Abstract
In this work, the influence of sucrose and fructose on the gel-forming capacity of kefiran was investigated as well as the physicochemical characteristics of the resulting gels. The addition of sugar to gel-forming solutions did not alter the pseudoplastic flow properties of kefiran solutions and after one freeze-thaw cycle translucent gels with high water-holding capability were obtained. A highly porous matrix was revealed by microscopy whose pore size varied with sugar concentration. Sucrose and fructose had different effects on the rheological characteristics of sugar-kefiran gels. An increment in the strength of the gels with progressive concentrations of sucrose was evidenced by an increase in the elastic modulus (G’), indicating that sucrose reinforces the binding interactions between the polymer molecules (p ≤ 0.05). A drastic reduction in elastic modulus occurred, however, when 50.0 % w/w sucrose was added to kefiran gels, resulting in less elasticity. In contrast, when fructose was added to kefiran gels, elastic modulus decreased slightly with progressive sugar concentrations up to 10 %, thereafter increasing up to 50 % (p ≤ 0.05). Supplementation with up to 30 % sugar contributed to water retention and increased the viscous modulus. The relative increment in the elastic and viscous moduli elevated the loss tangent (tanδ) depending on the type and concentration of sugar. Sugars (sucrose, fructose) present in the matrix of the polysaccharide networks modified water-polymer and polymer-polymer interactions and consequently changed the gels’ physicochemical characteristics, thus allowing the possibility of selecting the appropriate formulation through tailor-made kefiran cryogels.
Keywords: Kefiran, Exopolysaccharide, Kefir, Gels, Rheological properties
Introduction
In recent decades the inclusion of polysaccharides as a functional and/or bioactive ingredient has increased as an effective way to enhance the nutritional and health promoting features of commercial products. In addition, a polysaccharide component promotes the functionality of foods by influencing the rheological properties of the final product acting as thickening additives, gelling agents, or stabilizers for dispersions and solutions. Polysaccharides that have the ability to form gels, alone or in mixtures, offer a great potential for replacing unfavorable food ingredients that do not conform well to nutritional and health claims (Frøst and Janhøj 2007; Warrand 2006). A detailed knowledge of the gelling conditions is essential in order to fulfil the popular demands with respect to the functional and physicochemical characteristics of a gelled food (Ruas-Madiedo et al. 2008).
Kefiran is a water-soluble polysaccharide produced by the lactic-acid bacteria that conform the kefir grain (Maeda et al. 2004; Micheli et al. 1999; Rimada and Abraham 2001). It is composed of glucose and galactose and is not hydrolyzed by human digestive enzymes (Micheli et al. 1999). Kefiran has been reported to have biologic activities that are beneficial to human health (Abraham et al. 2010; Medrano et al. 2008, 2011).
The use of kefiran as a hydrocolloid in foods has been proposed upon consideration of the polysaccharide’s rheological attributes (Abraham et al. 2010). Kefiran can increase the viscosity of aqueous solutions and acidic milk gels (Rimada and Abraham 2006) and can form cryogels by freezing in dilute solution (0.5–2, w/v) (Piermaria et al. 2008) or in the presence of alcohol (Mukai et al. 1991). Cryogels are matrices with interconnected macropores or supermacropores that are formed from solutions of polymeric precursors (Lozinsky et al. 2003). The parameters affecting the final properties of gels prepared by sequential freeze-thaw cycling include the physical-chemical characteristics of the starting materials—e. g., the molecular size and concentration of the polymer—plus those variables that relate to the processing steps—e. g., the number of freeze-thaw cycles, the temperatures, and the number of times along with rate of both the freezing and the thawing (Lazaridou and Biliaderis 2004). In addition, the presence of cosolutes further determines the mechanism of gelation and the final mechanical properties of the gelled polymers (Lazaridou and Biliaderis 2007).
Sugars of low molecular weight are common additives or ingredients in a wide variety of gelled foods such as desserts, candy gums, jam. Along with their sweetening effect, these saccharides modify the physical properties of the food system. Sucrose is the most common sweetener used in foods it contributes to the physical and functional attributes of foods and beverages—such as flavor enhancement, humectancy, freezing-point depression, and osmotic stability—apart from sweetness (Davis 1995). Fructose is commonly used in food to reduce energy load on account of its high sweetening power. The combination of a gelling agent with other food components can change the physicochemical properties of the resulting gels and enlarge its application range (Russ et al. 2013). Sugars can change the conformational ordering and intermolecular interaction of polymers and affects the physical and mechanical properties of food gels as was described in agarose, starch, and ß-glucan or galactomannan gels (Doyle et al. 2006; Evageliou et al. 2000a; Lazaridou et al. 2008; Maurer et al. 2012).
Because of both the health-promoting properties of kefiran and the capability of this polysaccharide to form cryogels, kefiran becomes a promising matrix for developing foods with enhanced functional quality. Moreover, the cryogelation potential of kefiran has not been explored in the presence of sugars. In this study, we therefore investigated the effect of incorporating fructose and sucrose in kefiran solutions on the subsequent gel-network formation during freeze-thaw gelation and evaluated the rheological and water-holding properties of the resulting cryogels.
