Abstract
Fermented milk products containing probiotics and prebiotics can be used in management, prevention and treatment of some important diseases (e.g., intestinal- and immune-associated diseases). Microencapsulation has been used as an efficient method for improving the viability of probiotics in fermented milks and gastrointestinal tract. Microencapsulation of probiotic bacterial cells provides shelter against adverse conditions during processing, storage and gastrointestinal passage. Important challenges in the field include survival of probiotics during microencapsulation, stability of microencapsulated probiotics in fermented milks, sensory quality of fermented milks with microencapsulated probiotics, and efficacy of microencapsulation to deliver probiotics and their controlled or targeted release in the gastrointestinal tract. This study reviews the current knowledge, and the future prospects and challenges of microencapsulation of probiotics used in fermented milk products. In addition, the influence of microencapsulation on probiotics viability and survival is reviewed.
Keywords: Fermented milks, Microencapsulation, Probiotic microorganisms, Viability
Introduction
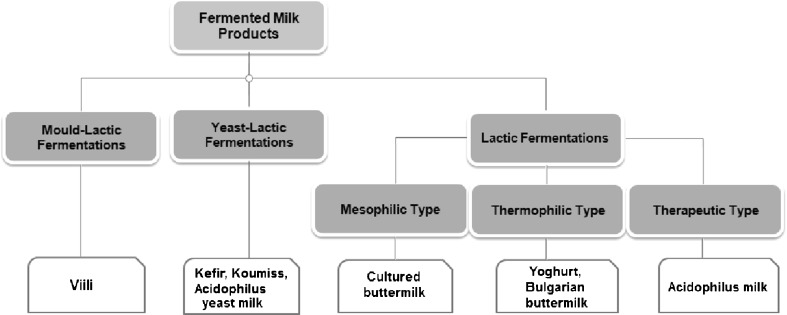
Nowadays, fermented milk products have been used as functional foods around the world because of their beneficial effects on human health (Divya et al., 2012). According to Codex Alimentarius, "fermented milk is a milk product obtained by fermentation of milk, which milk may have been manufactured from products obtained from milk with or without compositional modification as limited by the provision in Section 3.3 (of this standard), by the action of suitable microorganisms and resulting in reduction of pH values with or without coagulation" (CAC/RCP 243, 2003). Fermented milks are widely produced around the world, and approximately 400 generic names are applied to the traditional and commercial products, but in actual essence the list may include only a few variations. The most popular and industrially manufactured fermented milk products, based on the main microorganisms dominating the flora in the product and the main metabolites of the starter cultures are summarized in Figure 1 (Tamime, 2006; Tamime and Robinson, 1999).
Fig. 1.
Types of fermented milk products
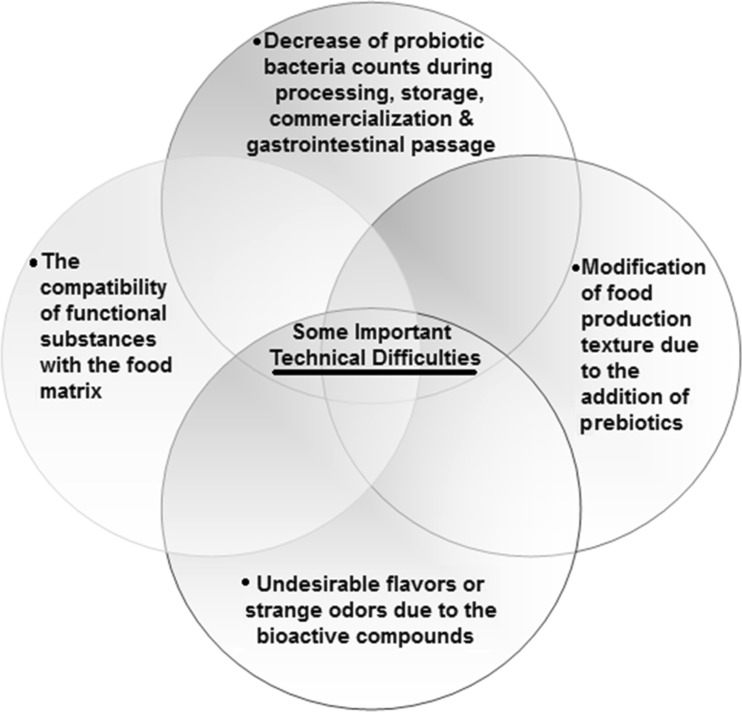
Technological aspects related to microbial systems and functional foods are the composition and processing of raw materials, the viability and productivity of the applied starter cultures, and technological and storage conditions of the final foods. Several parameters can control the safety aspects, sensory properties, organoleptic characteristics and stability of fermented milk products (O'grady and Gibson, 2005; Shah, 2001; Korbekandi et al., 2011; Beheshtipour et al., 2013; Mohammadi et al., 2012; Shah et al., 2000; Shah and Lankaputhra, 1997). In addition, development of fermented milk products containing probiotics and prebiotics has its technical difficulties. Some of the technical difficulties encountered by industries in the development of fermented milk products are mentioned in Figure 2. The viability of probiotic microorganisms in the final product until the time of consumption has been proposed with the descriptor minimum of biovalue (MBV). MBV is the minimum of viable probiotic cells per gram or milliliter of probiotic product, and is the most important qualitative parameter of probiotic products as it determines their pharmaceutical effectiveness (Mortazavian et al., 2007a; Mortazavian and Sohrabvandi, 2006). The viability of probiotic microorganisms is affected by factors such as the strain of probiotic bacteria, interactions among present species, pH, production of organic acids and volatile compounds (e.g., lactic acid, acetic acid, orotic acid, succinic acid, uric acid, citric acid, ethanol, pyruvate, acetaldehyde, diacetyl and acetoin). Other important factors are the metabolic products and acids produced during refrigerated storage, concentration of hydrogen peroxide and dissolved oxygen in fermented milks, concentration of sodium chloride in the media, inoculation level, incubation temperature and time, growth promoters (nutrients availability) and inhibitors, buffering capacity of the media, storage temperature, heat treatments, homogenization and packaging materials and conditions (Kosin and Rakshit, 2006; De vuyst, 2000; Korbekandi et al., 2011; Lucas et al., 2004; Oliveira et al., 2001; Donkor et al., 2006; Dave and Shah, 1997; Shah, 2000; Ravula and Shah, 1998). The viability of probiotics in fermented milks depends on the multiplication and survival rates of probiotic cells over the storage period, as well as during the fermentation period until the time of consumption. Therefore, loss of viability of probiotics could occur during two stages, i.e., production and storage. Unfortunately, probiotics show poor viability in fermented milks, because they grow slowly and also lose their viability (sometimes dramatically) during the fermentation and refrigerated storage. Therefore, the viable counts of probiotics in fermented milks at the moment of consumption are expected to be less than the minimum acceptable concentration of 106 cfu g−1 or mL−1. Moreover, a high viable population of probiotic bacteria in food products at the time of consumption does not guarantee the same survival rate after the arrival of the cells in the intestine. The low pH of the stomach and the presence of bile salts in the small intestine are the main reasons for the dramatic decline in viability of delivered cells (Tamime et al., 2005; Kosin and Rakshit, 2006; Korbekandi et al., 2011) .
Fig. 2.
Some of the technical difficulties encountered by industries in the development of fermented milk products
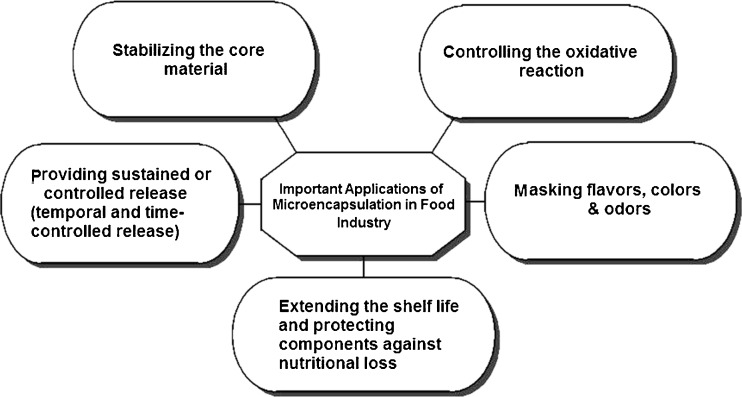
Many investigations have documented the positive effects of microencapsulation of probiotic microorganisms and their survival in fermented milks and gastrointestinal tract (Adhikari et al., 2000; Sultana et al., 2000; Sun and Griffiths, 2000; Krasaekoopt et al., 2004; Picot and Lacroix, 2004; Iyer and Kailasapathy, 2005; Kailasapathy, 2006). Some important applications of microencapsulation in the food industry are summarized in figure 3 (Anal and Singh, 2007). Probiotics must survive food processing and storage during product maturation and shelf-life for successful delivery in fermented milks. Furthermore, because viable and bioactive probiotic microorganisms are usually required at the target site in the host, it is important that probiotic cells withstand the host′s natural barriers against ingested bacteria. Microencapsulation technologies can be used to protect these sensitive bacteria against high oxygen levels, acidic environments, freezing and especially during transit through the gastrointestinal tract (Korbekandi et al., 2011). This study reviews the potential effects of microencapsulation on viability and survival of probiotics used in fermented milk products. The aim of this review article is, therefore, to reflect on the current state and future prospects, especially the potentials and limitations of the above mentioned techniques for food industries.
Fig. 3.
Important applications of microencapsulation in the food industry
Probiotic microorganisms
Probiotics are defined as “live microorganisms (bacteria or yeasts), which when administered in adequate amount confer a health benefit on the host” (World Health Organization and Food and Agriculture Organization of the United Nations WHO/FAO 2001). They can be used in management, prevention and treatment of some important diseases. Table 1 summarizes important health benefits from the consumption of probiotics (Parvez et al., 2006; Kalliomaki et al., 2003; Lee et al., 2003; Rinkinen et al., 2003). Lactobacillus and Bifidobacterium species are the most important probiotic microorganisms applied in probiotic fermented milks, because of some potential characteristics such as their good tolerance toward harmful environmental factors (e.g., low pH, hydrogen peroxide and molecular oxygen) and beneficial effects on human health (Mortazavian and Sohrabvandi, 2006; Lee and Wong, 1998; Oliveira et al., 2009; (Østlie et al., 2003, 2005) (Table 2). Bifidobacteria are gram-positive, strictly anaerobic bacteria and grow at pH 4.5–8.5. Lactobacilli are gram-positive, facultative anaerobic or microaerophilic rod-shaped bacteria (Holzapfel et al., 2001; Doleyres and Lacroix, 2005).
Table 1.
Potential health benefits from probiotics
| • Modulation/stimulation of the immune system • Improvement of mucosal immune function, mucin secretion & prevention of disease • Improvement of lactose digestion & symptoms of intolerance in lactose intolerance • Lowered serum cholesterol levels • Shortened the duration of acute gastroenteritis, acute diarrhea & antibiotic-associated diarrhea • Reduced pain & constipation of irritable bowel syndrome • Reduced bloating & flatulence in irritable bowel syndrome • Control of cancer (e.g., reduced risk factors for colon cancer) • Alleviation of food allergy symptoms in infants |
Table 2.
