Abstract
Germinated, fermented and raw Cucumeropsis mannii (melon) seeds were processed into flours. A portion of the flours were defatted using n-hexane; both the full fat and defatted flours were evaluated for proximate composition and functional properties. The proximate compositions of the full fat and defatted C. mannii seed flours were: moisture, 4.97–5.67 % and 6.17–8.13 %; total ash, 1.95–3.24 % and 4.38–7.19 %; crude protein, 36.62–39.91 % and 71.91–77.05 %; crude fat, 45.06–49.57 % and 1.56–2.57 %; crude fibre, 2.71–3.63 % and 4.34–4.59 %; carbohydrate, 3.78–4.07 % and 4.45–6.54 %, respectively. The functional properties of the flour from full-fat and defatted seed were: water absorption capacity, 116.67–183.33 % and 216.67–267.67 %; oil absorption capacity, 252.33–274.00 % and 292.00–345.00 %; foaming capacity 17.36–30.34 % and 34.78–44.69 %; foaming stability, 5.17–11.54 % and 11.41–14.55 %; least gelation concentration, 24.67–28.00 and 13.33–18.67 %; emulsion capacity, 49.73–79.28 mL/g and 40.34–65.61 mL/g; bulk density 0.65–0.81 g/mL and 0.36–0.39 g/mL; protein solubility 4.00–5.89 % and 5.21–7.11 %, respectively. Germination enhanced the water absorption capacity, foaming capacity and protein solubility while fermentation increased the emulsion capacity. Defatting improved the water and oil absorption capacities, foaming capacity and protein solubility. The flour from germinated seeds may find use as ingredients in food emulsion and salad dressing, while those from fermented seeds may be used as food thickeners.
Keywords: Cucumeropsis mannii, Fermentation, Functional properties, Germination, Proximate composition
Introduction
Cucumeropsis mannii is a tropical African plant which belongs to the cucurbitaceae family (Sanjur et al. 2002). It is an annual climbing stems that grows in wet humid climate particularly in the South Western part of Nigeria. Cucurbitaceae is widely grown in sub-Saharan Africa not for the pulp which is bitter, but for the seeds which are particularly rich in protein and oil content ranging from 43 to 48 % (Badifu and Ogunsua 1991; Koffi et al. 2008; Ogunbusola et al. 2012). The full fat meal is used in several dietary preparations that vary with the food habits of the people (Odibo et al. 1990; de Mello et al. 2000). In Sub-Saharan Africa, C. mannii is prized for its oleaginous seeds. The seed kernel is traditionally used for preparing ‘egusi’ soups in Cameroon, Nigeria and Benin, and pistachio soup in Cote d’Ivoire, in which it functions as thickening, emulsifying, fat binding and flavoring agents (Enujiugha and Ayodele-Oni 2003; Loukou et al. 2007). The seeds can be dried, roasted and eaten as snacks and it can be fermented and used as soup condiment called ‘Ogiri’ (Odibo et al. 2012). The oil is also used domestically and highly valued. In today’s food product development, melon and some other oil seeds could become a functional ingredients as well as vegetable protein source. Utilization of any plant protein sources in new food product development or as food supplement is based on the knowledge of their functional properties and nutritional composition. Studies on Cucurbitaceae have been limited to bitter guard (Citrullus colocynthis), water melon (Citrullus vulgaris) and fluted pumpkin (Telfairia occidentalis Hook) (Akobundu et al. 1982; Fagbemi and Oshodi 1991; Fagbemi 2007). C. mannii is one of the cheapest among the Cucurbitaceae family, yet highly underutilized. It is therefore imperative to study the effects of various processing techniques on the seeds in order to effectively exploit it in food system. This study examined the nutritional composition and functional properties of full fat and defatted germinated and fermented C. mannii seed flours to explore various utilization option for the underutilized seeds.