Materials and methods
Isolation of kefiran
The isolation and purification of kefiran was performed as previously reported by Rimada and Abraham (2006) from kefir grains belonging to the CIDCA culture collection and originally obtained from a household at La Plata, Argentina. Stated in brief, a weighed amount of CIDCA AGK1 kefir grains obtained by successive subcultures in low-fat milk (Sancor, Santa Fe, Argentina) was treated in boiling water (1:10 [w/v]) for 30 min with discontinuous stirring. The mixture was centrifuged (Avanti J25 Beckman Coulter Inc. centrifuge, Palo Alto, CA, USA) at 10,000 g for 20 min at 20 °C and the polysaccharide in the supernatant precipitated by the addition of two volumes of ice-cold ethanol. The mixture was left overnight at −20 °C then centrifuged at 10,000 g for 20 min at 4 °C. The pellet was dissolved in hot water and the precipitation and centrifugation repeated twice. The final precipitate was dissolved in hot distilled water to give the working kefiran solution. The polysaccharide concentration was determined by the anthrone method, involving a measurement of absorbance at 620 nm with glucose solutions as standards. All the samples were tested for the absence of other sugars by qualitative thin-layer chromatography. The concentrations of the proteins in kefiran solutions were determined by the Bradford method (Bradford 1976). Kefiran solutions with 99.9 % purity were lyophilized in a Heto FD4 (Lab Equipment, Denmark) and the polysaccharide obtained stored at 4 °C in hermetic bottles until use.
Preparation and rheological measurement of kefiran solutions
Water dispersions containing 2 % (All percent concentrations, both here and hereafter, are with respect to w/w.) kefiran and 0, 5.9, 10.0, 17.6, 30.0, and 50.0 % fructose or sucrose (reagent-grade) were prepared and then heated to 90 °C with continuous stirring until complete solubilization.
Rotational analysis of the solutions was performed in a Haake ReoStress 600 rheometer (Thermo Haake, Karlsruhe, Germany) with a 1-mm–gap in a plate-plate sensor system PP35. During testing the solutions were maintained at 20 °C by a circulating water bath (Circulator DC50 Thermo Haake) connected to the jacket surrounding the sensor system. Shear stress was determined as a function of the shear rate. The viscometer was programmed so that the rotor speed increased from 0 to 500 s−1 in 2 min, maintained 1 min at the maximum speed, and then decreased from 500 to 0 s−1 in 2 min. The apparent viscosity (ηapp) was calculated from the ascending curves at 300 s−1. The flow (n) and consistency (K) index were determined by adjusting the experimental results according to the Ostwald Waele models:
where τ is the shear stress (Pa), K is the consistency index (Pa.sn), is the shear rate (s−1), and n is the flow index (dimensionless).
Preparation of gels
Aliquots of kefiran, kefiran-sucrose or kefiran-fructose solutions were placed in cylindrical plastic containers of internal diameter 35 mm and closed with a plastic film. Then, aliquots were placed immediately in a freezer at −20 °C for 24 h and transferred to a refrigerator at 4 °C for a further 24 h (Lozinsky et al. 2003; Piermaria et al. 2008).
Gel characterization
Water activity (aw)
Water activity of the gels was evaluated with an AquaLab™ water-activity meter (Decagon Devices, Inc., Washington).
Scanning electron microscopy
Gels were cryofixed in liquid nitrogen (−196 °C) and immediately freeze-dried in a FD4 Heto lyophilizer (Lab Equipment, Denmark). The samples were coated with a 15- to 20-nm-thick layer of gold through the use of an ionic model SCD 030 sputter (Balzers, Liechtenstein) and then observed in a Philips 505 scanning electron microscope (Eindhoven, Netherlands) at an accelerated voltage of 20 kV. The mean pore diameter was calculated through an analysis of the photographs by means of the ImageJ processing software (Schneider, Rasband, and Eliceiri 2012).
Water-holding capacity (WHC)
Weighed gel samples were centrifuged at 3,000 g for 5 min at 20 °C. After removal of the supernatant by pipetting, the tubes were inverted to drain for 15 min. WHC (%) was defined as 100 times a pellet’s weight divided by the original gel’s weight (Wu et al. 2009).
Dynamic rheological measurements
Small-deformation oscillatory measurements were carried out in the controlled stress RheoStress 600 rheometer with a serrated plate-and-plate geometry (35-mm diameter, 1-mm gap). Samples were removed with as little disturbance as possible from a vessel and placed onto the bottom plate of the rheometer. Low-viscosity silicone oil was applied to the edges to prevent evaporation. The linear viscoelasticity region was determined through a stress-sweep test at a fixed frequency (1 Hz) at temperatures of 5, 20, and 40 °C. Isothermal assays were performed at 20 °C with the temperature of the plates maintained by a circulating water bath (DC50, Haake). The elastic (G’) and viscous (G”) moduli and the loss tangent (tanδ) were evaluated as a function of frequency (between 0.1 and 10 Hz) at a stress of 0.1 Pa. Temperature sweeps were also performed and G’, G” and tanδ were measured at a fixed frequency of 1 Hz as a function of the temperature between 5 and 50 °C. All measurements were performed within the linear viscoelastic range.
Differential scanning calorimetry
A Q100, TA Instrument-Waters LLC (New Castle, USA) calorimeter was used. The samples were placed in previously weighed aluminum pans and the pans sealed and reweighed. The scanning temperature and heating rates were 4–100 °C and 10 °C/min, respectively. An empty pan was used as reference for all measurements. After scanning all the pans were perforated and dried to constant weight in an oven at 105 ± 1 °C to obtain the sample dry weight. In the thermograms the transition temperatures and enthalpy of transition (ΔH) were obtained by means of the TA Universal Analysis 2000 software.
Texture profile analysis
Cilindric gels of 20 mm length and 30 mm diameter were equilibrated to ambient temperature and subjected to an instrumental texture profile analysis (TPA) in a TA.XT2 Texture Analyzer (Stable Micro Systems, Surrey, UK). The gels were compressed twice at 0.5 mm/s to 30 % of their original height. Textural parameters such as hardness (the peak force during the first compression cycle), cohesiveness (the ratio of the area under the first and second compression), adhesiveness (the negative area from peak between first and second compression) and springiness (the distance compressed during the second compression to the peak force, divided by the initial sample height, reported as a percentage) were considered.