Some examples of lactic acid bacteria and bifidobacteria
| Lactic acid bacteria | Bifidobacteria |
|---|---|
|
L. acidophilus
L. amylovorus L. casei L. crispatus L. delbrueckii subsp. bulgaricus L. gasseri L. johnsonii L. paracasei L. plantarum L. reuteri L. rhamnosus L. salivarius |
B. adolescentis
B. animalis B. bifidum B. breve B. infantis B. lactis B. longum B. pseudolongum |
Protecting probiotics by microencapsulation
Microencapsulation is a physicochemical or mechanical process in which the bacterial cells are entrapped within coatings of hydrocolloidal materials, providing protection from adverse conditions such as high acidity and low pH, bile salts, cold shock (induced by process conditions such as deep freezing and freeze drying), molecular oxygen (in the case of obligatory anaerobic microorganisms), heat shock (caused by the process conditions such as spray drying), and chemical antimicrobial agents applied in order to inhibit or reduce cell injuries/loss and increase viability (Korbekandi et al., 2008; Adhikari et al., 2000; Krasaekoopt et al., 2006; Sultana et al., 2000; Mortazavian et al., 2008; Krasaekoopt et al., 2004; Hansen et al., 2002; Lee and Heo, 2000; Rao et al., 1989; Wenrong and Griffiths, 2000; Champagne 2012; Heidebach et al., 2012). Other advantages including increase in sensory stability and/or its improvement and immobilization of the cells for their homogeneous distribution throughout the product, can also be achieved (Mortazavian et al., 2007b). High acidity and low pH of fermented products are the main factors that cause viability loss of probiotics, especially during refrigerated storage (Shah et al., 1995; Dave and Shah, 1997). Techniques commonly applied for probiotic microencapsulation are emulsion, extrusion, spray drying, freeze drying and adhesion to starch. Some important examples of emulsion, extrusion and spray-drying techniques applied in microencapsulation studies are mentioned in Table 3.
Table 3.
Some important examples of emulsion, extrusion, and spray drying techniques applied in microencapsulation studies
| Encapsulating materials | Probiotic microorganisms | Size of capsules | Reference | |
|---|---|---|---|---|
| Emulsion Techniques | 2–4 % alginate | Lactobacillus casei NCDC-298 | - | (Mandal et al., 2006) |
| κ-Carrageenan/locust bean gum | Bifidobacterium longum | 1–2 mm | (Maitrot et al., 1997) | |
| 2 % alginate, 5 % glycerol, 0.26 % xanthan gum + 0.8 % chitosan | Lactobacillus bulgaricus KFRI 673 | 40–80 μm | (Lee et al., 2004) | |
| 1 % Alginate, glycerol + preservatives in micro porous glass (MGP) membrane | Lactobacillus casei YIT 9018 | - | (Song et al., 2003) | |
| 3 % alginate | Bifidobacterium longum Bifidobacterium bifidum Bifidobacterium infantis Bifidobacterium breve Bifidobacterium adolescentis | 20–70 μm | (Truelstrup Hansen et al., 2002) | |
| - 2 % alginate + 2 % corn starch - 1 % Xanthan + 0.5 % gellan | Lactobacillus reuteri | - | (Muthukumarasamy et al., 2006) | |
| 2 % κ-carrageenan | Bifidobacterium longum B6 | 5–100 μm | (Adhikari et al., 2000) | |
| Bifidobacterium longum ATCC 15708 | ||||
| 32 % Oil, 20 % caseinate, 20 % fructo-oligosaccharides, 20 % glucose syrup or starch (MicroMAX) | Bifidobacterium infantis | 15–20 μm | (Crittenden et al., 2006) | |
| Alginate/starch | Bifidobacterium infantis | 0.5–1 mm | (Sultana et al., 2000) | |
| Alginate/starch | Bifidobacterium infantis | 0.5–1 mm | (Godward and Kailasapathy, 2003) | |
| Milk fat + 10 % whey protein isolate | Bifidobacterium breve R070 (BB R070) | 3–80 μm | (Picot and Lacroix, 2004) | |
| Bifidobacterium longum R023 (BL R023) | ||||
| Milk proteins | Lactobacillus paracasei ssp. paracasei F19 | 68 ± 5 μm | (Heidebach et al., 2009) | |
| Bifidobacterium lactis Bb12 | ||||
| 13 % gelatin, 1.25 mM genipin + 1 % alginate | Bifidobacterium adolescentis 15703 T | 49–53 μm | (Annan et al., 2008) | |
| 4 % sodium alginate + 2 % starch | Lactobacillus acidophilus LA1 | 100–300 μm | (Sabikhi et al., 2010) | |
| 1 % gum Arabic, gellan gum or mesquite seed gum | Lactobacillus sp. | 30, 17 & 10 μm | (Yáñez-Fernández et al., 2008) | |
| Extrusion Techniques | 1.5 % alginate + 0.1 % poly-L-lysine & 0.1 % alginate | Lactobacillus reuteri Bifidobacterium longum | 619 ± 31 μm | (Martoni et al., 2008) |
| Alginate | Bifidobacterium lactis | 2–2.5 mm | (Favaro Trindade and Grosso, 2000) | |
| - 2 % alginate + 0.17 % alginate | Bifidobacterium bifidum ATCC 1994 | 1.89 mm | (Krasaekoopt et al., 2004) | |
| - 2 % alginate + 0.05 % poly-L-lysine | Lactobacillus acidophilus 547 Lactobacillus casei 01 | |||
| Gellan/xanthan | Bifidobacterium infantis | 3 mm | (Sun and Griffiths, 2000) | |
| 2 % Alginate, 1 % gellan, 0.86 % peptides, 0.2 % fructo-oligosaccharides | Bifidobacterium bifidum | - | (Chen et al., 2007) | |
| Alginate/starch | Bifidobacterium lactis | 2–2.5 mm | (Talwalkar and Kailasapathy, 2003) | |
| 2–4 % sodium alginate | Bifidobacterium longum KCTC 3128 Bifidobacterium longum HLC 3742 | 1.03–2.62 mm | (Lee and Heo, 2000) | |
| 3 % κ-carrageenan | Bifidobacterium bifidum (ATCC 15696) | - | (Dinakar and Mistry, 1994) | |
| Spray-drying Techniques | 10 % Gelatin, gum Arabic, soluble starch, or skim milk | Bifidobacterium infantis CCRC 14633 Bifidobacterium infantis CCRC 14661 Bifidobacterium longum ATCC 15708 Bifidobacterium longum CCRC 14634 Bifidobacterium longum B6 | 10–20 μm | (Lian et al., 2002; Lian et al., 2003; Hisiao et al. 2004) |
| Oil (32 %), 20 % caseinate, 20 % fructo-oligosaccharides, 20 % glucose syrup or starch (MicroMAX) | Bifidobacterium infantis | 15–20 μm | (Crittenden et al., 2006) | |
| Milk fat/whey proteins Whey proteins | Bifidobacterium longum Bifidobacterium breve | 20–75 μm | (Picot and Lacroix, 2003a, 2004) | |
| 10 % Waxy maize starch | Bifidobacterium PL1 | 5 μm | (O′Riordan et al., 2001) | |
| 30 % maltodextrin & 20 % gum Arabic | Lactobacillus acidophilus BCRC 14079 Bifidobacterium longum BCRC 14605 | 10 μm | (Su et al., 2007) | |
| Cellulose acetate phthalate |
Lactobacillus acidophilus (La-05) Bifidobacterium lactis (Bb-12) |
22 μm | (Favaro-Trindale and Grosso, 2002) | |
| Gum acacia (gum Arabic) | Lactobacillus paracasei NFBC 338 | 5–15 μm | (Desmond et al., 2002) |
Microencapsulation of L. acidophilus and bifidobacteria with calcium alginate did not considerably increase their viability after being subjected to the intense acid (pH 2) and bile (2 %) environment, vice versa, however, at mild acidic conditions (natural acidity of yoghurt), throughout 8 weeks of refrigerated storage improving the probiotics survivability was noticeable. Kebary et al. (1998) showed that encapsulation of bifidobacteria with alginate could significantly increase their viability in frozen ice milk, whereas, using k-carrageenan for this reason was not as successful as the previous one. Encapsulated B. longum in milk showed higher viability compared with free cells during storage time (Truelstrup Hansen et al., 2002). Higher survivability of B. infantis in yoghurt during the refrigerated storage was reported when the cells were encapsulated by mixture of gelan-xanthan. The average size of the beads was 3 mm after the encapsulation process (Sun and Griffiths, 2000). Encapsulated probiotics with an alginate-starch mixture and a bead size range of 0.5 to 1.0 mm were considerably more viable in yoghurt during the storage period (Sultana et al., 2000). Increase in the viability of lactobacilli in frozen ice milk after encapsulation with alginate (size range from 25 to 62 μm) has been reported (Sheu and Marshall, 1993). Good efficiency for encapsulation process after the encapsulation of B. infantis with xanthan-gelan mixture in yoghurt with pH 4 during the 6 weeks of storage period at 4 °C has been reported. Mentioned cells showed higher survivability during the pasteurization process (Sun and Griffiths, 2000). Because microencapsulation of probiotic starter cultures considerably decreases their metabolic activity, viability of the cells would increase due to the slower acid production rate. For instance, it has been reported that incubation time for yoghurt made with L. casei and L. acidophilus up to the end point of pH 5, increased from 6 h in the case of free cells to 30 h in the case of encapsulated cells (Sultana et al., 2000).
Viability and survival of probiotics in gastrointestinal conditions
Microencapsulated probiotics should survive passage through the upper digestive tract in large numbers sufficient enough to produce desired beneficial effects in the host intestine (Cook et al, 2012; Gilliland, 1989). The effect of microencapsulation on the survival of probiotic bacteria under gastrointestinal conditions has been investigated by researchers (Table 4). Kim et al., (2008) have investigated the effect of microencapsulation on viability of L. acidophilus ATCC 43,121 during exposure to artificial gastrointestinal. They also investigated the effect of microencapsulation on the heat susceptibility of this microorganism during the heat treatment. Other characteristics of non-encapsulated and encapsulated L. acidophilus ATCC 43,121 were studied (such as cholesterol assimilation and intestinal adhesion). As a result, the encapsulated cells exhibited a significantly higher resistance to artificial intestinal juice and heat treatment than non-encapsulated samples. The assimilative reductions of cholesterol by non-encapsulated and encapsulated L. acidophilus ATCC 43,121 were 35.98 % and 32.84 %, respectively. Moreover, encapsulation did not significantly (P > 0.05) affect the adherence of L. acidophilus ATCC 43,121 onto the human intestinal epithelial cell lines HT-29. Studies suggest that microencapsulation of free probiotic cells can increase their viability by ≥ 2 log cycles in fermented milks during a refrigerated storage period. In fermented milk drinks with pH values of less than 4.2, free cells of L. acidophilus La-5 lost their viability to less than 106 cfu mL−1 after 1 week and in the case of B. lactis Bb-12, a similar loss occurred after 2 weeks of storage. For encapsulated cells, viable population of L. acidophilus and bifidobacteria remained higher than 105 and 106 cfu mL−1 after 42 days of refrigerated storage (Mortazavian et al., 2008). In simulated gastrointestinal conditions, the viability of the mentioned probiotic cells after encapsulation was increased from 0.6 and 0.2 % as free cells to 18.0 and 9.5 % under harsh gastrointestinal conditions (pH 1.5, 90 min/2 % bile salts, 90 min). Under normal simulated gastrointestinal conditions (pH 2.0, 30 min/0.6 % bile salts, 60 min), the cell survival rates were 16 % for L. acidophilus and 21 % for bifidobacteria before encapsulation and 26 and 34 % (L. acidophilus and bifidobacteria, respectively) after encapsulation (Mortazavian et al., 2008).
Table 4.