Materials and methods
Sample preparation
Cucumeropsis mannii seeds were obtained from ‘Bisi’ market in Ado Ekiti, Ekiti State, Nigeria. The unshelled C. mannii seeds were sorted to remove the unwholesome and extraneous materials. The seeds were divided into three portions. The first was germinated as described by Giami and Bekebain (1992). Briefly, the seeds were soaked in water for 1 h and then spread on a wet cotton wool, moistened daily and observed for sprouting. Sprouted kernels (≤1 cm) were spread on a tray and oven dried at 50 °C for 6 h. The dried germinated seeds were shelled manually and dried further for 1 h at 50 °C, the seeds were milled into flour using Amalex blender and sieved to pass through 300 μm mesh size. The second portion was shelled manually and fermented by soaking in warm water at ambient temperature for 4 days without changing the water. It was then dried in a hot air oven at 50 °C for 6 h, milled and sieved to pass through 300 μm mesh size. The last portion was shelled manually, washed and dried in a hot air oven at 50 °C for 6 h, milled and sieved to pass through 300 μm mesh size. The flours obtained from the different portions were divided into two. One portion was defatted continuously using soxhlet apparatus for 8 h with n-hexane as solvent. The defatted flours were further milled into fine powder, packaged and kept in cool dry area for further analysis.
Proximate analyses
Moisture, total ash, crude fat and crude fibre contents were determined using the standard methods of Association of Official Analytical Chemists (AOAC 2005). The crude protein content was determined by the micro Kjeldahl nitrogen method and the nitrogen content was converted to protein using a 6.25 conversion factor. Carbohydrate content was determined by difference. This was carried out by adding together the value of moisture, total ash, crude fat, crude fibre and protein in percentages, and subtracting from 100 %.
Functional properties
The water and oil absorption capacity (WAC, OAC) of the seed flours were determined as described by Fagbemi et al. (2012) adapted from Sathe et al. (1982). Distilled water (ρ = 1 g/ml) and Executive Chef® vegetable oil (ρ = 0.92 g/ml) were used for WAC and OAC determinations, respectively. Briefly, to 1.0 g of the flour sample 10 ml of water (for WAC) of oil (for OAC) was added in a beaker and stirred using magnetic stirrer for 5 min. The resulting suspension was centrifuged for 30 min at 2,500 × g. The supernatant was decanted and the volume measured. The water or oil absorbed was calculated as the difference between the initial volume of water or oil used and the final volume of the decanted supernatant. The result was expressed in percentages.
Bulk density was determined as described by Ige et al. (1984). Briefly some quantity of the flour sample was transferred to a pre-weighed measuring cylinder (W1) and the new weight (W2) was recorded. The volume occupied by the flour in the measuring cylinder was recorded as V. The bulk density was expressed by the equation below:
The emulsion capacity was determined using turbidimetric technique as described by Chavan et al. (2001). Seed flour of known concentration (0.5 % m/v) was dispersed in water and stirred at 500 rpm for 20 min. An aliquot of 30 ml of the sample suspension was pipetted into 100 mL measuring cylinder followed by the addition of 10 mL Chef ®vegetable oil. The mixture was homogenized at 2,000 rpm and the emulsion volume observed after 1 min of homogenizing was expressed as the emulsion capacity and calculated as emulsion volume (mL) per gram sample.
Protein solubility was determined as described by Ige et al. (1984). Briefly, 2 g of the seed flour was dispersed in 10 mL distilled water and mixed thoroughly with magnetic stirrer at 500 rpm for 10 min. The mixture was centrifuged at 3,500 rpm for 30 min and the supernatant decanted. The protein in the supernatant was then determined using standard micro-Kjeldahl procedure. The result was presented as percentage of the soluble protein in the flour used.
The foaming capacity and foaming stability was determined as described by Coffman and Garcia (1977). A measured quantity of the flour was dispersed in 100 mL distilled water and stirred using magnetic stirrer at 1,500 rpm for 5 min. The foaming mixture was immediately transferred into a 250 mL graduated measuring cylinder and the foam volume was measured. The foaming capacity was expressed as the percentage volume increase (v/v, %) as shown below:
The foaming stability was expressed as foam volume remaining after 10, 20, 30, 40, 50 and 60 min and calculated as shown below:
The least gelation concentration (LGC) was determined using the procedure of Coffman and Garcia (1977). Seed flour sample suspensions of 2–20 % (m/v) were prepared in distilled water. An aliquot of 10 mL from each sample suspension was transferred to different test tubes which was heated in a gentle boiling water bath for 60 min. The test tubes were then cooled rapidly in a water bath for 2 h, followed by further cooling in 4 °C water bath. The LGC was taken as the concentration when the samples in the test tubes did not fall or slip when inverted.