Statistical analysis
Differences in the properties and parameters were statistically tested by means of the Analysis of Variance (ANOVA) and by Fisher’s least-significant-difference (LSD) mean-discrimination test, at a p ≤ 0.05 as the threshold level of significance (SYSTAT software, version 10.0). All experiments were performed in at least triplicate.
Results
Rheological evaluation of kefiran solutions containing sucrose or fructose
The gel-forming solutions exhibited flow curves corresponding to a shear-thinning behavior with no hysteresis area. The Ostwald de Waele model fitted the experimental data at a correlation coefficient of more than 0.999 in all trials. The flow indices (n) were all less than 1—as was expected for the polymer concentration used in the solutions—and decreased when the sugar concentration increased, giving values of 0.6818 for the kefiran solution without sugars and 0.4595 and 0.4797 for solutions supplemented with 50.0 % sucrose or fructose, respectively. A significant increment in the apparent viscosity at 300 s−1 (ηapp 300 s−1) was observed with increased concentrations of either sugar (Table 1).
Table 1.
Apparent viscosities at 300 s−1 (ηapp 300 s-1), consistency index (K), flow index (n) and correlation coefficient (r2), obtained for experimental data fitted to Ostwald-de-Waele model, as a function of sugar (sucrose or fructose) concentration in gel forming solutions. Measurements were performed at 20 °C
| Sugar | Concentration % wt | K * (Pa.s-n) | n * | r2 | ηapp 300 s−1 *(mPa.s) |
|---|---|---|---|---|---|
| 0 | 0.2845 ± 0.0221 a | 0.6818 ± 0.0110 a | 0,9996 | 46.59 ± 0,62 a | |
| sucrose | 5.9 | 0.4745 ± 0.0351 a | 0.6268 ± 0.0048 a, b, c | 0.9994 | 56.96 ± 5.81 a, b |
| 10.0 | 0.5711 ± 0.0171 a | 0.6188 ± 0.0017 a, b | 0.9995 | 65.40 ± 1.30 b, c | |
| 17.6 | 0.7541 ± 0.1003 a | 0.5967 ± 0.0055 b, c, d | 0.9995 | 75.96 ± 7.81 c, d | |
| 30.0 | 1.5615 ± 0.1874 a | 0.5439 ± 0.0018 d, e | 0.9996 | 116.10 ± 12.87 e | |
| 50.0 | 9.0800 ± 2.9400 b | 0.4595 ± 0.1318 e, f | 0.9993 | 612.85 ± 38.82 g | |
| fructose | 5.9 | 0.4875 ± 0.0025 a | 0.6325 ± 0.0018 a, b | 0.9995 | 60.34 ± 0.27 a, b, c |
| 10.0 | 0.5155 ± 0.0369 a | 0.6245 ± 0.0088 b, c | 0.9995 | 60.91 ± 1.21 a, b, c | |
| 17.6 | 0.6882 ± 0.0195 a | 0.6134 ± 0.0049 b, c | 0.9995 | 76.46 ± 4.35 c, d | |
| 30.0 | 1.0260 ± 0.0198 a | 0.5741 ± 0.0088 c, d | 0.9996 | 90.74 ± 2.64 d | |
| 50.0 | 4.9235 ± 0.9991 a | 0.4797 ± 0.0474 f | 0.9994 | 249.30 ± 16.97 f |
* Different letters in the same column indicate a significant difference (p ≤ 0.05)