Survival of encapsulated probiotics under gastrointestinal conditions
| Encapsulation materials & Methods | Probiotic microorganisms | Survival of probiotics under gastrointestinal conditions | Reference |
|---|---|---|---|
| 2 % sodium alginate with poly-L-lysine or chitosan (Extrusion technique) |
Bifidobacterium bifidum | Higher than 106 cfu mL−1 | (Cui et al., 2000) |
| 2 % sodium alginate with chitosan (Extrusion technique) |
Lactobacillus bulgaricus | Higher than 106 cfu mL−1 | (Lee et al., 2004) |
| 2–4 % alginate (Extrusion technique) |
Bifidobacterium longum | Depending on alginate concentration and bead size | (Lee and Heo, 2000) |
| 2 % sodium alginate with chitosan | Lactobacillus acidophilus | 1.5 × 106 cfu g−1 | (Krasaekoopt et al., 2004) |
| Alginate | 1.3 × 104 cfu g−1 | ||
| PLL-alginate (Extrusion technique) |
1.0 × 104 cfu g−1 | ||
| 2 % sodium alginate with chitosan | Lactobacillus casei | 1.6 × 106 cfu g−1 | (Krasaekoopt et al., 2004) |
| Alginate | 6.7 × 103 cfu g−1 | ||
| PLL-alginate (Extrusion technique) |
7.0 × 103 cfu g−1 | ||
| 0.75 % gellan/1 % xanthan gum (Extrusion technique) |
Bifidobacterium infantis | Higher than 106 cfu mL−1 | (Sun and Griffiths, 2000) |
| 0.75 % gellan/1 % xanthan gum (Extrusion technique) |
Bifidobacterium lactis | Higher than 106 cfu mL−1 | (McMaster et al., 2005) |
| Sodium alginate with poly-L-lysine or chitosan Addition of Hi-maize starch or Raftiline®/Raftilose® (Extrusion technique) |
Lactobacillus acidophilus | Higher than 106 cfu mL−1 | (Iyer and Kailasapathy, 2005) |
| 1.8 % sodium alginate (Extrusion technique) |
Lactobacillus acidophilus | 105–106 cfu mL−1 | (Chandramouli et al., 2004) |
| 2 % alginate with Hi-maize starch (Emulsion technique) |
Lactobacillus acidophilus Bifidobacterium spp. | Higher than 106 cfu mL−1 | (Sultana et al., 2000) |
| 3 % alginate (Emulsion technique) |
Bifidobacterium adolescentis | 8.2–1.0 log cfu mL−1 | (Truelstrup Hansen et al. 2002) |
| Bifidobacterium breve | |||
| Bifidobacterium lactis | |||
| Bifidobacterium longum | |||
| 1 % alginate with micro porous glass (MPG) membrane | Lactobacillus casei | Higher than 106 cfu mL−1 | (Song et al., 2003) |
| (Emulsion technique) | |||
| 35 % gum Arabic | Bifidobacterium infantis | 89.17 % | (Lian et al., 2003) |
| 15 % skim milk | CCRC 14633 | 65.16 % | |
| 30 % gelatin | 92.73 % | ||
| 35 % soluble starch | 92.70 % | ||
| (Spray-drying technique) | |||
| 35 % gum Arabic | Bifidobacterium longum B6 | 93.53 % | (Lian et al., 2003) |
| 15 % skim milk | 81.26 % | ||
| 30 % gelatin | 87.15 % | ||
| 35 % soluble starch | 95.47 % | ||
| (Spray-drying technique) | |||
| 10 % heat-denatured whey protein isolate (Spray-drying/emulsion technique) |
Bifidobacterium breve Bifidobacterium longum | 1.0 log cfu mL−1 3.8 log cfu mL−1 | (Picot and Lacroix, 2004) |
Lee and Heo (2000) showed that survivability of B. longum encapsulated with calcium alginate in the simulated conditions of gastric juice (pH 1.5) could be considerably increased. They found that the death rate of the probiotics in the capsules decreased proportionally with an increase in the alginate concentration (1 ~ 3 %), bead size (1 ~ 3 mm) and initial cell numbers. Experiments indicated that coating of the calcium chloride on sodium alginate capsules containing L. acidophilus increased tolerance of the mentioned bacteria against harsh acidic (pH 2) and bile (1 %) conditions (Chandramouli et al., 2004). Simulated conditions of the stomach (pH 1.5) led to a dramatic loss in the viable counts of B. infantice (from 1.23 × 109 to <10 cfu/ml after 30 min), nevertheless, its viability loss under the same conditions after microencapsulation did not exceed the 0.67 % of the first viable cell amount (Sun and Griffiths, 2000). Findings have revealed that resistant starch is an efficient component for the purpose of probiotics encapsulation, because it is not dissolved or decomposed in the gastric acid, neutral pH and by the enzymatic activity of pancreas, but releases its cells when the beads enter the intestine (Englyst et al., 1992; Sun and Griffiths, 2000). It should be mentioned that apart from the type and concentration of coating materials; diameter of capsules or coats is also a determinable factor for improving the viability of probiotics. For instance, it was reported that survivability of encapsulated probiotics with alginate capsules under the acidic-bile conditions showed no significant difference when the diameter of gel-beads were 20 and 70 μm compared with the bigger sizes (Sultana et al., 2000). Furthermore, the survival of encapsulated probiotics is dependent on the initial cell numbers and bacterial species (Sultana et al., 2000; Truelstrup-Hansen et al., 2002).
Microencapsulation materials
Encapsulation of probiotic microorganisms in polymer systems seems to be easy lab-scale process in order to protect probiotics against low pH and high bile concentrations (Anal and Singh, 2007; Heidebach et al., 2010; Lian et al., 2002; Peres et al., 2012; Huq et al., 2013). However, scaling up of this process needs industrial investments. The selection of different types of encapsulating materials usually depends on the functional properties of the microcapsules and the coating process used. Materials commonly used in encapsulation of probiotic microorganisms include κ-carrageenan, alginate, cellulose acetate phthalate, modified starch, chitosan, gellan, xanthan, gum Arabic and animal proteins (milk, gelatin). Coating the microcapsules produced by different microencapsulation technologies with an additional film can prevent their exposure to oxygen during storage as well as improve their stability at acidic pH (Jung et al., 2007; Krasaekoopt et al., 2004; Lee et al., 2004). For instance, chitosan-coated alginate beads were reported to provide better protection in simulated gastric juices poly-L-lysine or alginate coating (Krasaekoopt et al., 2004). Coating of microcapsules with alginate produces a uniform 1–2-μm thin exterior layer and can improve the survival of bifidobacteria (Annan et al., 2008). Coating the beads with poly-L-lysine and alginate was reported to limit Lactococcus lactis release and reduce the acidifying activity of the culture. Low-molecular weight chitosan has been found to show better control of bacterial cell release than high-molecular weight chitosan and to result in more spherical beads without changing their size (Rokka and Rantamäki, 2010).
Carrageenan
κ-Carrageenan is a natural polysaccharide extracted from marine macro algae. κ-Carrageenan is composed of repeating D-galactose-4-sulphate units and 3,6-anhydro-D-galactose joined by alternating α1 3 and β1 4 glycosidic linkages. The combination of this polymer with locust beam gum, which produces more flexible gels through interaction of the galactomannan chains of locust bean gum with carrageenan, was recently used to encapsulate probiotic bacteria (Anal and Singh, 2007; Lian et al., 2002).
Alginate
Alginate is widely used for encapsulating probiotics due to its biocompatibility, cost-effectiveness, simplicity, non-toxicity and good intestinal digestibility (Mortazavian et al., 2007b). However, the use of alginate is limited due to its low physical stability in the presence of anti-gelling cations (e.g., sodium and magnesium ions) or chelating agents (e.g., phosphate) (Lee et al., 2004; Krasaekoopt et al., 2006). Moreover, under low pH conditions, cross-linked alginate matrices can undergo degradation of the alginate molecule and subsequent reduction in its molecular weight, causing faster release of entrapped active ingredients. Alginic acid is a polyuronic acid extracted from seaweeds and is composed of various proportions of 1–4 linked β-D-mannuronic and α-L-guluronic acids (Mortazavian et al., 2007b). The functional properties of alginate as a supporting material are strongly associated with the composition and sequence of D-mannuronic and L-guluronic acids. It was demonstrated that sodium alginate in calcium chloride could be used to encapsulate L. acidophilus to protect this organism from harsh acidic conditions in gastric fluid (Chandramouli et al., 2004; Lee et al., 2004). Krasaekoopt et al. (2004) have investigated the effect of coating on the survival of probiotics encapsulated in alginate beads as compared to free cells during storage. The survival of encapsulated probiotic bacteria was higher than that of free cells. In comparison with κ-carrageenan, it was reported that B. bifidum survived better in frozen milk in beads made from alginate (Kebary, 1996). Several investigations have shown that microencapsulation of probiotic bacteria with alginate or whey proteins protects them against acid stress, allowing the cells to survive in the stomach and be delivered in the intestines (Gerez et al., 2012; Doherty et al., 2012). It was reported that an optimal combination of capsule for probiotic survival in gastric conditions included 3 % sodium alginate, 1 % pancreatic digested casein and 3 % sodium alginate. Moreover, caseinate and fructo-oligosaccharides with either dried glucose syrup or resistant starch were found to provide protection (Picot and Lacroix, 2004; Lee et al., 2004; Muthukumarasamy et al., 2006; Rokka and Rantamäki, 2010).
Starch
Starch as a dietary component consists of D-glucose units joint together with glycosidic bonds and has protective role against colorectal cancer (Cassidy et al., 1994). Resistant starch has been used in protection of probiotic bacteria to reach the large intestine. L. rhamnosus and bifidobacteria with starch have been shown to survive passage through the human gastrointestinal tract (Lian et al., 2003). But, Hi-Maize starch encapsulation did not protect L. acidophilus and B. infantis from high acid conditions (Sultana et al., 2000). Researchers found that encapsulation of lactic acid bacteria (LAB) with starch (large potato starch granules enzymatically treated to obtain porous structure) could increase the survivability of these organisms (Mattila-Sandholm et al., 2002). Talwalkar and Kailasapathy (2003) have reported that encapsulation of L. acidophilus and B. lactis in alginate-starch systems (dropping a mixture of alginate-starch-bacteria into a CaCl2 coagulation bath) prevented bacteria from oxygen toxicity. Sultana et al., (2000) have evaluated the effects of encapsulation of probiotic bacteria with alginate-starch in simulated gastrointestinal conditions and in yoghurts. As a result, the survival of encapsulated cultures of L. acidophilus and Bifidobacterium spp. showed a decline in viable count of approximately 0.5 log over a period of 8 weeks while there was a decline of approximately 1 log in cultures incorporated as free cell in yoghurts.
Gelatine
Gelatine can be used as thermally reversible gelling agent for encapsulation of probiotics. Researchers used high concentrations of gelatine (24 % w/v) to encapsulate L. lactis by cross-linking with toluene−2, 4-diisocyanate for biomass production (Hyndman et al., 1993). Moreover, encapsulation of Bifidobacterium cells in a mixed gel composed of alginate, pectin and whey proteins has been investigated (Guerin et al., 2003). As a result, the encapsulated cells survived better that free cells in simulated gastric pH and bile solutions. Annan et al. (2008) have reported that encapsulation in alginate-coated gelatin microspheres improved survival of the probiotic B. adolescentis 15,703 T during exposure to simulated gastrointestinal conditions. Gelatin microspheres were cross-linked with the non-cytotoxic genipin and coated with alginate cross-linked by Ca2+ from external or internal sources. The alginate coat prevented pepsin-induced degradation of the gelatin microspheres in simulated gastric juice (pH 2.0, 2 h), resulting in significantly (P < 0.05) higher numbers of survivors due to the buffering effect of intact microspheres.