Statistical analysis
All determinations were carried out in triplicates for each test and analysis of variance (ANOVA) was used to analyze the result and means were separated by Duncan’s method. The statistical package SPSS version 17.0 (SPSS Inc., Chicago, Illinois USA) computer program was used and significant differences was noted at 95 % confidence limit.
Results and discussion
Proximate composition of the full Fat and defatted seed flours
The moisture content of the defatted samples ranged from 6.17 ± 0.29 to 8.13 ± 0.71 % (Table 1) and compares favorably well with values of 6.39 % reported for defatted pumpkin seed (Olaofe et al. 1994) and 8.12 % for gourd seed (Badifu and Ogunsua 1991). Among the flours from defatted sample seeds, flour from the raw seed had the lowest moisture content of 6.17 ± 0.29 %. There was no significant difference in the moisture content of the flour from full fat germinated or fermented seeds (Table 1). For the full fat samples, moisture content of 5.53 ± 0.35 % obtained for the flour from raw sample compared well with earlier reports for Cucumeropsis mannii, Citrullus colocynthis and fluted pumpkin (Fokou et al. 2004; Akobundu et al. 1982). This low moisture content confers extended shelf life on the seed flour of C. mannii.
Table 1.
Proximate composition (g/100 g) of full-fat and defatted Cucumeropsis mannii under different processing conditions
| Composition | Full-fat | Defatted | ||||
|---|---|---|---|---|---|---|
| CF | GF | FF | CD | GD | FD | |
| Moisture content | 5.53 ± 0.35bc | 5.67 ± 0.29bc | 4.97 ± 0.25c | 6.17 ± 0.29b | 8.13 ± 0.71a | 8.00 ± 0.50a |
| Ash content | 3.24 ± 0.40c | 1.95 ± 0.60d | 2.69 ± 0.35cd | 7.19 ± 0.58a | 4.38 ± 0.25b | 6.41 ± 0.45a |
| Protein content | 37.59 ± 1.22d | 39.91 ± 0.48c | 36.62 ± 0.99d | 74.78 ± 1.05b | 77.05 ± 1.55a | 71.91 ± 1.04b |
| Fat content | 46.86 ± 2.08ab | 45.06 ± 1.25b | 48.57 ± 0.76a | 1.94 ± 0.31c | 1.56 ± 0.17c | 2.57 ± 0.30c |
| Crude fibre | 2.71 ± 0.25c | 3.63 ± 0.18b | 3.34 ± 0.33b | 4.34 ± 0.23a | 4.43 ± 0.14a | 4.57 ± 0.25a |
| Carbohydrate | 4.07 ± 0.11d | 3.78 ± 0.13e | 3.81 ± 0.17e | 5.58 ± 0.28a | 4.45 ± 0.16c | 6.54 ± 0.31b |
Value are mean ± SD (n = 3). Values with similar superscripts are not significant (p ≤ 0.05) according to Duncan Multiple range test. CF raw-dried full fat, GF germinated full fat, FF fermented full fat, CD raw-dried defatted, GD germinated defatted, FD fermented defatted
The crude fat content of the defatted samples were significantly reduced. The flour from full fat fermented seeds had the highest fat content of 48.57 ± 0.76 % while the germinated sample had the least among the full fat samples (Table 1). The full fat seed flours have crude fat content ranging from 45.06 ± 1.25 to 48.57 ± 0.76 % which were higher than the 18.3–21.5 % reported for soybean (Vasconceios et al. 1997). This indicates that C. mannii seed is a higher source of oil when compared with soyabean seed. Fermented seeds will produce more oil than the germinated seeds. Thus, C. mannii seeds could be used as a source of vegetable oil for industrial and domestic purposes. The crude fat content of the defatted samples compared favourably with 2.80 % for gourd seed as reported by Ogungbenle (2006) and higher than 1.86 % for Chinese kernel reported by Olaofe et al. (2009). Defatted samples have high flour volume per unit weight than full fat samples due to fat removal thus contributing to the increase in fibre content of the defatted samples.