Gel characterizations
The inclusion of sucrose or fructose in kefiran solutions did not hinder gel formation: on the contrary, after a single freeze-thaw cycle gelatinous, translucent, and self-supporting gels were obtained from all the solutions analyzed. Kefiran gels had aw values at 20 °C close to 1, with the water activity (aw) of the gel containing the highest sucrose or fructose concentrations being 0.9465 ± 0.0025 and 0.9245 ± 0.0005, respectively.
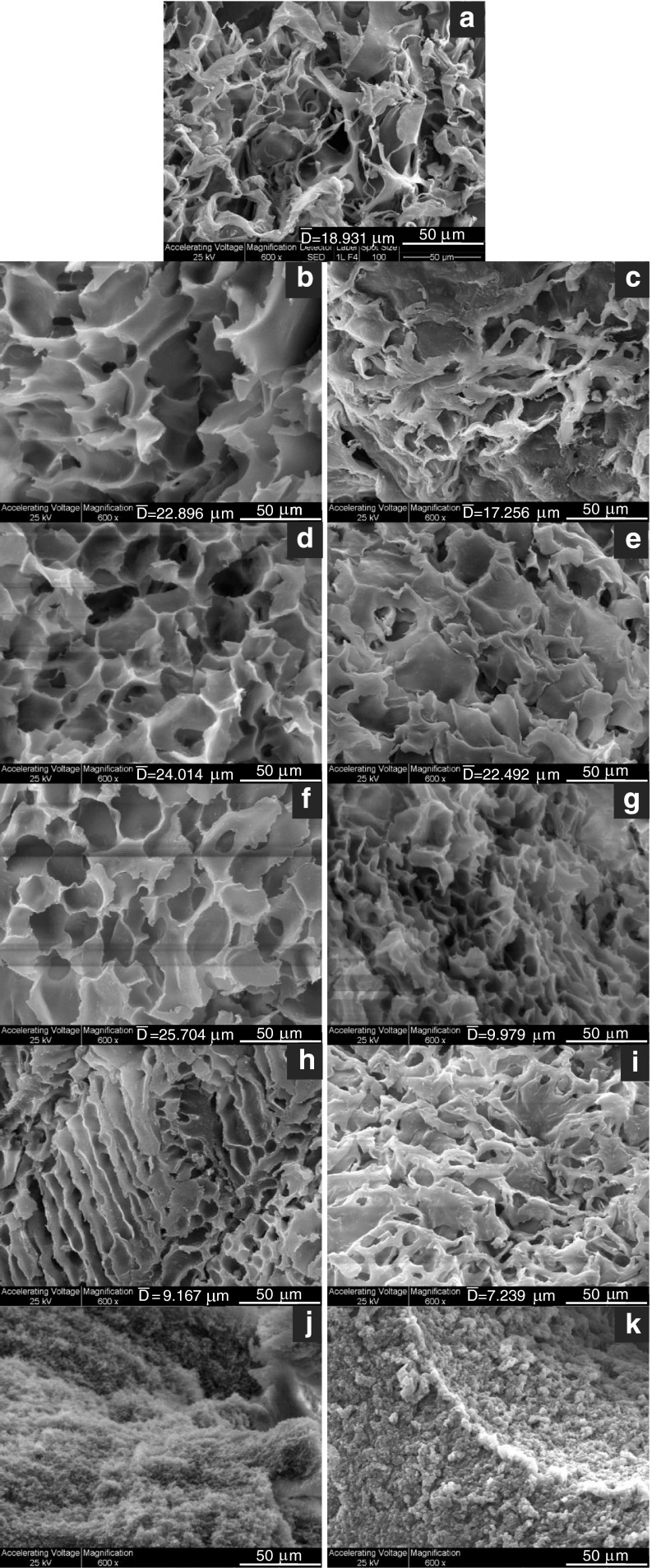
The microstructure of the gels with the included sugars was evaluated by scanning electron microscopy at 600X magnification (Fig. 1). The gels exhibited a three dimensional highly porous structure except for those containing the highest sugar concentrations assayed, whose microstructure had the appearance of overlaid sheets of polysaccharide. The pore size of the gels containing low sugar concentrations was greater than that of the gels without sugars. At concentration values of 30.0 % for sucrose or 17.6 % for fructose, the pore sizes decreased down to dimensions even smaller than those found for kefiran gels without sugars.
Fig. 1.
SEM micrographs of 2 % (w/w) kefiran gels (a) without sugars and with (b and c) 5.9 % (w/v), (d and e) 10.0 %, (f and g) 17.6 % (w/v), (h and i) 30.0 % (w/v), and (j and k) 50.0 % (w/v) sucrose and fructose, respectively. Magnification, 600X
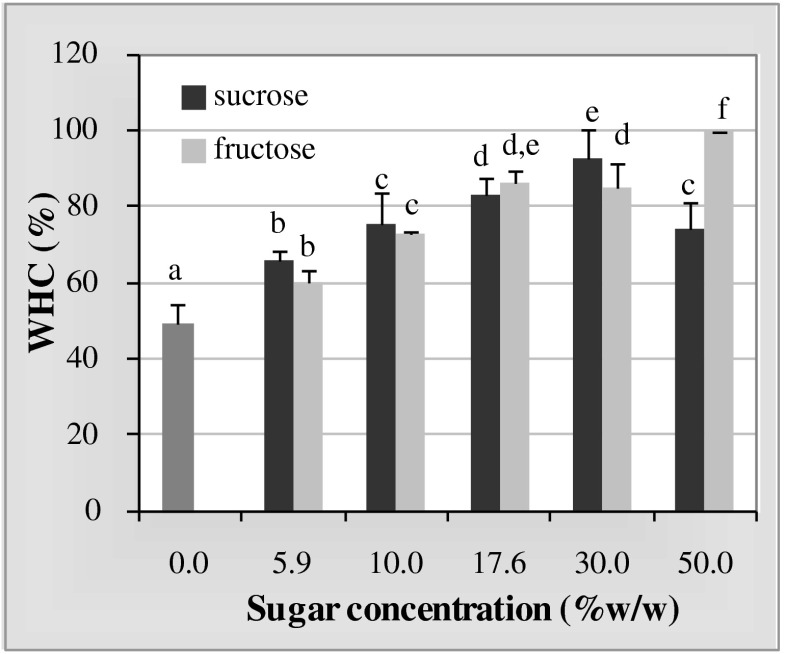
When subjected to centrifugal force, water was expelled from the kefiran gels, and this property was modified by the type and concentration of sugar. The water holding capacity of fructose-kefiran gels increased with sugar concentration throughout the entire concentration range studied, attaining a value close to 100 % at a concentration of 50.0 % sucrose (Fig. 2). In contrast, the sucrose-kefiran gels exhibited the highest water holding capacity value (92.5 %) at a concentration of 30.0 %.
Fig. 2.
Water-holding capacity (WHC) of kefiran gels containing different sucrose or fructose concentrations. Different letters above the error bars indicate significant differences (p ≤0.05)
The mechanical spectrum of samples provides information about the behavior of polymeric gels obtained within the linear viscoelastic region. The elastic modulus (G’) of kefiran gels, with and without sugars, was higher than the viscous modulus (G”) throughout the entire frequency range studied, and both moduli were virtually independent of frequency indicating a true gel-like behaviour (data not shown).