Chitosan
Chitosan (the N-deacetylated product of the polysaccharide chitin) is an important coating material which can be isolated from crustacean shells, insect cuticles and the membrane of fungi. Chitosan is a cationic linear polysaccharide composed essentially of β (1-4)-linked glucosamine units together with some proportion of N-acetyl glucosamine units. Lee et al. (2004) have investigated the effects of chitosan-alginate microparticles on the survival of L. bulgaricus KFRI 673 in simulated gastric juices and intestinal fluid and on their stability during storage at 4 and 22 °C. These coating materials are effective to protect bacteria from harsh environment such as acidic pH. The stirring rate, level of the gelling agent, temperature, concentration of the surfactant polymer and the viscosities of the phases were reported to influence morphology and size of particles (Peniche et al., 2003).
Whey proteins
Gbassi et al. (2009) used whey proteins as a coating material to improve encapsulation of L. plantarum strains (L. plantarum 299v, L. plantarum 800 and L. plantarum CIP A159) in calcium alginate beads. It was concluded that whey proteins are a convenient, cheap and efficient material for coating alginate beads loaded with bacteria. Picot and Lacroix (2004) successfully encapsulated Bifidobacterium strains (B. breve R070 and B. longum R023) in whey protein-based microcapsules. B. breve R070 exhibited high survival rate during spray drying and encapsulated cells showed high viability than none-capsulated ones during 28 days storage in low pH yoghurts and simulated gastrointestinal environment.
Miscellaneous compounds
Heidebach et al. (2009) have investigated the microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Rennet could be used to prepare water-insoluble microcapsules based on milk proteins without significant loss of cells during encapsulation process. In another study, Lactobacillus F19 and Bifidobacterium Bb12 were encapsulated in casein-based microcapsules produced by enzymatic gelation with transglutaminase (Heidebach et al., 2010). Lactobacillus F19 survived in higher numbers in the encapsulated state compared to free cells. Encapsulation improved the survivability of Bifidobacterium Bb12 during storage for up to 90 days.
Chan and Zhang (2002) have investigated the use of methacrylic acid copolymer for the compression coating of L. acidophilus. It appears that this coating material when used together with pectin can be useful in order to target delivery of the probiotics to the terminal ileum and the beginning of the colon in human gastrointestinal tract. The coating material used in this study was a mixture of sodium alginate and hydroxylpropyl cellulose in the weight ratio 9:1. Moreover, cellulose acetate phthalate (a cellulose derivative polymer) is physiologically inert and can be used as an enteric coating material for target delivery of core substances in intestinal tract. Cellulose acetate phthalate has proven effective in microencapsulation of probiotic bacteria by both spray-drying and emulsion techniques (Anal and Singh, 2007). It was demonstrated that encapsulation of B. pseudolongum in cellulose acetate phthalate using emulsion method protected bacteria from acidic environment (simulated gastric environment) (Rao et al., 1989). In another study, B. lactic and L. acidophilus were encapsulated in cellulose acetate phthalate polymer using spray-drying technique (Favaro-Trindale and Grosso, 2002). This method was effective in protection of both these probiotic bacteria from low pH media similar to human stomach.
Moolman et al. (2006) reported the encapsulation of probiotics with an inter-polymer complex in supercritical carbon dioxide. This method was used to encapsulate indomethacin and B. lomgum in a poly (vinyl pyrrolidone)-poly (vinyl acetate-co-crotonic acid) inter-polymer complex. Ding and Shah (2009) have investigated the effect of various encapsulating materials (alginate, guar gum, xanthan gum, locust bean gum and carrageenan gum) on the stability of probiotic bacteria, including L. rhamnosus, B. longum, L. salivarius, L. plantarum, L. acidophilus, L. paracasei, B. lactis type B1-04, B. lactis type Bi-07, HOWARU L. rhamnosus and HOWARU B. bifidum. Consequently, probiotic bacteria encapsulated in alginate, carrageenan gum and xanthan gum survived better (P < 0.05) than free probiotic bacteria under acidic conditions. Moreover, these encapsulating materials improved the survival of probiotic bacteria when exposed to bile salts. Xanthan gum is an exopolysaccharide derived from the plant-pathogenic bacterium Xanthomonas campestris and is composed of glucose, mannose and glucuronic acid (Nisperos-Carriedo, 1994). Gellan gum is a microbial polysaccharide derived from Pseudomonas elodea and is constituted of a repeating unit of four monosaccharide molecules (glucose, glucuronic acid, glucose and rhamnose). Several investigations have reported the combination of gellan and xanthan as microencapsulating materials (Muthukumarasamy et al., 2006; McMaster et al., 2005). The combination of xanthan and gellan gums to form bead is not only acid resistant but also is stabilized by calcium ions which may protect probiotic cells from acid injury. For instance, it was reported that at pH 2.5, the viable count of encapsulated Bifidobacterium spp. with gellan-xanthan gum decreased only 0.67 log in 30 min (Sun and Griffiths, 2000).
Common methods for microencapsulation
Extrusion and emulsion
Extrusion and emulsion techniques, which are called droplet and two-phase system methods, respectively, are two basic manners of encapsulation of probiotic microorganisms. In general, the extrusion technique is simple and inexpensive method with gentle operations, minimizing cell injuries and supporting relatively high viability of probiotic cells. Biocompatibility and flexibility are the other virtues of this method. The most important disadvantage of this method is that it could not be feasible for large-scale production due to slow formation of the microbeads. In general, the size of beads formed from this method (~2–5 mm) is larger than those produced by the emulsion method (e.g., spherical beads made by emulsification techniques have bead diameter ranging from 25 μm to 2 mm). With the extrusion method, the size of the capsules is dependent on the viscosity of sodium alginate solution, the extruder orifice diameter and the distance between the syringe and the calcium chloride collecting solution (Smidsrod and Skjak-Braek, 1990). A higher concentration of sodium alginate results in high viscosity which leads to large particle sizes. Low-temperature extrusion technique could be used in microencapsulation of microorganisms and enzymes. In this technique, encapsulation is accomplished in a plasticized composite matrix consisting of fat, flour and starch. After addition of mentioned mixture to the encapsulated solution, the resulting paste (approximately 20 % moisture content) is chopped in a chopping system till particles with a diameter range of 0.5–1.5 mm are produced (Mortazavian et al., 2007b; Mortazavian and Sohrabvandi, 2006).
The emulsion technique is more expensive than the extrusion method due to the need for using vegetable oil for emulsion formation (Mortazavian et al., 2007b). In this method, a small volume of cell/polymer slurry (dispersed phase) is added to the large volume of vegetable oil (continuous phase) such as sun flower, soy, corn, mille or light paraffin oil. Sheu and Marshall (1993) developed a method to entrap bacteria using a water/oil system. The encapsulation material is first mixed with probiotic bacteria and the mixture is suspended in an oil bath containing Tween 80 (as the emulsifying agent). Then, the produced emulsion is broken by adding CaCl2 and the produced microcapsules are collected by centrifugation. Sodium alginate, κ-carrageenan with KCl as the emulsion breaker and genipin cross-linked gelatin can be used to microencapsulate probiotic bacteria by emulsion method (Adhikari et al., 2002; Adhikari et al., 2000; Annan et al., 2008). It has been reported that concentration and viscosity of the encapsulation mix before gelation, agitation rate of mixture and type of emulsifier used are the main important parameters which control the diameter of the final formed microbeads. Very large beads (approximately 1,000 μm or larger) can give a coarse texture and a weakness in coated structures (Mortazavian et al., 2008). Diameter of beads significantly influences the viability of probiotic cells and their metabolic rate and sensory properties of the final product. It also affects distribution and dispersion quality of microbeads within the product (Mortazavian et al., 2007b; Mortazavian and Sohrabvandi, 2006).
Freeze drying and spray drying
The use of freeze-drying and spray-drying methods and the key parameters of these two processes are critical in providing high viability levels (Anal and Singh, 2007). Both freeze-drying and spray-drying methods can be used in microencapsulation of probiotics on a large-scale, but both approaches expose the cultures to extreme environmental conditions. Semyonov et al. (2010) evaluated the implementation of spray-freeze drying (SFD) to produce dry microcapsules of L. paracasei with high viability. They investigated the survival of the cells encapsulated in a matrix of maltodextrin and trehalose. Consequently, SFD can be used to produce dry microcapsules of probiotic cells with high viability (>60 %). It seems that trehalose concentration and maltodextrin molecular weight were critical factors which affected on final probiotic viability.
In summary, the principle of spray-drying technique involves dissolving a polymer, in the continuous phase, which surrounds the core material particles inside the sprayed droplets. The drying process causes this solution to shrink into a pure polymer envelope enclosing the core material. Spray drying can be used as a cost-effective method to produce large quantities of probiotic cultures when critical parameters such as the type of atomization, air pressure and outlet-air temperature have been controlled (Gardiner et al., 2000; Champagne and Møllgaard, 2008). Different polysaccharides were used as the matrix and the nozzle temperature of the spray dryer as well as the water activity of the microcapsules had an important impact on the survival of probiotic bacteria.
In freeze drying, the product is frozen to below the critical temperature of the formulation. Then, the freezing process is followed by primary drying, where the chamber pressure is lowered, the shelf temperature usually increased and the unbound water removed by sublimation. Finally, a secondary drying step is done to remove the bound water by desorption and the product is gradually brought back to ambient temperature (Jennings, 1999; Oetjen, 1999). In the freeze-drying technique, the important factors including pH of the medium, content of protective compounds (e.g., carbohydrates), prehistory of biomass (such as medium stress, harvest time), initial cell concentration, freezing rate and temperature (e.g., the use of liquid nitrogen−196 °C) should be controlled (Carvalho et al., 2004). For instance, Schoug et al. (2006) demonstrated that the freezing survival of L. coryniformis Si3 was affected by the sucrose concentration, cell density and freezing rates. The critical and important contributors to viability loss during freeze drying are osmotic shock and membrane injury resulting from intracellular ice formation and recrystallisation.
Among the numerous methods proposed for manufacturing microcapsules, spray drying is the most appropriate technique for developing water-insoluble dry microcapsule preparations with small and controlled particle size (Picot and Lacroix, 2003a; Groboillot et al., 1994). This system is desirable for incorporating immobilized probiotic bacteria in food products because of various important reasons, including higher stability, easier handling and storage of cultures and limited effects on sensorial and organoleptic properties of foods. Nevertheless, microencapsulation by spray drying for protecting active materials is rarely considered for cell immobilization because of the high mortality resulting from simultaneous dehydration and thermal inactivation of probiotic microorganisms. The exposure to high air temperature during spray-drying process, which are required to facilitate water evaporation during the passage of the bacteria in the spray-drying chamber has negative impact on their viability and hence their biological activity and effectiveness in the spray-dried product. On the other hand, because of the important role of water in stability of biological molecules, the removal of water may cause irreversible changes in functional and structural integrity of bacterial membranes and proteins (Picot and Lacroix, 2003a). Picot and Lacroix proposed easy scaled-up and low-cost microencapsulation method to improve the stability of probiotic lactic cultures and to overcome these aforementioned limits. This technique consisted of coating milk fat droplets containing powder particles of freeze-dried bacteria with whey protein polymers, using emulsification and spray drying in a continuous two-step process (Picot and Lacroix, 2003b).