The defatted flours had significantly higher (p < 0.05) protein content when compared with the full fat samples. The germinated sample had protein content of 77.05 ± 1.55 % and this was significantly higher than raw and fermented samples with values of 74.78 ± 1.05 % and 71.91 ± 1.04 % respectively. The germinated full fat and defatted samples were higher in protein content than the raw dried and fermented samples which may be due to protein synthesis and hydrolysis during sprouting (Asiedu et al. 1993). The fermented samples had the least protein content which may be as a result of leaching and breakdown of proteins during fermentation. The protein content of the full fat samples were higher than the values of 31.1 %, 30.8 % and 33.0 % reported for melon, gourd and pumpkin seeds respectively (Badifu and Ogunsua 1991; Olaofe et al. 1994). It, however, compares fairly well with 39.5 % reported for Chinese kernel (Olaofe et al. 2009). Therefore, germinated C. mannii could be an alternative source of dietary protein followed by the raw dried and fermented seed flours and would be good supplement if the amino acid profile is highly balanced where malnutrition arises, especially in developing countries of Africa where the majority of the populace live on starchy foods.
The carbohydrate content was obtained by difference. The defatted raw and fermented flours had carbohydrate contents of 5.58 ± 0.28 % and 6.54 ± 0.31 % respectively, and both were significantly higher than the value obtained for the germinated sample. The full fat flours had carbohydrate contents that were significantly lower than the defatted samples. Those values were low when compared with the values of 9.89 % and 6.97 % reported for gourd and pumpkin seeds respectively (Ogungbenle 2006; Olaofe et al. 1994). Generally C. mannii is not a good source of carbohydrate (Ogunbusola et al. 2012); it is greatly affected by processing. The fermented full fat sample is significantly (P < 0.05) lower in carbohydrate than the raw dried sample probably due to utilization of the sugar for fermentation. Hydrolysis of carbohydrate to sugar during germination might have led to the low carbohydrate content in the germinated flour. The higher value recorded for defatted samples may probably due to the removal of fat which lead to higher flour volume per unit mass hence, higher amount carbohydrate content.
The crude fibre and total ash contents of the full fat samples were significantly lower when compared to the defatted samples (Table 1). The total ash contents of full fat samples were slightly lower than the 4.66 % and 4.40 % reported for fluted pumpkin and melon seeds respectively (Badifu and Ogunsua 1991; Olaofe et al. 1994). After the seeds were defatted the values obtained for the total ash content were higher. This suggests that defatted samples will be able to provide more essential minerals when consumed than the same quantity of full fat samples.
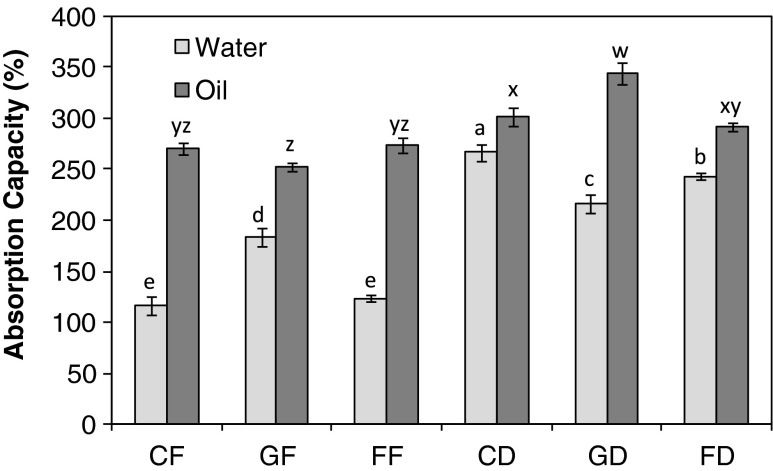
Water and Oil absorption capacity