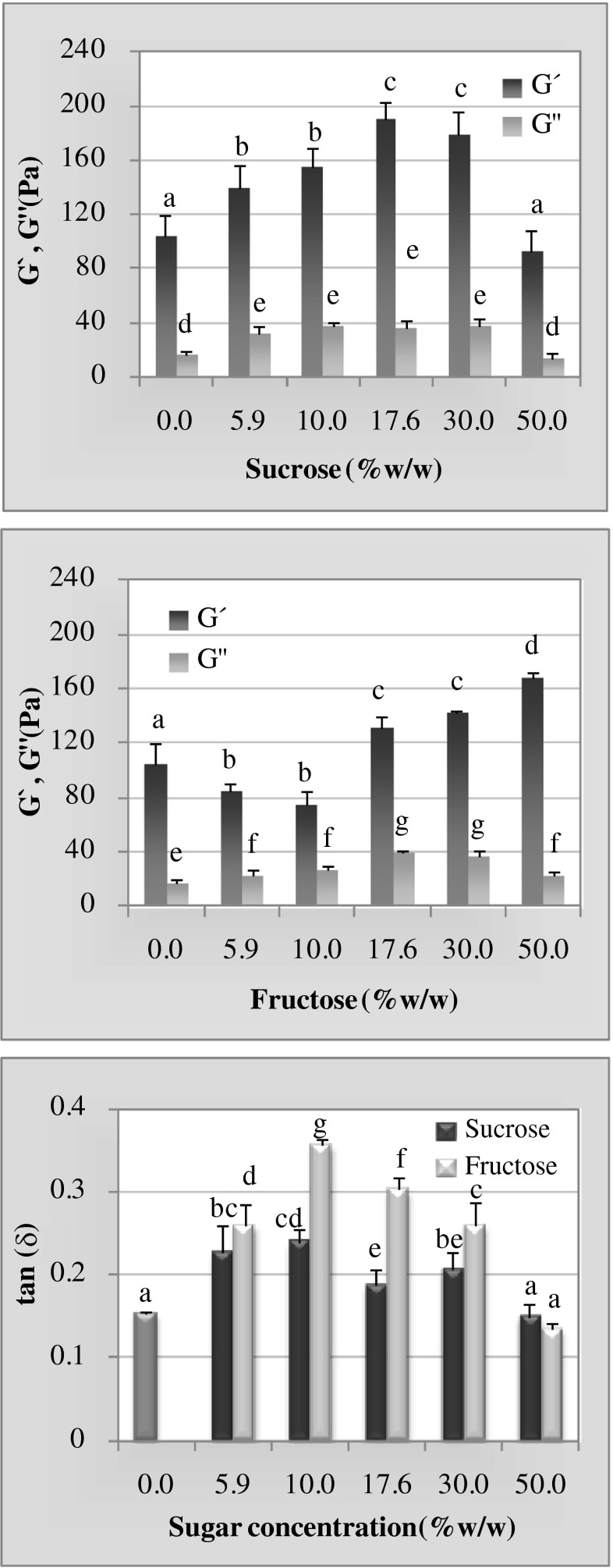
The frequency of 1 Hz was chosen for comparison of the elastic (G’) and viscous moduli (G”) along with the loss tangent (tanδ) of gels containing different concentrations of sugar (Fig. 3). When sucrose was present in the gels (Fig. 3a), an increase in G’ was observed, except at the highest sucrose concentration evaluated (50.0 %), where the G’ value became the same as obtained for the kefiran gel without sugar. The same pattern was observed for the G” modulus. For the fructose-kefiran gels a diminution in the elastic modulus obtained at the two lower sugar concentrations evaluated (5.9 and 10.0 %), but thereafter G’ increased at higher sugar concentrations (Fig. 3b). The viscous modulus increased with sugar concentration up to 30.0 %, then decreased to a value comparable to that of the gel with 5.9 % fructose.
Fig. 3.
The elastic (G’) and viscous (G”) moduli at different sugar concentrations for sucrose (a) or fructose (b) and the loss tangent (tanδ) at the same concentrations of both sugars (c)—all at a frequency of 1 Hz and 20 °C. Within the same graph and in the same parameter different letters above the error bars indicate significant differences (p ≤0.05)
Figure 3c shows the tanδ values of kefiran gels as a function of sugar concentration for both fructose and sucrose. In comparison to gels without sugars, the inclusion of fructose or sucrose produced higher tanδ values except for the highest concentration assayed—i. e., 50.0 %—where the gels had the same tanδ value as the sugar-free kefiran gels. For the other concentrations assayed, the fructose-containing gels exhibited higher tanδ values than those supplemented with sucrose.
Texture profile analysis of kefiran gels without and with 30 % of fructose or sucrose were performed. The hardness values of all gels samples were in the range 0.186 - 0.460 N. A trend to lower hardness in the gels containing sugars was observed, but no significant differences were found. Likewise, there are not significant differences for the others textural parameters (data not sown).