Important parameters affecting microencapsulation
In order to protect probiotics from stresses and heat treatment, researchers have investigated the tolerance of probiotic microorganisms to stress by using different composites of carrier matrix systems (Leverrier et al., 2005; Boza et al., 2004). Lian et al. (2002) have investigated the effects of types of probiotic strains and carriers on survival of bifidobacteria after spray drying. As a result, spray drying at 10 % (w/w) gelatine, gum Arabic or soluble starch showed the highest survival of bifidobacteria. Among the organisms, B. longum B6 was the least susceptible to spray drying under the test conditions. Moreover, B. longum B6 and B. infantis CCRC 14633 were microencapsulated by spray drying the cell suspension containing the test organisms and 10 % (w/w) of the carrier material of gelatine, soluble starch, skim milk or gum Arabic. Survivability of these organisms was tested in simulated gastric juice (pH 2.0 and 3.0) and bile solution (0.5 % and 2.0 %). It was concluded that encapsulation of probiotic microorganisms with spray drying caused higher survival than free cells specially when exposed to gastric juice. Kearney et al. (2009)) monitored the viability of probiotic L. paracasei NFBC 338 during spray drying. They concluded that live bacterial cell counts in spray-dried yoghurt powders depended on their susceptibility to the spray drying temperature and also on the numbers in the yoghurt prior to drying process. The important reason for manufacturing yoghurt in a spray-dried powder form is to improve its shelf-life by preserving the product in a stable and readily usable state. Ananta et al. (2005) evaluated the feasibility and applicability of spray drying to produce dry skim-milk-based preparations containing probiotic bacteria L. rhamnosus GG (ATCC 53103). Moreover, polydextrose-based and oligofructose-based prebiotic substances were also included in the carrier matrix to investigate their protection capacity. Using reconstituted milk as the drying medium achieved bacteria survival rate of > 60 % at an outlet temperature of 80 °C. The incorporation of mentioned commercial prebiotic substances in the skim-milk powder did not exert any adverse effect on bacterial survival upon spray drying, but impaired the stability of bacteria during long-term storage. Therefore, the maintenance of the structural and functional integrity of the bacterial cell membranes when drying in reconstituted skim-milk/Raftilose® P95 and reconstituted skim-milk/Polydextrose blends was not as effective as when drying in skim-milk alone. Using L. paracasei NFBC 338, it was demonstrated that survival rate of >80 % was achievable during spray drying in reconstituted skim milk, while under similar conditions (outlet temperature of 80 °C), Ananta et al. (2005) reported a survival rate of >60 % for probiotic bacteria L. rhamnosus GG (ATCC 53103).
Several important factors can influence the viability and survival of probiotic bacteria during dehydration, such as stress treatment, growth phase of probiotic culture prior to dehydration, genetic modification and growth media. It has been revealed that bacteria respond to changes in their immediate surroundings by a metabolic reprogramming which leads to a cellular state of enhanced resistance. For instance, pre-adaptation of L. paracasei NFBC 338 by exposure to 0.3 M NaCl resulted in significant resistance to heat stress associated with spray drying (at outlet temperatures of 95–100 °C) in comparison with non-adapted control cells (33.46 ± 2.3 % versus 8.27 ± 4.42 % survival, respectively) (Desmond et al., 2001). It has been reported that the viability of the heat-adapted L. paracasei NFBC 338 in reconstituted skim milk was enhanced 18-fold during spray drying at outlet temperatures of 95–105 °C (Desmond et al., 2001). It is well known that the stress responses of bacterial cultures depend on the growth phase. The optimal growth phase for dehydration survival is the stationary phase. For instance, it has been reported that stationary phase cells of L. rhamnosus yielded the highest recovery rate after drying (31–50 % survival), but early log-phase cells exhibited only14% survival and lag phase cells showed the highest susceptibility, with only a 2 % cell survival under similar conditions of drying (Corcoran et al., 2004). Saarela et al. (2004) reported that freeze drying and storage stability performance of B. animalis subsp. lactis cells grown to a late-logarithmic growth phase (15 h) or to an early stationary phase. On the other hand, Carvalho et al. (2003) reported that starvation of stationary phase L. bulgaricus cultures resulted in improved resistance during storage in the dried state. Moreover, the final pH value of the growth media of the probiotic cultures also influences the survival during desiccation. It was demonstrated that the highest viability (approximately 80 % survival) was obtained following freeze-drying process, when L. reuteri cells were grown at pH 5 and harvested after 2.5 h in the stationary phase (Palmfeldt and Hahn-Hägerdal, 2000). Viability of probiotic cultures during dehydration could be enhanced by over-expression of the genes encoding various stress inducible proteins. New findings in the field of genomics and proteomics have led to the identification of genes involved in Lactobacillus stress responses such as the molecular chaperone groESL and dnaK. Walker et al. (1999) demonstrated that features of the groESL operon are shared between various LAB. It has also been reported that groESL over-expression in L. paracasei NFBC 338 resulted in improved performances during spray drying and freeze drying but did not contribute to enhanced survival of probiotic cultures during storage in the powder form (Corcoran et al., 2006). Furthermore, strains expressing betL exhibited a significant increase in resistance to several stresses. For example, the percent survival of UCC118-betL+ during freeze drying was 36 %, compared to 18 % for UCC118-betL− (Sheehan et al., 2006). Moreover, the tre locus plays critical role in freeze drying of L. acidophilus. Analysis of the L. acidophilus NCFM genome revealed that a putative trehalose utilization locus consisting of a transcriptional regulator (treR), a trehalose phosphoenolpyruvate transferase system transporter (treB) and a trehalose-6-phosphate hydrolase (treC). It was demonstrated that disruption of both the hydrolase genes and trehalose transporter abolished the ability of L. acidophilus NCFM to grow on trehalose and reduced the survival of this microorganism when subjected to repeated cycles of freezing and thawing in the presence of trehalose, demonstrating that not only is the internalization of trehalose important, but also its subsequent hydrolysis is an important contributing factor (Duong et al., 2006).
It has been demonstrated that different bacterial species vary with respect to spray-drying tolerance, indicating the importance of strain selection. The criteria for the selection of probiotic microorganisms includes acid tolerance, bile tolerance, heat tolerance and ability to metabolize probiotics, adherence and colonization to intestinal epithelium/tissue, stimulating immune response, antimicrobial activity/antagonisms to pathogens, improving host digestion, etc. It is well known that thermal and osmotic resistance of LAB is species-dependent. The survival of probiotics after spray drying also depends on the kinds and concentrations of carriers used as well as on the outlet temperature of the spray dryer (Ananta et al., 2005; Hisiao et al., 2004; Lian et al., 2003; Lian et al., 2002). Among different probiotic microorganisms, thermophilic or thermotolerant ones such as S. salivarius subsp. thermophilus, L. helveticus and L. delbrueckii ssp. bulgaricus or lactis have an advantage in withstanding higher temperatures during processing and storage (Gardiner et al., 1998; Gardiner et al., 2000; Rodtong and Tannock, 1993; Drake et al., 1996). For instance, L. paracasei NFBC 338 survived significantly better that L. salivarius UCC 118 at similar spray-drying conditions, which may be attributed to the greater thermal tolerance of L. paracasei compared to L. salivarius (Gardiner et al., 2000). Furthermore, it has been shown that B. animalis subsp. lactis survived spray drying at ~70 % or greater in reconstituted skim milk (20 % w/v) at an outlet temperature of 85–90 °C (Simpson et al., 2005).
A variety of protectants have been added to drying media before the processes of freeze drying and spray drying to protect the viability of probiotics during dehydration (Morgan et al., 2006). These protectants including skim milk powder, whey protein, trehalose, glycerol, betaine, sucrose, glucose, lactose, adonitol and polymers (e.g., polyethylene glycol and dextran) (Anal and Singh, 2007; Heidebach et al., 2010; Lian et al., 2002). For instance, it has been reported that survival of L. helveticus during vacuum drying was improved by addition of 1 % sorbitol (Santivarangkna et al., 2006). It was well documented that the addition of carbohydrates can improve the viability of probiotics and has protective effects for probiotic bacteria during freeze drying. These cryoprotectants can raise the glass-phase transition temperature and therefore viable cells can reach the glassy phase without nucleating intracellular ice (Fowler and Toner, 2005). It also has been reported that trehalose is an effective cryoprotectant during freeze drying, enabling higher survival of L. acidophilus, due to the remarkably high glass transition temperature of trehalose and the strong ion-dipole interactions and hydrogen bonding between trehalose and the biomolecules (Conrad et al., 2000; Patist and Zoerb, 2005). Moreover, it was demonstrated that trehalose, trehalose/lactose and lactose/maltose were the most efficacious disaccharides during freeze drying (Meng et al., 2008). In one study, the influence of microencapsulation, prebiotics and cryoprotectants on the viability of probiotic bacteria (L. acidophilus, L. casei, L. rhamnosus and Bifidobacterium spp.) in yoghurt and freeze-dried yoghurt after processing and storage have been investigated (Capela et al., 2006). There was a 7 % improvement in the viability of L. casei 1,520 when cryoprotectant (Unipectine TM RS 150) was added at 2.5 % (w/v). The prebiotic (Raftilose® P95) when added at 1.5 % w/v to yoghurt improved the viability of the combined selected probiotic organisms by 1.42 log during four weeks of storage at 4 °C. Microencapsulation with alginate, improved viability of combined selected probiotic organisms by 0.31 log in freeze-dried yoghurt stored at 21 °C. Skim milk and sucrose which have been used commonly as cryoprotectants are considered to be capable of preventing cellular injury. The protective activity of sucrose is suggested to be due to its ability to prevent injurious eutectic freezing of cell fluids by trapping salts in a highly viscous or glass-like phase, whereas skim milk is capable of preventing cellular injury by stabilizing the cell membrane (Saarela et al., 2006).
True selection of capsule materials regarding their purpose surrounding environment is very important. For instance, leakage of calcium ions from alginate capsules structure leads to its decomposition. Therefore, alginate capsules should be avoided from environments containing high acidity and chelating agents. However, in milk-based media such as liquid milk, cream and yoghurt, due to availability of high levels of calcium ions, leaching of calcium ions from gel-bead structures could be considerably inhibited. Therefore, gel-beads maintain their shape and structure (Truelstrup-Hansen et al., 2002). Using resistant starch as a capsule material makes beads resistant against enzymatic digestion (Dimantov et al., 2003). Sometimes it is necessary to use especial types of hydrophobic components for encapsulation to make the beads tolerant against the high moisture conditions of products (Truelstrup-Hansen et al., 2002).
Concentration of the capsule-making solution and final beads diameter are important factors in the encapsulation effectiveness. In parallel with increasing beads diameter, their protective effects against the violent environmental factors increase (Truelstrup-Hansen et al., 2002). Sultana et al. (2000) reported that alginate capsules with the range of 0.5–1.0 mm in diameter significantly increased viability of bifidobacteria in yoghurt with normal pH during refrigerated storage, but not at the simulated stomach pH. Increasing beads diameter more than the especial limit (regarding types of capsule and product) is inapplicable because of causing inappropriate mouth-feel and flavor. Furthermore, increasing capsule diameter leads to decreasing its digestibility by pancreatic enzymes. Increasing of beads diameter especially when resistant starch is used for capsule formation should be under attention because this component is resistant to enzymatic digestion of pancreas (Dimantov et al., 2003). Research relevant to the concentration of capsule-making solutions has revealed that raising concentration of alginate solution from 0.75 % to 1.8 % has noticeable effects on L acidophilus viability under the simulated gastric conditions; but at >2 %, it was impossible to generate spherical and homogeneous beads due to increase in solution viscosity and decrease in its of mass diffusivity (Chandramouli et al., 2004). Moreover, increasing the solution concentration containing calcium alginate and HACS (>2 %, of even up to 4 %) did not have any considerable effect on the protective properties of beads against intensive environmental factors (Sultana et al., 2000).
Type and severity of detrimental environmental factors are some of the most important parameters that reduce encapsulation effectiveness. For instance, capsules tolerate low acidic environments such as yoghurt medium much more than violent acidic conditions such as gastric juices (Sultana et al., 2000; Truelstrup-Hansen et al., 2002). It has been reported that alginate capsules with a mean diameter of 100 μm are effective enough for the most types of fermented products, but not for gastric acid (Cui et al., 2000). There is a report regarding digestion of starch capsules by encapsulated bacteria (Takata et al., 1977). Therefore, prior to selection of capsule materials for encapsulation, ability of the enclosed bacteria to digest starch should be considered.