Water absorption capacity (WAC) is the ability of moist material to retain water when subjected to an external centrifugal gravity force or compression. It consists of the sum of bound water, hydrodynamic water and, mainly, physically trapped water (Ku and Mun 2008). The WAC of the germinated full fat seed flour was 183 % and it was higher (p < 0.05) than those of the raw and fermented seed flours which were 116 % and 123 %, respectively (Fig. 1). The increase in WAC of germinated full fat C. mannii seed flour may be due to increase in protein content which absorbs more water probably due to protein synthesis during germination (Asiedu et al. 1993). Similar result has been reported on mung bean flour (Del Rosario and Flores 1981). Defatting of C. mannii seed significantly increased the WAC of the flour irrespective of the processing method as the WAC of the defatted seed flours at different treatments were significantly higher (p < 0.05) than those of the full fat seed flours. However the WAC of the defatted raw seed flour was 280 % and this was significantly higher than the values obtained for the fermented and germinated seed flours with values of 250 % and 230 % respectively (Fig. 1). Defatting (especially oil seeds) reduced the hydrophobic tendencies of the flour proteins and enhanced better interaction between the proteins and solvent which resulted in high WAC (Chau and Cheung 1998). Thus flour from defatted C. mannii seed flour will enhance thickening capacity than the flour from full fat seeds in food system. The oil absorption capacities (OAC) were generally higher than those of the WAC. There was no significant difference in the OAC of the full fat samples. The defatted germinated seed flour had an OAC of 345 % and was significantly higher than all the other samples (Fig. 1) and was similar to the observations in fluted pumpkin seed flours (Oshodi and Fagbemi 1992). The high values for the defatted samples may be due to the increase in protein content, which enhanced hydrophobicity by exposing more polar amino acids to the oil (Chau and Cheung 1998). OAC is of great importance in retaining flavour, improving mouth feel of baked goods and in the preparation and formulation of ground meat (Del Rosario and Flores 1981).
Fig. 1.
The water and oil absorption capacities of Cucumeropsis mannii seed flours. Value represent mean ± sd (n = 3). Values with similar superscripts are not significant (p ≤ 0.05) according to Duncan Multiple range test. The samples analysed are CF raw-dried full fat, GF germinated full fat, FF fermented full fat, CD raw-dried defatted, GD germinated defatted, FD fermented defatted
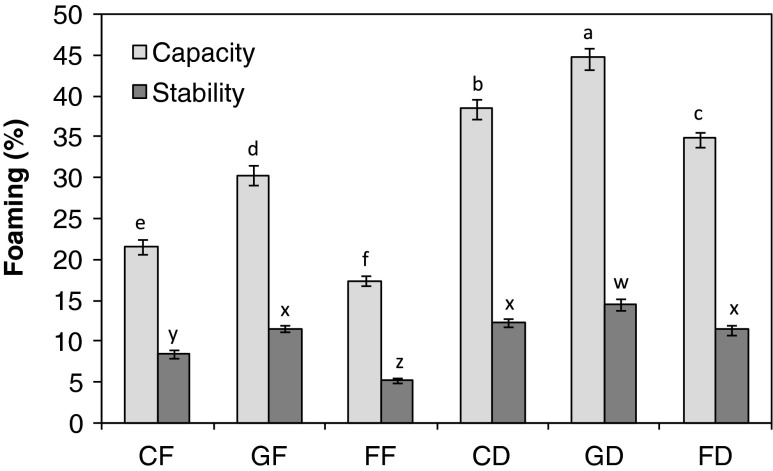
Foaming capacity and stability
Figure 2 describes the foaming capacity and stability of C. manni seed flours. The foaming capacity (FC) of the full fat seed flours which ranged from 17.36 to 30.34 % and were low when compared with the defatted seed flours which range from 34.78 to 44.69 %. The FC of the defatted seed flours is comparable with that of cashew nut flours reported by Fagbemi (2008). The trend observed in the full fat is similar to the trend in the defatted samples. Oil seed protein (particularly soybean) is used as aerating agent that replaces or complements egg white in whipped toppings and frozen desserts. Defatted C. mannii seed flours in the raw dried, germinated or fermented forms may be used as aerating agents in such food systems. Defatted seed flours, especially the germinated one, have higher foaming stability when compared with the full fat. Fatty materials generally collect at the liquid gas interface thus interfering with the protein alignment and leading to a decrease in the stability of foam. The removal of fat led to the observed increase in the foaming stability of C. manni seed flour.