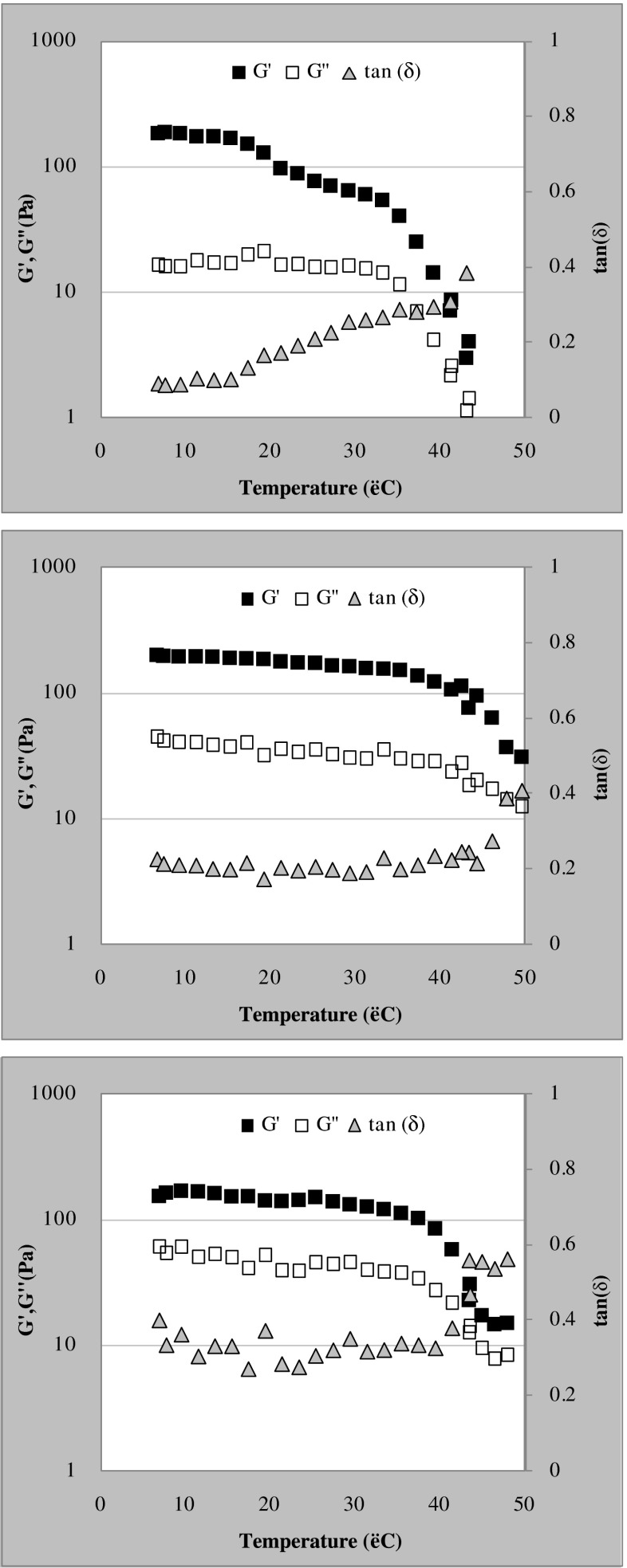
Figure 4 shows the elastic and viscous moduli and the loss tangent as a function of temperature for kefiran gels in the absence of sugars or containing either 30.0 % sucrose or fructose and assessed at 1 Hz. Without a sugar supplement (Fig. 4a) both moduli (G’ and G”) decreased slightly and progressively with increasing temperature during heating, but then above a threshold temperature dropped steeply. When sucrose (Fig. 4b) or fructose (Fig. 4c) was included in the gels, the decline in the slope of the initial shoulder of the plot was less pronounced than that seen with the gels without sugars; and the temperature at which the sharper decrease in G’ began was shifted to the right towards higher values. For the purpose of comparison, the temperature at which the G’ value was reduced to 1/5 of the value at 20 °C for each gel was determined. This temperature shifted from 38 °C for the kefiran gel without sugar, to 47 or 42 °C when the gel contained 30.0 % sucrose or fructose, respectively. Thermograms corresponding to the kefiran gels without sugar indicated an endothermic transition at 39.7 ± 6.6 °C. The enthalpy of this process was of 32.5 ± 7.5 mJ/mg. No endothermic transition was observed in the thermograms corresponding to gels with either sugar.
Fig. 4.
The elastic modulus (■), the viscous modulus (□), and the loss tangent (▲) at 1 Hz and 0.1 Pa as a function of temperature during the heating of kefiran gels either without sugars (a) or supplemented with 30.0 % (w/w) sucrose (b) or fructose (c)
Discussion
Since the formation and properties of food gels depend on interactions between the macromolecules themselves and between those gel structural elements and the water plus the other food components present, we investigated the ability of kefiran to form gels in presence of sucrose or fructose and determined the rheological and water-holding properties of the resulting supplemented gels. Solutions of kefiran containing sucrose or fructose at a concentration up to 50.0 % were able to form gels after one freeze-thaw cycle. The characteristics of the gels had become modified by the inclusion of either of the two sugars at different concentrations as was evidenced by microscopy, water holding capacity and rheological measurements but no difference in texture profile were observed.
Differences in the characteristics of gels have been attributed, at least in part, to different forms of gelation as a result of solvent characteristics (Vaikousi and Biliaderis 2005). The viscosity of a gel-forming solution affects the cryogelation and consequently the gel characteristics. The apparent viscosity of kefiran-sugar solutions increased with successive increments in sugar concentration. The pattern of viscosity increment is different depending on type of sugar. The number and/or orientation of the individual hydroxyl groups of each sugar can influence the structure of water molecules in the surrounding solvation layer (Dashnau, Sharp, and Vanderkooi 2005). Such effect could exert a consequent alteration in the competition between the polymer-water and polymer-polymer interactions modifying apparent viscosity. As a result of a higher solution viscosity, the chains of the dissolved polysaccharide could become restrained from diffusing freely during the cryogelation process (Maurer et al. 2012); thus modifying the kefiran-gel characteristics.
The molecules of sucrose and fructose contribute to water retention. Cryogels obtained with 50 % sucrose-kefiran solutions did not show the ordered structure obtained at lower sucrose concentration may be due to the interaction of these molecules with kefiran as well as with water, decreasing water-holding capacity. When fructose was included on kefiran gel, microstructure observed by SEM indicates the same effect that sucrose but in this case an increment in water-holding capacity was observed in all fructose-kefiran gels indicating a different interaction of this sugar in kefiran network.
Introducing large concentrations of a cosolute into aqueous biopolymer systems led to a reduction in the concentration of water, promoting a self-association of the polymer chains resulting in an enhancement in the strength of gels. This increase in elastic modulus (G’) could also be explained by a direct interaction of low-molecular-weight carbohydrates with kefiran via hydrogen bonds that led to an apparent stiffening of the flexible chains of the polysaccharides. Stiffer chains produce a higher elastic modulus, since additional bending forces would be required to deform the flexible part of the chains within the networks (Maurer et al. 2012).