Sensory quality of fermented milks with microencapsulated probiotics
Microencapsulation of probiotics has certain consequences for sensory quality of fermented milk products. The shape and size of capsules affecting the sensory quality of final products are important issues for industrial production. Furthermore, in spray-drying process, the outlet temperature may affect the colour of the capsules due to a Maillard reaction (a form of non-enzymatic browning similar to caramelization) (McMaster et al., 2005; O′Riordan et al., 2001; Su et al., 2007). The addition of encapsulated probiotic bacteria did not significantly change the appearance and color, acidity, flavor, or aftertaste of the yoghurts, but significantly affected their textural properties (smoothness) (Krasaekoopt et al., 2006). Although microencapsulation of probiotic cells can be applied as an efficient method to improve the sensory attributes of the probiotic fermented milks, its unsuitable usage might lead to the off flavor and/or off texture of the final product. For example, encapsulation of B. longum and B. lactis in milk led to an especial off-flavor which was not observed in the product containing free cells of the same bacteria. This fact was attributed to changes in the metabolic pathways of the encapsulated cells which caused production of small-bitter peptides (Truelstrup Hansen et al. 2002).
Adhikari et al. (2002) observed that encapsulation lowered the acetic acid content in yoghurt significantly if bifidobacteria were added to the product before fermentation. Acetic acid produced by Bifidobacterium spp. gives a vinegar taint to the fermented probiotic products such as yoghurt (Adhikari et al., 2000). This off flavor which is mainly produced during the fermentation period develops within storage time. Microencapsulation of bifidobacteria has been used to overcome this problem, because the amount of produced acetic acid in yoghurt generated with encapsulated bifidobacteria was considerably lower than those produced by non-encapsulated ones thereby, improving the flavor properties of fermented probiotic products (Adhikari et al., 2000).
It has been understood that microbeads with diameters more than the special limit (>100 μm, particularly more than 1 mm) can deteriorate mouth-feel properties of products such as liquid milk and yoghurt due to the appearance of the special sense of coarseness. Beads with the range of 1–3 mm in diameter can adversely affect both texture and flavor of the final product might be adversely affected (Chandramouli et al., 2004). It should be mentioned that increasing the beads diameter to more than the particular limit (regarding type of capsule and microorganism) has been proved to have no significant effect on the viability of the cells (Truelstrup-Hansen et al., 2002).
Conclusions
Fermented milk products have been consumed for nutrition and maintenance of good health. These functional foods containing probiotics and prebiotics can be used to prevent and/or cure some important degenerative diseases. Therefore, it is necessary to optimize the technological and economical aspects of manufacturing of these probiotic foods. In general, a successful stabilization method should be able to immobilize the growth of cells to prevent product spoilage and should retain their viability and survival during storage and the product shelf-life. Several methods have been used to improve the viability of probiotics such as selection of resistant strains (e.g., acid and bile resistant strains), incorporation of micronutrients (such as amino acids and peptides), use of oxygen impermeable containers, two-step fermentation, stress adaptation and microencapsulation. Prebiotics (e.g., lactulose, inulin and a range of oligosaccharides) can also be incorporated with probiotics (symbiotic system) to improve the viability of probiotics. However, the process stability of probiotic microorganisms is not always optimal. Microencapsulation can be applied to maintain the viability of probiotics during food product processing, storage and gastrointestinal passage. When fermented milk products containing microencapsulated probiotics are consumed, the protective barrier must ensure probiotics survival during gastrointestinal passage and at the same time it must ensure their proper release in the intestine in order to ultimately achieve suitable benefits. Several microencapsulation techniques were found to adequately protect the probiotic bacteria and improve the viability and survival of them in fermented milk products. Selection of the best microencapsulation technique plays an important role in maintaining viable cell counts of probiotic microorganisms at sufficiently high levels to assure their therapeutic activity throughout shelf-life. General industrial application of microencapsulation technologies in the case of probiotic bacteria seems still far from achieved and many of details are under the question. Future studies should be concentrated on the aspects such as applying more efficient encapsulation materials and techniques or improving the commonly used ones, minimizing the extra costs incurred by microencapsulation, studying correlations between process factors and microencapsulation effectiveness in fermented milk products and optimization of the process factors to reach the highest viability of probiotics and the most satisfactory sensory quality of the products. The crucial factors affecting microencapsulation must be identified, controlled and optimized in order to protect the viability and enhance the survival of bacteria against adverse environmental conditions. Innovative microencapsulation technologies which can generate large quantities of food-grade materials at low cost are still needed to improve viability and stability of probiotics in fermented milk products and during gastrointestinal transit.
Acknowledgments
This review was supported by Isfahan University of Medical Sciences, Faculty of Pharmacy and Pharmaceutical Sciences.
References
- Adhikari K, Gruen IU, Mustapha A, Fernando LN. Changes in the profile of organic acids in plain set and stirred yogurts during manufacture and refrigerated storage. J Food Qual. 2002;25:435–451. [Google Scholar]
- Adhikari K, Mustapha A, Grun IU, Fernando L. Viability of microencapsulated bifidobacteria in set yogurt during refrigerated storage. J Dairy Sci. 2000;83:1946–1951. doi: 10.3168/jds.S0022-0302(00)75070-3. [DOI] [PubMed] [Google Scholar]
- Anal AK, Singh H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Technol. 2007;18:240–251. [Google Scholar]
- Ananta E, Volkert M, Knorr D. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int Dairy J. 2005;15:399–409. [Google Scholar]
- Annan NT, Borza AD, Hansen LT. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res Int. 2008;41:184–193. [Google Scholar]
- Beheshtipour H, Mortazavian AM, Mohammadi R, Sohrabvandi S, Khosravi-Darani K. Supplementation of Spirulina platensis and Chlorella vulgaris Algae into Probiotic Fermented Milks. Comprehensive Reviews in Food Science and Food Safety. 2013;12:144–154. [Google Scholar]
- Boza Y, Barbi D, Scamparini A. Survival of Beijerinckia sp. microencapsulated in carbohydrates by spray-drying. J Microencapsul. 2004;21:15–24. doi: 10.1080/02652040310001599751. [DOI] [PubMed] [Google Scholar]
- CAC/RCP 243: 2003, Codex Standard for Fermented Milks.
- Capela P, Hay TKC, Shah N. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Res Int. 2006;39:203–211. [Google Scholar]
- Carvalho AS, Silva J, Ho P, Teixeira P, Gibbs P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int Dairy J. 2004;14:835–847. [Google Scholar]
- Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Effect of various factors upon thermotolerance and survival during storage of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. J Food Sci. 2003;68:2538–2541. doi: 10.1021/bp034165y. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham SA, Cummings J. Starch intake and colorrectal cancer risk: an international comparison. Br J Cancer. 1994;69:119–125. doi: 10.1038/bjc.1994.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CP, Møllgaard H. Production of probiotic cultures and their addition in fermented foods. In: Franworth ER, editor. Handbook of Fermented Functional Foods. United States of America: CRC Press Taylor & Francis Group; 2008. [Google Scholar]
- Champagne CP. Microencapsulation of probiotics in food: challenges and future prospects. Ther Deliv. 2012;3:1249–1251. doi: 10.4155/tde.12.121. [DOI] [PubMed] [Google Scholar]
- Chan ES, Zhang Z. Encapsulation of probiotic bacteria Lactobacillus acidophilus by direct compression. Food and Bioproducts Processing. 2002;80:78–82. [Google Scholar]
- Chandramouli V, Kailasapathy K, Peiris P, Jones M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J Microbiol Methods. 2004;56:27–35. doi: 10.1016/j.mimet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Chen KN, Kuo YT. Optimal thermotolerance of Bifidobacterium bifidum in gellan–alginate microparticles. Biotechnol Bioeng. 2007;98:411–419. doi: 10.1002/bit.21450. [DOI] [PubMed] [Google Scholar]
- Conrad PB, Miller DP, Cielenski PR, Pablo JJ. Stabilization and preservation of Lactobacillus acidophilus in saccharide matrices. Cryobiology. 2000;41:124–127. doi: 10.1006/cryo.2000.2260. [DOI] [PubMed] [Google Scholar]
- Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. J Control Release. 2012;162:56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Corcoran BM, Ross RP, Fitzgerald GF, Dockery P, Stanton C. Enhanced survival of GroESL-overproducing Lactobacillus paracasei NFBC 338 under stressful conditions induced by drying. Appl Environ Microbiol. 2006;72:5104–5107. doi: 10.1128/AEM.02626-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran BM, Ross RP, Fitzgerald GF, Stanton C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J Appl Microbiol. 2004;96:1024–1039. doi: 10.1111/j.1365-2672.2004.02219.x. [DOI] [PubMed] [Google Scholar]
- Crittenden R, Weerakkody R, Sanguansri L, Augustin MA. Synbiotic microcapsules that enhance microbial viability during nonrefrigerated storage and gastrointestinal transit. Appl Environ Microbiol. 2006;72:2280–2282. doi: 10.1128/AEM.72.3.2280-2282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Goh J, Kim P, Choi S, Lee B. Survival and stability of Bifidobacteria loaded in alginate poly-L-lysine microparticles. Int J Pharm. 2000;210:51–59. doi: 10.1016/s0378-5173(00)00560-3. [DOI] [PubMed] [Google Scholar]
- Dave RI, Shah N. Viability of yogurt and probiotic bacteria in yoghurts made from commercial starter culture. Int Dairy J. 1997;7:31–41. [Google Scholar]
- De Vuyst L. Technology aspects related to the application of functional starter cultures. Food Technol Biotechnol. 2000;38:105–112. [Google Scholar]
- Desmond C, Ross RP, O′Callaghan E, Fitzgerald G, Stanton C. Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. J Appl Microbiol. 2002;93:1003–1011. doi: 10.1046/j.1365-2672.2002.01782.x. [DOI] [PubMed] [Google Scholar]
- Desmond C, Stanton C, Fitzgerald GF, Collins K, Ross RP. Environmental adaptation of probiotic lactobacilli towards improvement of performance during spray drying. Int Dairy J. 2001;11:801–808. [Google Scholar]
- Dimantov A, Greenberg M, Kesselman E, Shimoni Study of high amylase corn starch as food grade enteric coating in a microcapsule model systems. Innov. Food Sci Eng Technol. 2003;5:93–100. [Google Scholar]
- Dinakar P, Mistry VV. Growth and viability of Bifidobacterium bifidum in Cheddar cheese. J Dairy Sci. 1994;77:2854–2864. doi: 10.3168/jds.S0022-0302(94)77225-8. [DOI] [PubMed] [Google Scholar]
- Ding WK, Shah N. Effect of various encapsulating materials on the stability of probiotic bacteria. J Food Sci. 2009;74:M100–M107. doi: 10.1111/j.1750-3841.2009.01067.x. [DOI] [PubMed] [Google Scholar]
- Divya JB, Varsha KK, Nampoothiri KM, Ismail B, Pandey A. Probiotic fermented foods for health benefits. Eng Life Sci. 2012;12:377–390. [Google Scholar]
- Doherty SB, Auty MA, Stanton C, Ross RP, Fitzgerald GF, Brodkorb A. Survival of entrapped Lactobacillus rhamnosus GG inwhey protein micro-beads during simulated ex vivo gastro-intestinal transit. Int Dairy J. 2012;22:31–43. [Google Scholar]