Fig. 2.
The foaming stability and foaming capacity of Cucumeropsis mannii seed flours. Value represent mean ± sd (n = 3). Values with similar superscripts are not significant (p ≤ 0.05) according to Duncan Multiple range test. The samples analysed are CF raw-dried full fat, GF germinated full fat, FF fermented full fat, CD raw-dried defatted, GD germinated defatted, FD fermented defatted
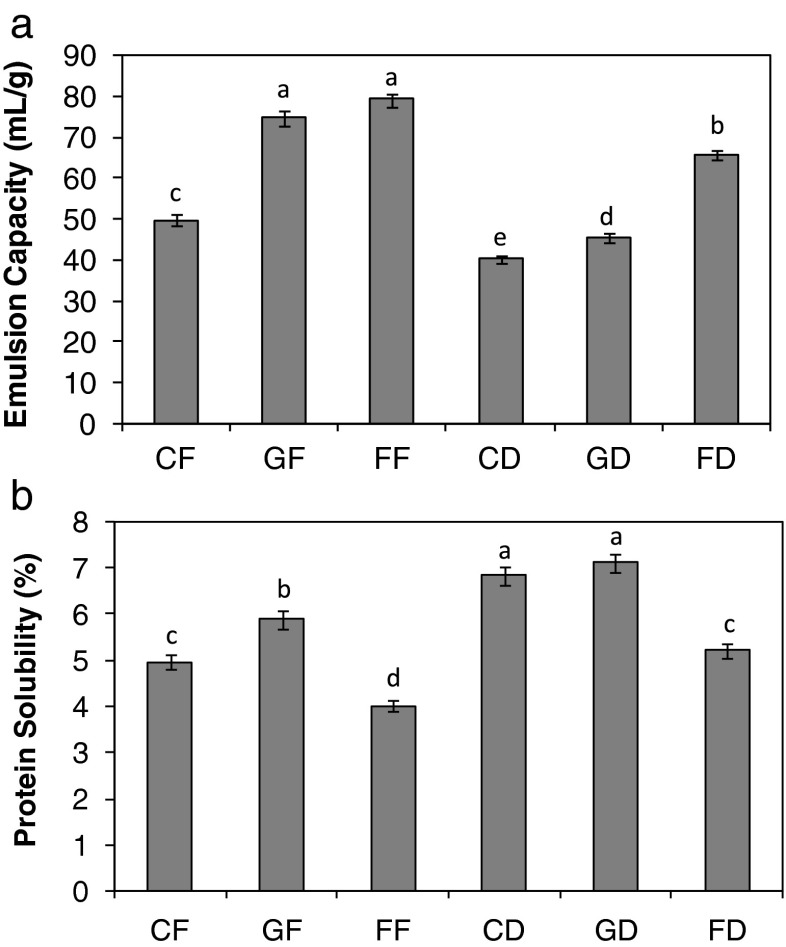
Emulsion capacity and protein solubility
Emulsion capacity was expressed as the maximum amount of oil that the seed flour in solution would emulsify without losing its emulsion characteristics. The emulsion capacity of the full fat fermented seed flour was 79.28 mL/g and was not significantly different from the germinated seed flour; however both were significantly higher than the raw seed flour with value of 49.7 mL/g (Fig. 3a). This is comparable with our previous findings of 21.7–78.3 % in cashew nut (Fagbemi 2008). When the samples were defatted, the flour from fermented seeds still had the highest emulsion capacity of 65.6 mL/g, followed by the germinated seed flour (Fig. 3a). The germinated as well as the fermented seed flours may be used as good emulsifying agents as well as in the production of vegetable milk, pastries and as ingredient in frozen desserts. The fermented sample will serve a good purpose in the production of soups and gravies. Fermentation reduced the protein solubility of C. mannii seed flour as shown in Fig. 3b. Similar trend was observed with the protein solubility of the defatted seed flours. The germinated sample was the most soluble with a value of 7.11 % while the fermented sample was the least soluble (Fig. 3b). The high protein solubility observed for the germinated C. mannii seed flour may be due to the high protein content of the germinated seed due to protein synthesis and hydrolysis during sprouting (Asiedu et al. 1993; Del Rosario and Flores 1981). The low protein solubility of the fermented C. mannii seed flour may be due to protein denaturation and precipitation. The germinated C. mannii seed flours may be useful in vegetable milk production and other food systems that required high protein solubility (Del Rosario and Flores 1981).