Different sugars, however, can differ substantially in their effectiveness in promoting the association of biopolymers in intermolecular junctions. Consequently, the addition of 0 to 30.0 % sucrose to kefiran gel-forming solutions produced gels with higher elastic moduli than gels prepared with fructose at the same concentrations. The difference between the effect of fructose and sucrose seen here is in agreement with the report by Evageliou et al. (2000a, b); who, working with starch gels and high-methoxy pectin gels, observed that equivalent concentrations of sucrose, glucose, or fructose led to substantial differences in gel strength, with increases in the order of fructose — sucrose — glucose. Since primary hydroxyl groups can form stronger hydrogen bonds, the inhibition of intermolecular association would depend on the number of those groups per monosaccharide (Doyle et al. 2006).
The difference between sugars became more pronounced when the sucrose and fructose supplementation was at 50.0 %: there the presence of fructose reinforced the structure so as to increase the G’ value, whereas the inclusion of sucrose interfered in the gel-network formation as resulting in less elastic kefiran gels. As the sucrose concentration becomes increased, the force to enhance the polymer-polymer association through a reduction in the water content continues, but is eventually outweighed by the inhibitory effect of sugar molecules binding to the polymer chains and blocking the chain-chain associations required to form the junctions of the cryogel network. In this respect, the sucrose-kefiran gels had a similar behavior to that previously described for sucrose-agarose cryogels (Maurer et al. 2012).
In agarose-sucrose gels at high sucrose content, the glass transition had been postulated to become sufficiently close to the gelation temperatures to inhibit gel formation, thus slowing down the development of the gel network (Normand et al. 2003). This mechanism would also explain why increasing sucrose content led to a gradual increase in the viscous properties of the present kefiran gels. The modification in both moduli can be visualized by analyzing loss tangent (tanδ = G”/G’) which is an indicator of the global viscoelastic behaviour of gels. The corresponding tanδ values of the kefiran cryogel with sucrose or fructose indicated that the addition of sugar up to 30.0 % produced gels with higher viscous moduli that, in turn, led to higher tanδ, compared to gels with kefiran alone. Nevertheless, at the highest sugar concentration evaluated, a decrease in tanδ was observed. In fructose-kefiran gels it was produced by an increase in the elastic modulus; whereas the decrease in tanδ observed for the sucrose-kefiran gels occurred because of a greater decrease in the viscous modulus relative to that seen with the elastic modulus.
When the rheological parameters of the gels were evaluated during the heating of the gels, the magnitude of the changes and the temperature at which they occurred were both found to be modified by the inclusion of sugars in the formulation. The large reduction in G’ observed at around 37 °C for the kefiran gels without sugars, which transition has been interpreted as a melting phenomenon (Piermaria et al. 2008), would be relevant within the context of the application of these gels in food formulations because this endothermic change becomes minimized and shifted to higher temperatures when either sugar was included in the gels. Likewise, only an extremely small transition or none at all, had been observed by differential scanning calorimetry for agarose gels containing sugars (Normand et al. 2003).
Conclusions
The present results demonstrate that the polysaccharide kefiran maintain its ability to form gels in the presence of sucrose or fructose at a concentration up to 50.0 % after a single cryogenic treatment. The molecules of sucrose and fructose contribute to water retention and impact differentially on the rheological characteristics gels. Since these sugars provide easily interchangeable linkages, they would act as a type of plasticizer to interfere with the formation of hydrogen bonds between the polysaccharide molecules, thus modifying the viscoelasticity of the gel.
Kefiran cryogels network can be modified by adding simple sugars like sucrose and fructose thus enabling the possibility of choosing the appropriate formulation for producing a kefiran cryogel that is tailor-made for a particular commercial purpose.
Acknowledgments
Lucía Zavala is a fellow of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Judith Piermaria and Analía G. Abraham are members of the Carrera de Investigador Científico y Tecnológico of CONICET. This work was supported by the Agencia Nacional de Investigaciones Científicas y Técnicas (ANPCyT), CONICET, and UNLP. The authors are grateful to Diana Velasco for bibliographic assistance and to Dr. Donald F. Haggerty, a retired career investigator and native English speaker, for editing the final version of the manuscript.