- Doleyres Y, Lacroix C. Technologies with free and immobilised cells for probiotic bifidobacteria production and protection. Int Dairy J. 2005;15:973–988. [Google Scholar]
- Donkor ON, Henriksson A, Vasiljevic T, Shah N. Effect of acidification on the activity of probiotics in yoghurt during cold storage. Int Dairy J. 2006;16:1181–1189. [Google Scholar]
- Drake M, Small CL, Spence KD, Swanson B. Rapid detection and identification of Lactobacillus spp. in dairy products by using the polymerase chain reaction. J Food Prot. 1996;59:1131–1136. doi: 10.4315/0362-028X-59.10.1031. [DOI] [PubMed] [Google Scholar]
- Duong T, Barrangou R, Russell WM, Klaenhammer TR. Characterization of the tre locus and analysis of trehalose cryoprotection in Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2006;72:1218–1225. doi: 10.1128/AEM.72.2.1218-1225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englyst HN, Kingman SM, Gummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;2(46):33–50. [PubMed] [Google Scholar]
- Favaro-Trindale CS, Grosso C. Microencapsulation of L. acidophilus (La-05) and B lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. J Microencapsul. 2002;19:485–494. doi: 10.1080/02652040210140715. [DOI] [PubMed] [Google Scholar]
- Favaro Trindade CS, Grosso CRF. The effect of the immobilization of Lactobacillus acidophilus and Bifidobacterium lactis in alginate on their tolerance to gastro-intestinal secretions. Milchwissenschaft. 2000;55:496–499. [Google Scholar]
- Fowler A, Toner M. Cryo-injury and biopreservation. Ann N Y Acad Sci. 2005;1066:119–135. doi: 10.1196/annals.1363.010. [DOI] [PubMed] [Google Scholar]
- Gardiner G, O′Sullivan E, Kelly J, Auty MAE, Fitzgerald GF, Collins JK, Ross RP. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Appl Environ Microbiol. 2000;66:2605–2612. doi: 10.1128/aem.66.6.2605-2612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner G, Ross RP, Collins JKG, Fitzgerald GF, Stanton C. Development of a probiotic Cheddar cheese containing human-derived Lactobacillus paracasei strains. Appl Environ Microbiol. 1998;64:2192–2199. doi: 10.1128/aem.64.6.2192-2199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbassi GK, Vandamme T, Ennahar S, Marchioni E. Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins. Int J Food Microbiol. 2009;129:103–105. doi: 10.1016/j.ijfoodmicro.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Gerez CL, Font de Valdez G, Gigante ML, Grosso CRF. Whey protein coating bead improves the survival of the probiotic Lactobacillus rhamnosus CRL 1505 to low pH. Lett Appl Microbiol. 2012;54:552–556. doi: 10.1111/j.1472-765X.2012.03247.x. [DOI] [PubMed] [Google Scholar]
- Gilliland SE. Acidophilus milk products: A review of potential benefits to consumers. J Dairy Sci. 1989;72:2483–2494. doi: 10.3168/jds.S0022-0302(89)79389-9. [DOI] [PubMed] [Google Scholar]
- Godward G, Kailasapathy K. Viability and survival of free, encapsulated and co-encapsulated probiotic bacteria in ice cream. Milchwissenschaft. 2003;58:161–164. [Google Scholar]
- Groboillot AF, Boadi DK, Poncelet D, Neufeld RJ. Immobilization of cells for application in the food industry. CRC critical reviews in biotechnology. 1994;14:75–107. doi: 10.3109/07388559409086963. [DOI] [PubMed] [Google Scholar]
- Guerin D, Vuillemard JC, Subirade M. Protection of bifidobacteria encapsulated in polysaccharide-protein gel beads against juice and bile. J Food Prot. 2003;66:2076–2084. doi: 10.4315/0362-028x-66.11.2076. [DOI] [PubMed] [Google Scholar]
- Hansen LT, Allan-Wojtas PM, Jin YL, Paulson A. Survival of Ca-alginate microencapsulated Bifidobacterium ssp. in milk and simulated gastrointestinal conditions. Food Microbiol. 2002;19:35–45. [Google Scholar]
- Heidebach T, Forst P, Kulozik U. Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocoll. 2009;23:1670–1677. [Google Scholar]
- Heidebach T, Först P, Kulozik U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J Food Eng. 2010;98:309–316. [Google Scholar]
- Heidebach T, Först P, Kulozik U. Microencapsulation of Probiotic Cells for Food Applications. Crit Rev Food Sci Nutr. 2012;52:291–311. doi: 10.1080/10408398.2010.499801. [DOI] [PubMed] [Google Scholar]
- Hisiao H-C, Lian W-C, Chou CC. Effect of packaging conditions and temperature on viability of microencapsulated bifidobacteria during storage. Journal of Food Science and Agriculture. 2004;84:134–139. [Google Scholar]
- Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr. 2001;73:365–373. doi: 10.1093/ajcn/73.2.365s. [DOI] [PubMed] [Google Scholar]
- Huq T, Khan A, Khan RA, Riedl B, Lacroix M. Encapsulation of Probiotic Bacteria in Biopolymeric System. Crit Rev Food Sci Nutr. 2013;53:909–916. doi: 10.1080/10408398.2011.573152. [DOI] [PubMed] [Google Scholar]
- Hyndman CL, Groboillot AF, Poncelet D, Champagne CP, Neufeld R. Microencapsulation of Lactococcus lactis within cross-linked gelatin membranes. J Chem Technol Biotechnol. 1993;56:259–263. [Google Scholar]
- Iyer C, Kailasapathy K. Effect of co-encapsulation of probiotics with prebiotics on increasing the viability of encapsulated bacteria under in vitro acidic and bile salt conditions and in yogurt. J Food Sci. 2005;70:18–23. [Google Scholar]
- Jennings TA. Lyophilisation-Introduction and basic principles. Boca Raton: CRC press; 1999. [Google Scholar]
- Jung JK, Kil JH, Kim SK, Jeon JT, Park KY. Survival of Double-Microencapsulated Bifidobacterium breve in Milk in Simulated Gastric and Small Intestinal Conditions. J Food Sci Nutr. 2007;12:58–63. [Google Scholar]
- Kailasapathy K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT Food Sci Technol. 2006;39:1221–1227. [Google Scholar]
- Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- Kearney N, Meng XC, Stanton C, Kelly J, Fitzgerald GF, Ross R. Development of a spray dried probiotic yoghurt containing Lactobacillus paracasei NFBC 338. Int Dairy J. 2009;19:684–689. [Google Scholar]
- Kebary K. Viability of Bifidobacterium bifidum and its effect on quality of frozen Zabady. Food Res Int. 1996;29:431–437. [Google Scholar]
- Kebary KMK, Hussein SA, Badawi RM. Improving viability of Bifidobacterium and their effect on frozen ice milk. J Dairy Sci. 1998;26:319–337. [Google Scholar]
- Kim S-J, Cho SY, Kim SH, Song O-J, Shin l-S, Cha DS, Park H. Effect of microencapsulation on viability and other characteristics in Lactobacillus acidophilus ATCC 43121 LWT. LWT Food Sci Technol. 2008;41:493–500. [Google Scholar]
- Korbekandi H, Jahadi M, Maracy M, Abedi D, Jalali M. Production and evaluation of a probiotic yogurt using Lactobacillus casei ssp. casei. Int J Dairy Technol. 2008;62:75–79. [Google Scholar]
- Korbekandi H, Mortazavian AM, Iravani S (2011) Technology and stability of probiotic in fermented milks containing probiotics and prebiotics In: Probiotic and prebiotic foods: technology, stability and benefits to the human health. Shah, N.P., da Cruz, A.G., Faria, J.A.F. (Eds.), Nova Science Publishers, Inc. USA.
- Kosin B, Rakshit SK. Microbial and processing criteria for production of probiotics: A review. Food Technol Biotechnol. 2006;44:371–379. [Google Scholar]
- Krasaekoopt W, Bhandari B, Deeth H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int Dairy J. 2004;14:737–743. [Google Scholar]
- Krasaekoopt W, Bhandari B, Deeth H. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT- and conventionally treated milk during storage. LWT Food Sci Technol. 2006;39:177–183. [Google Scholar]
- Lee JS, Cha DS, Park H. Survival of freezed-dried Lactobacillus bulgaricus KFRI 673 in chitosan-coated calcium alginate microparticles. J Agric Food Chem. 2004;52:7300–7305. doi: 10.1021/jf040235k. [DOI] [PubMed] [Google Scholar]
- Lee KY, Heo T. Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salts solution. Appl Environ Microbiol. 2000;66:869–873. doi: 10.1128/aem.66.2.869-873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-K, Puong KY, Ouwehand AC, Salminen S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by Lactobacilli. J Med Microbiol. 2003;52:925–930. doi: 10.1099/jmm.0.05009-0. [DOI] [PubMed] [Google Scholar]
- Lee Y-K, Wong SF. Stability of lactic acid bacteria in fermented milk. In: Salminen S, Wright A, editors. Lactic acid bacteria: microbiology and functional aspects. Inc, New York: Marcel Dekker; 1998. pp. 103–115. [Google Scholar]
- Leverrier P, Fremont Y, Rouault A, Boyaval P, Jan G. In vitro tolerance to digestive stresses of propionibacteria: Influence of food matrices. Food Microbiol. 2005;22:11–18. [Google Scholar]
- Lian WC, Hsiao HC, Chou CC. Survival of bifidobacteria after spray-drying. Int J Food Microbiol. 2002;74:79–86. doi: 10.1016/s0168-1605(01)00733-4. [DOI] [PubMed] [Google Scholar]
- Lian WC, Hsiao HC, Chou CC. Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. Int J Food Microbiol. 2003;86:293–301. doi: 10.1016/s0168-1605(02)00563-9. [DOI] [PubMed] [Google Scholar]
- Lucas A, Sodini I, Monnet C, Jolivet P, Corrieu G. Probiotic cell counts and acidification in fermented milks supplemented with milk protein hydrolysates. Int Dairy J. 2004;14:47–53. [Google Scholar]
- Maitrot H, Paquin C, Lacroix C, Champagne CP. Production of concentrated freeze-dried cultures of Bifidobacterium longum in k-carrageenan-locust bean gum gel. Biotechnol Tech. 1997;11:527–531. [Google Scholar]
- Mandal S, Puniya AK, Singh K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int Dairy J. 2006;16:1190–1195. [Google Scholar]
- Martoni C, Bhathena J, Urbanska AM, Prakash S. Microencapsulated bile salt hydrolase producing Lactobacillus reuteri for oral targeted delivery in the gastrointestinal tract. Appl Microbiol Biotechnol. 2008;81:225–233. doi: 10.1007/s00253-008-1642-8. [DOI] [PubMed] [Google Scholar]
- Mattila-Sandholm T, Myllarinen P, Crittenden RGM, Fonden R, Saarela M. Technological challenges for future probiotic foods. Int Dairy J. 2002;12:173–182. [Google Scholar]
- McMaster LD, Kokott SA, Slatter P. Micro-encapsulation of Bifidobacterium lactis for incorporation into soft foods World. J Microbiol Biotechnol. 2005;21:723–728. [Google Scholar]
- Meng XC, Stanton C, Fitzgerald GF, Daly C, Ross R. Anhydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2008;106:1406–1416. [Google Scholar]
- Mohammadi R, Sohrabvandi S, Mortazavian AM. The starter culture characteristics of probiotic microorganisms in fermented milks. Engineering in Life Sciences. 2012;12:399–409. [Google Scholar]
- Moolman FS, Labuschagne PW, Thantsa MS, Van Der Merwe TL, Rolfes H, Cloete T. Encapsulating probiotics with an interpolymer complex in supercritical carbon dioxide. S Afr J Sci. 2006;102:349–354. [Google Scholar]
- Morgan CA, Herman N, White PA, Vesey G. Preservation of micro-organisms by drying: A review. J Microbiol Meth. 2006;66:183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Mortazavian AM, Ehsani MR, Azizi A, Razavi SH, Mousavi SM, Sohrabvandi S. Viability of calcium alginate-microencapsulated probiotic bacteria in Iranian yogurt drink (Doogh) during the refrigerated storage period and under the simulated gastrointestinal conditions. Aust J Dairy Technol. 2008;63:24–29. [Google Scholar]
- Mortazavian AM, Ehsani MR, Mousavi SM, Sohrabvandi S, Reinheimer J. Effect of refrigerated storage temperature on the viability of probiotic micro-organisms in yoghurt. Int J Dairy Technol. 2007;60:123–127. [Google Scholar]
- Mortazavian AM, Razavi SH, Ehsani MR, Sohrabvandi S. Principles and methods of microencapsulation of probiotic microorganisms.Iranian. J Biotechnol. 2007;5:1–18. [Google Scholar]
- Mortazavian AM, Sohrabvandi S. Probiotics and Food Probiotic Products; based on dairy probiotic products. Tehran: Eta Publication; 2006. [Google Scholar]
- Muthukumarasamy P, Allan WP, Holley RA. Stability of Lactobacillus reuteri in different types of microcapsules. J Food Sci. 2006;71:M20–M24. [Google Scholar]
- Nisperos-Carriedo MO (1994) In: Edible coatings and films to improve food quality. Krochta, J.M., Baldwin, E.A., Nisperos-Carriedo, M. (Eds.), Technomic Publishing Company, Lancaster.
