadherin (ECAD) was recombinantly fused to the assembly domain of rat cartilage oligomeric matrix protein (COMP), resulting in the chimeric protein ECAD-COMP. The COMP domain formed a five-stranded alpha-helical coiled-coil. This enabled the formation of a pentameric ECAD with bundled C-termini and free N-termini. The pentameric protein construct ECAD-COMP and the monomeric ECAD were expressed in human embryonal kidney 293 cells. Electron microscopy, analytical ultracentrifugation, solid phase binding and cell attachment assays revealed that pentamers showed strong self-association and cell attachment, whereas monomers exhibited no activity. At the high internal concentration in the pentamer the N-terminal EC1 domains of two E-cadherin arms interact to form a ring-like structure. Then the paired domains interact with a corresponding pair from another pentamer. None of the four other extracellular domains of E-cadherin is involved in this interaction. Based on these results, an in vivo mechanism is proposed whereby two N-terminal domains of neighbouring E-cadherins at the cell surface first form a pair, which binds with high affinity to a similar complex on another cell. The strong dependence of homophilic interactions on C-terminal clustering points towards a regulation of E-cadherin mediated cell-cell adhesion via lateral association.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberle H., Butz S., Stappert J., Weissig H., Kemler R., Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994 Dec;107(Pt 12):3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- Behrens J., Vakaet L., Friis R., Winterhager E., Van Roy F., Mareel M. M., Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993 Feb;120(3):757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W., Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994 May 27;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Efimov V. P., Lustig A., Engel J. The thrombospondin-like chains of cartilage oligomeric matrix protein are assembled by a five-stranded alpha-helical bundle between residues 20 and 83. FEBS Lett. 1994 Mar 14;341(1):54–58. doi: 10.1016/0014-5793(94)80239-4. [DOI] [PubMed] [Google Scholar]
- Engel J. Electron microscopy of extracellular matrix components. Methods Enzymol. 1994;245:469–488. doi: 10.1016/0076-6879(94)45024-2. [DOI] [PubMed] [Google Scholar]
- Herrenknecht K., Kemler R. Characterization of recombinant E-cadherin (uvomorulin) expressed in insect cells. J Cell Sci Suppl. 1993;17:147–154. doi: 10.1242/jcs.1993.supplement_17.21. [DOI] [PubMed] [Google Scholar]
- Hyafil F., Morello D., Babinet C., Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980 Oct;21(3):927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993 Sep;9(9):317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992 Apr 17;69(2):225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Larue L., Ohsugi M., Hirchenhain J., Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Duquette M., Urry L., McHenry K., Smith T. F. The evolution of the thrombospondin gene family. J Mol Evol. 1993 Jun;36(6):509–516. doi: 10.1007/BF00556355. [DOI] [PubMed] [Google Scholar]
- McNeill H., Ryan T. A., Smith S. J., Nelson W. J. Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion. J Cell Biol. 1993 Mar;120(5):1217–1226. doi: 10.1083/jcb.120.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A. P., Askari J. A., Craig S. E., Garratt A. N., Clements J., Humphries M. J. Integrin alpha 4 beta 1-mediated melanoma cell adhesion and migration on vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced IIICS region of fibronectin. J Biol Chem. 1994 Nov 4;269(44):27224–27230. [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Tsuji K., Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990 Apr 6;61(1):147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Overduin M., Harvey T. S., Bagby S., Tong K. I., Yau P., Takeichi M., Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science. 1995 Jan 20;267(5196):386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Hoschützky H., Herrenknecht K., Kemler R. A possible new adhesive site in the cell-adhesion molecule uvomorulin. Mech Dev. 1990 Dec;33(1):49–56. doi: 10.1016/0925-4773(90)90134-8. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyriéras N., Hyafil F., Louvard D., Ploegh H. L., Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S., Herrenknecht K., Kemler R., Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994 Aug 1;223(3):1019–1026. doi: 10.1111/j.1432-1033.1994.tb19080.x. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Morrow J. S. Molecular cloning of human E-cadherin suggests a novel subdivision of the cadherin superfamily. Biochem Biophys Res Commun. 1994 May 16;200(3):1754–1761. doi: 10.1006/bbrc.1994.1656. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Fannon A. M., Kwong P. D., Thompson A., Lehmann M. S., Grübel G., Legrand J. F., Als-Nielsen J., Colman D. R., Hendrickson W. A. Structural basis of cell-cell adhesion by cadherins. Nature. 1995 Mar 23;374(6520):327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Kwong P. D., Fannon A. M., Colman D. R., Hendrickson W. A. Considerations on the folding topology and evolutionary origin of cadherin domains. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6793–6797. doi: 10.1073/pnas.92.15.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappert J., Kemler R. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes Commun. 1994 Aug;2(4):319–327. doi: 10.3109/15419069409014207. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Gossler A., Boller K., Kemler R. Expression and distribution of cell adhesion molecule uvomorulin in mouse preimplantation embryos. Dev Biol. 1987 Dec;124(2):451–456. doi: 10.1016/0012-1606(87)90498-2. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Kemler R. Identification of a putative cell adhesion domain of uvomorulin. EMBO J. 1985 Dec 16;4(13A):3393–3398. doi: 10.1002/j.1460-2075.1985.tb04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Kemler R. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp Cell Res. 1984 May;152(1):169–178. doi: 10.1016/0014-4827(84)90241-6. [DOI] [PubMed] [Google Scholar]
- Winkel G. K., Ferguson J. E., Takeichi M., Nuccitelli R. Activation of protein kinase C triggers premature compaction in the four-cell stage mouse embryo. Dev Biol. 1990 Mar;138(1):1–15. doi: 10.1016/0012-1606(90)90171-e. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]