References
- Abraham AG, Medrano M, Piermaria JA, Mozzi F. Novel applications of polysaccharides from lactic acid bacteria: a focus on kefiran. In: Hollingworth CS, editor. Food hydrocolloids: characteristics, properties and structures. New York: Nova Science Publishers Hauppauge; 2010. pp. 253–271. [Google Scholar]
- Bradford M. A rapid and sensitive method for quantization of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dashnau JL, Sharp KA, Vanderkooi JM. Carbohydrate intramolecular hydrogen bonding cooperativity and its effect on water structure. J Phys Chem B. 2005;109:24152–24159. doi: 10.1021/jp0543072. [DOI] [PubMed] [Google Scholar]
- Davis EA. Functionality of sugars: physicochemical interactions in foods. Am J Clin Nutr. 1995;62:170S–177S. doi: 10.1093/ajcn/62.1.170S. [DOI] [PubMed] [Google Scholar]
- Doyle JP, Giannouli P, Martin EJ, Brooks M, Morris ER. Effect of sugars, galactose content and chainlengthon freeze–thaw gelation of galactomannans. Carbohydr Polym. 2006;64:391–401. doi: 10.1016/j.carbpol.2005.12.019. [DOI] [Google Scholar]
- Evageliou V, Richardson RK, Morris ER. Effect of sucrose, glucose and fructose on gelation of oxidised starch. Carbohydr Polym. 2000;42:261–272. doi: 10.1016/S0144-8617(99)00158-7. [DOI] [Google Scholar]
- Evageliou V, Richardson RK, Morris ER. Effect of pH, sugar type and thermal annealing on high-methoxy pectin gels. Carbohydr Polym. 2000;42:245–259. doi: 10.1016/S0144-8617(99)00191-5. [DOI] [Google Scholar]
- Frøst M, Janhøj T. Understanding creaminess. Int Dairy J. 2007;17:1298–1311. doi: 10.1016/j.idairyj.2007.02.007. [DOI] [Google Scholar]
- Lazaridou A, Biliaderis CG. Cryogelation of cereal B-glucans: structure and molecular size effects. Food Hydrocoll. 2004;18:933–947. doi: 10.1016/j.foodhyd.2004.03.003. [DOI] [Google Scholar]
- Lazaridou A, Biliaderis CG. Cryogelation phenomena in mixed skim milk powder-barley-glucan-polyol aqueous dispersions. Food Res Int. 2007;40:793–802. doi: 10.1016/j.foodres.2007.01.016. [DOI] [Google Scholar]
- Lazaridou A, Vaikousi H, Biliaderis CG. Effects of polyols on cryostructurization of barley β-glucans. Food Hydrocoll. 2008;22:263–277. doi: 10.1016/j.foodhyd.2006.11.012. [DOI] [Google Scholar]
- Lozinsky V, Galaev IY, Plieva FM, Savina IM, Jungvid H, Mattiasson B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003;21:445–451. doi: 10.1016/j.tibtech.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Maeda H, Zhu X, Suzuki S, Suzuki K, Kitamura S. Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens WT-2BT. J Agric Food Chem. 2004;52:5533–5538. doi: 10.1021/jf049617g. [DOI] [PubMed] [Google Scholar]
- Maurer S, Junghans A, Vilgis TA. Impact of xanthan gum, sucrose and fructose on the viscoelastic properties of agarose hydrogels. Food Hydrocoll. 2012;29:298–307. doi: 10.1016/j.foodhyd.2012.03.002. [DOI] [Google Scholar]
- Medrano M, Pérez PF, Abraham AG. Kefiran antagonizes cytopathic effects of Bacillus cereus extracellular factors. Int J Food Microbiol. 2008;122:1–7. doi: 10.1016/j.ijfoodmicro.2007.11.046. [DOI] [PubMed] [Google Scholar]
- Medrano M, Racedo SM, Rolny IS, Abraham AG, Perez PF. Oral administration of kefiran induces changes in the balance of immune cells in a murine model. J Agric Food Chem. 2011;59:5299–5304. doi: 10.1021/jf1049968. [DOI] [PubMed] [Google Scholar]
- Micheli L, Ucelletti D, Palleschi C, Crescenzi V. Isolation and characterization of a ropy Lactobacillus strain producing the exopolysaccharide kefiran. Appl Microbiol Biotechnol. 1999;53:69–74. doi: 10.1007/s002530051616. [DOI] [PubMed] [Google Scholar]
- Mukai T, Watanabe N, Toba T, Itoh T, Adachi S. Gel-forming characteristics and rheological properties of kefiran. J Food Sci. 1991;56:1017–1026. doi: 10.1111/j.1365-2621.1991.tb14630.x. [DOI] [Google Scholar]
- Normand V, Aymard P, Lootens DL, Amici E, Plucknett KP, Frith WJ. Effect of sucrose on agarose gels mechanical behaviour. Carbohydr Polym. 2003;54:83–95. doi: 10.1016/S0144-8617(03)00153-X. [DOI] [Google Scholar]
- Piermaria J, de la Canal M, Abraham AG. Gelling properties of kefiran, a food grade polysaccharide obtained from kefir grain. Food Hydrocoll. 2008;22:1520–1527. doi: 10.1016/j.foodhyd.2007.10.005. [DOI] [Google Scholar]
- Rimada PS, Abraham AG. Polysaccharide production by kefir grains during whey fermentation. J Dairy Res. 2001;68:653–661. doi: 10.1017/S0022029901005131. [DOI] [PubMed] [Google Scholar]
- Rimada PS, Abraham AG. Kefiran improves rheological properties of glucono-delta-lactone induced skim milk gels. Int Dairy J. 2006;16:33–39. doi: 10.1016/j.idairyj.2005.02.002. [DOI] [Google Scholar]
- Ruas-Madiedo P, Abraham AG, Mozzi F, de los Reyes-Gavilán CG (2008) Functionality of exopolysaccharides produced by lactic acid bacteria. In Molecular aspects of lactic acid bacteria for traditional and new applications. pp. 137–166. Eds.: Mayo B, López P, Pérez-Martínez G. Kerala: Research Signpost
- Russ N, Zielbauer BI, Koynov K, Vilgis TA. Influence of nongelling hydrocolloids on the gelation of agarose. Biomacromolecules. 2013;14:4116–4124. doi: 10.1021/bm4012776. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaikousi H, Biliaderis CG. Processing and formulation effects on rheological behaviour of barley-glucan aqueous dispersions. Food Chem. 2005;91:505–516. doi: 10.1016/j.foodchem.2004.04.042. [DOI] [Google Scholar]
- Warrand J. Healthy polysaccharides the next chapter in food products. Food Technol Biotechnol. 2006;44:355–370. [Google Scholar]
- Wu M, Xiong YL, Chen J, Tang X, Zhou G. Rheological and Microstructural properties of porcine myofibrillar protein-lipid emulsion composite gels. J Food Sci. 2009;74:207–217. doi: 10.1111/j.1750-3841.2009.01140.x. [DOI] [PubMed] [Google Scholar]