- O′grady BG, Gibson R. Microbiota of the human gut. In: Tamime A, editor. Probiotic Dairy Products. UK: Blackwell Publishing Ltd; 2005. [Google Scholar]
- O′Riordan K, Andrews D, Buckle K, Conway P. Evaluation of microencapsulation of a Bifidobacterium strain with starch as an approach to prolonging viability during storage. J Appl Microbiol. 2001;91:1059–1066. doi: 10.1046/j.1365-2672.2001.01472.x. [DOI] [PubMed] [Google Scholar]
- Oetjen GW. Freeze-drying. Weinheim: Wiley-VCH; 1999. [Google Scholar]
- Oliveira MN, Sodini I, Remeuf F, Corrieu G. Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. Int Dairy J. 2001;11:935–942. [Google Scholar]
- Oliveira RPS, Florence ACR, Silva RC, Perego P, Converti A, Gioielli LA, Oliveira M. Effect of different prebiotics on the fermentation kinetics, probiotic survival and fatty acids profiles in nonfat symbiotic fermented milk. Int J Food Microbiol. 2009;128:467–472. doi: 10.1016/j.ijfoodmicro.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Østlie MH, Helland MH, Narvhus J. Growth and metabolism of selected strains of probiotic bacteria in milk. Int J Food Microbiol. 2003;87:17–27. doi: 10.1016/s0168-1605(03)00044-8. [DOI] [PubMed] [Google Scholar]
- Østlie MH, Treimo J, Narvhus J. Effect of temperature on growth and metabolism of probiotic bacteria in milk. Int Dairy J. 2005;15:989–997. [Google Scholar]
- Palmfeldt J, Hahn-Hägerdal B. Influence of culture pH on survival of Lactobacillus reuteri subjected to freeze-drying. Int J Food Microbiol. 2000;55:235–238. doi: 10.1016/s0168-1605(00)00176-8. [DOI] [PubMed] [Google Scholar]
- Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100:1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- Patist A, Zoerb H. Preservation mechanisms of trehalose in food and biosystems. Colloids Surf B: Biointerfaces. 2005;40:107–113. doi: 10.1016/j.colsurfb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Peniche C, Argüelles-Monal W, Peniche H, Acosta N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol Biosci. 2003;3:511–520. [Google Scholar]
- Peres CM, Peres C, Hernández-Mendoza A, Malcata FX. Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria – With an emphasis on table olives. Trends Food Sci Technol. 2012;26:31–42. [Google Scholar]
- Picot A, Lacroix C. Effects of micronization on viability and thermotolerance of probiotic freeze-dried cultures. Int Dairy J. 2003;13:455–462. [Google Scholar]
- Picot A, Lacroix C. Optimization of dynamic loop mixer operating conditions for production of O/W emulsion to be used for cell encapsulation. Lait. 2003;83:237–250. [Google Scholar]
- Picot A, Lacroix C. Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. Int Dairy J. 2004;14:505–515. [Google Scholar]
- Rao AV, Shivnarain N, Maharaj I. Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Can Inst Food Sci Tech J. 1989;22:345–349. [Google Scholar]
- Ravula RR, Shah N. Viability of probiotic bacteria in fermented frozen dairy desserts. Food Aust. 1998;50:136–139. [Google Scholar]
- Rinkinen M, Jalava K, Westermarck E, Salminen S, Ouwehand AC. Interaction between probiotic LAB and canine enteric pathogens: a risk factor for intestinal Enterococcus faecium colonization. Vet Microbiol. 2003;92:111–119. doi: 10.1016/s0378-1135(02)00356-5. [DOI] [PubMed] [Google Scholar]
- Rodtong S, Tannock G. Differentiation of Lactobacillus strains by ribotyping. Appl Environ Microbiol. 1993;59:3480–3484. doi: 10.1128/aem.59.10.3480-3484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokka S, Rantamäki P. Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur Food Res Technol. 2010;231:1–12. [Google Scholar]
- Saarela M, Rantala M, Hallamaa K, Nohynek L, Virkajärvi I, Mättö J. Stationary-phase acid and heat treatments for improvement of the viability of probiotic lactobacilli and bifidobacteria. J Appl Microbiol. 2004;96:1205–1214. doi: 10.1111/j.1365-2672.2004.02286.x. [DOI] [PubMed] [Google Scholar]
- Saarela M, Virkaja¨ rvi I, yAlakomi H-L, Sigvart-Mattila P, Mättö J. Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. Int Dairy J. 2006;16:1477–1482. [Google Scholar]
- Sabikhi L, Babu R, Thompkinson DK, Kapila S. Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food Bioprocess Technol. 2010;3:586–593. [Google Scholar]
- Santivarangkna C, Kulozik U, Foerst P. Effect of carbohydrates on the survival of Lactobacillus helveticus during vacuum drying. Lett Appl Microbiol. 2006;42:271–276. doi: 10.1111/j.1472-765X.2005.01835.x. [DOI] [PubMed] [Google Scholar]
- Schoug A, Olsson J, Carlfors J, Schnu¨rer J, Hakansson S. Freeze-drying of Lactobacillus coryniformis Si3-effects of sucrose concentration, cell density, and freezing rate on cell survival and thermophysical properties. Cryobiology. 2006;53:119–127. doi: 10.1016/j.cryobiol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Semyonov D, Ramon O, Kaplun Z, Levin-Brener L, Gurevich N, Shimoni E. Microencapsulation of Lactobacillus paracasei by spray freeze drying. Food Res Int. 2010;43:193–202. [Google Scholar]
- Shah N. Probiotic bacteria: selective enumeration and survival in dairy foods. J Dairy Sci. 2000;83:894–907. doi: 10.3168/jds.S0022-0302(00)74953-8. [DOI] [PubMed] [Google Scholar]
- Shah N. Functional foods from probiotics and prebiotics. Food Technol. 2001;55:46–53. [Google Scholar]
- Shah NP, Lankaputhra WEV (1997) Improving viability of Lactobacillus acidophilus and Bifidobacterium ssp. in yogurt. Int Dairy J 7:349–359
- Shah NP, Ali JF, Ravula R (2000) Populations of L. acidophilus, Bifidobacterium spp., and Lactobacillus casei in commercial fermented milk products. Biosci Microflora 19:35–39
- Shah NP, Lankaputhra WEV, Britz ML, Kyle WSA (1995) Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in commercial yoghurt during refrigerated storage. Int Dairy J 5:515–521.
- Sheehan VM, Sleator RD, Fitzgerald GF, Hill C. Heterologous expression of BetL, a betaine uptake system, enhances the stress tolerance of Lactobacillus salivarius UCC118. Appl Environ Microbiol. 2006;72:2170–2177. doi: 10.1128/AEM.72.3.2170-2177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu TY, Marshall RT. Microentrapment of Lactobacilli in calcium alginate gels. J Food Sci. 1993;58:557–561. [Google Scholar]
- Simpson PJ, Stanton C, Fitzgerald GF, Ross RP. Intrinsic tolerance of Bifidobacterium species to heat and oxygen and survival following spray drying and storage. J Appl Microbiol. 2005;99:493–501. doi: 10.1111/j.1365-2672.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- Song SH, Cho YH, Jiyong P. Microencapsulation of Lactobacillus casei YIT 9018 using a microporous glass membrane emulsification system. J Food Sci. 2003;68:195–200. [Google Scholar]
- Su L, Lin C, Chen M. Development of an Oriental-style dairy product coagulated by microcapsules containing probiotics and filtrates from fermented rice. Int J Dairy Technol. 2007;60:49–54. [Google Scholar]
- Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yogurt. Int J Food Microbiol. 2000;62:47–55. doi: 10.1016/s0168-1605(00)00380-9. [DOI] [PubMed] [Google Scholar]
- Sun W, Griffiths MW. Survival of bifidobacteria in yogurt and simulated gastric juice following immobilization in gellan–xanthan beads. Int J Food Microbiol. 2000;61:17–25. doi: 10.1016/s0168-1605(00)00327-5. [DOI] [PubMed] [Google Scholar]
- Takata I, Tosa T, Chibata I. Screening of matrix suitable for immobilization microbal cells. J Solid-phase Biochem. 1977;2:225–236. [Google Scholar]
- Talwalkar A, Kailasapathy K. Effect of microencapsulation on oxygen toxicity in probiotic bacteria. Aust J Dairy Technol. 2003;58:36–39. [Google Scholar]
- Tamime A. Fermented milks. Ltd: Blackwell Science; 2006. [Google Scholar]
- Tamime AY, Robinson R. Yoghurt: science and technology. 2. Cambridge: Woodhead publishing; 1999. [Google Scholar]
- Tamime AY, Saarela M, Korslund Sondergaard A, Mistry VV, Shah N. Production and maintenance of viability probiotics micro-organism in dairy products. In: Tamime A, editor. Probiotic Dairy Products. UK: Blackwell Publishing Ltd; 2005. pp. 39–72. [Google Scholar]
- Truelstrup Hansen L, Allan-Wojtas PM, Jin YL, Paulson AT. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastro-intestinal conditions. Food Microbiol. 2002;19:35–45. [Google Scholar]
- Walker DC, Girgis HS, Klaenhammer TR. The groESL Chaperone Operon of Lactobacillus johnsonii. Appl Environ Microbiol. 1999;65:3033–3041. doi: 10.1128/aem.65.7.3033-3041.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenrong S, Griffiths M. Survival of bifidobacteria in yogurt and simulated gastric juice following immobilization in gellan-xanthan beads. Int J Food Microbiol. 2000;61:17–25. doi: 10.1016/s0168-1605(00)00327-5. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Food and Agriculture Organization of the United Nations (WHO/FAO) (2001) Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO Food and Nutrition Paper 85; Rome 2006. Online accessed on July 3, 2007: ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf.
- Yáñez-Fernández J, Ramos-Ramírez EG, Salazar-Montoya JA. Rheological characterization of dispersions and emulsions used in the preparation of microcapsules obtained by interfacial polymerization containing Lactobacillus sp. Eur Food Res Technol. 2008;226:957–966. [Google Scholar]