Fig. 3.
The emulsion capacity (a) and protein solubility (b) of Cucumeropsis mannii seed flours. Value represent mean ± sd (n = 3). Values with similar superscripts are not significant (p ≤ 0.05) according to Duncan Multiple range test. The samples analysed are CF raw-dried full fat, GF germinated full fat, FF fermented full fat, CD raw-dried defatted, GD germinated defatted, FD fermented defatted
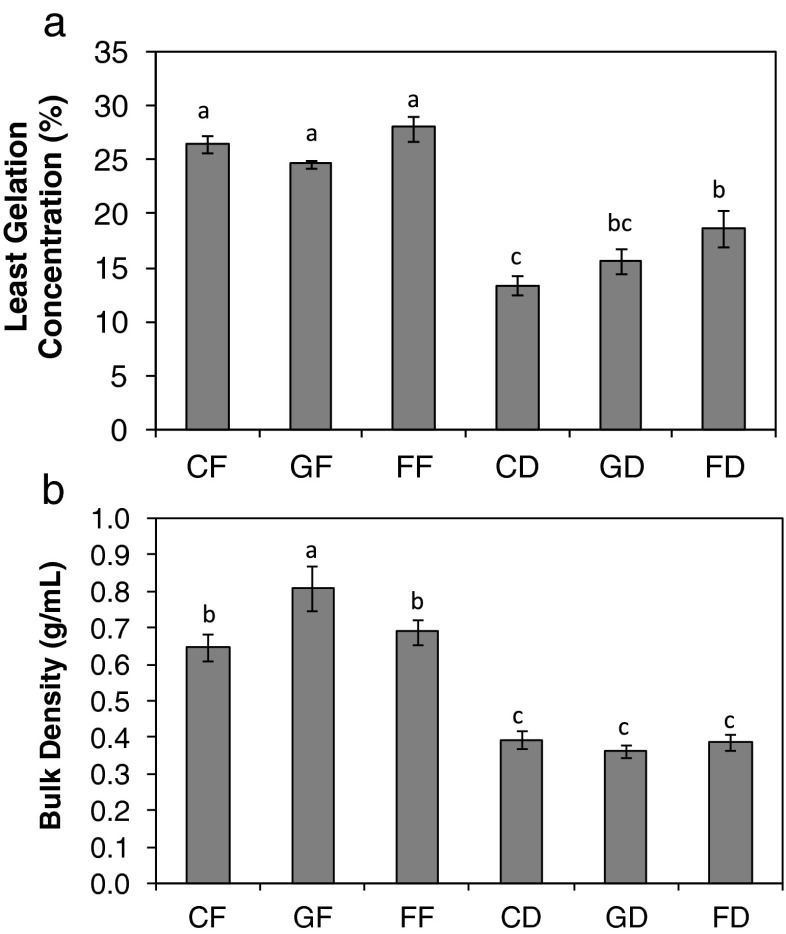
Least gelation concentration and bulk density
The lower the least gelation concentration (LGC) the better the gelling ability of the flour. The LGC of the full fat seed flours were all significantly higher than those of the defatted seed flours. Comparing the LGC of the full fat raw dried C. mannii seed flour (Fig. 4a) with other oil seeds showed that it is higher than 18 %, 17 % and 10 % reported for lupin seed, soybean and cashew nut, respectively (Sathe et al. 1982; Chau and Cheung 1998; Fagbemi 2008). Gelation property is dependent on the nature of the protein, starch and gums in the flour as well as their interaction during treatments. C. mannii has been reported as a poor gelating agent when compared with other oil seeds (Ogunbusola et al. 2012). Defatting significantly improved the gelation ability of the seed flours. The ability of seed flour to form gel is desirable in the preparation of extended meat products. The defatted seed flours especially raw dried sample may find use in such food preparation because lower concentrations are required to form gel. The bulk densities (BD) of the full fat seed flours were (p ≤ 0.05) higher than the defatted seed flours (Fig. 4b). Germination increased the BD of full fat C. mannii seed flours while there were reductions in the values obtained for the defatted samples (Fig. 4a). The results agreed with the observations of previous workers on cowpea (Padmashree et al. 1987) and fluted pumpkin seed flours (Giami and Bekebain 1992). The BD of the defatted samples will not offer packaging advantage. Removal of substantial amount of oil from the oil seeds make the weight of the defatted flour to be very low without reduction in its volume thereby resulting in low BD.
Fig. 4.
The least gelation concentration (a) and bulk desity (b) of Cucumeropsis mannii seed flours. Value represent mean ± sd (n = 3). Values with similar superscripts are not significant (p ≤ 0.05) according to Duncan Multiple range test. The samples analysed are CF raw-dried full fat, GF germinated full fat, FF fermented full fat, CD raw-dried defatted, GD germinated defatted, FD fermented defatted
Conclusion
The present study showed that defatting significantly (P < 0.05) increased the WAC, OAC, foaming capacity, foaming stability, LGC and protein solubility of the C. mannii seed flour, while, emulsion capacity and bulk density were decreased. These findings showed that the underutilized C. mannii is a high source of protein and fat with potential for use as functional ingredients in soups, frozen desert and as an emulsifier in vegetable milk production.
References
- Akobundu ENT, Cherry JP, Simmons JG. Chemical, functional and nutritional properties of egusi (Colocynthis citrillus L.) seed protein products. J Food Sci. 1982;47(3):829–835. doi: 10.1111/j.1365-2621.1982.tb12725.x. [DOI] [Google Scholar]
- AOAC (2005) Association of official analytical chemists official methods of analysis. 20th Edition. Washington DC
- Asiedu M, Lied E, Nilsen R, Sandnes K. Effect of processing (sprouting and fermentation) on sorghum and maize, vitamin and amino acid composition, biological utilization of maize protein. Food Chem. 1993;48(2):201–204. doi: 10.1016/0308-8146(93)90058-N. [DOI] [Google Scholar]
- Badifu GIO, Ogunsua AO. Chemical composition of kernels from some species of Cucurbitaceae grown in Nigeria. Plant Food Hum Nutr. 1991;41(1):35–44. doi: 10.1007/BF02196380. [DOI] [PubMed] [Google Scholar]
- Chau CF, Cheung PCK. Functional properties of flours prepared from three Chinese indigenous legume seed. Food Chem. 1998;61(4):429–433. doi: 10.1016/S0308-8146(97)00091-5. [DOI] [PubMed] [Google Scholar]
- Chavan UD, Mckenzie DB, Shahidi F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.) Food Chem. 2001;74:177–187. doi: 10.1016/S0308-8146(01)00123-6. [DOI] [Google Scholar]
- Coffmann CW, Garciaj VV. Functional properties and amino acid content of a protein isolate from mung bean flour. J Food Technol. 1977;12(5):473–484. doi: 10.1111/j.1365-2621.1977.tb00132.x. [DOI] [Google Scholar]
- Del Rosario RR, Flores DM. Functional properties of four types of mung bean flour. J Sci Food Agric. 1981;32(2):175–180. doi: 10.1002/jsfa.2740320213. [DOI] [Google Scholar]
- de Mello MLS, Narain N, Bora PS. Characterisation of some nutritional constituents of melon (Cucumis melo hybrid AF-522) seeds. Food Chem. 2000;68(4):411–414. doi: 10.1016/S0308-8146(99)00209-5. [DOI] [Google Scholar]
- Enujiugha VN, Ayodele-Oni O. Evaluation of nutrients and some anti-nutrients in lesser-known, underutilized oilseeds. Int J Food Sci Technol. 2003;38(5):525–528. doi: 10.1046/j.1365-2621.2003.00698.x. [DOI] [Google Scholar]
- Fagbemi TN, Oshodi AA. Chemical composition and function properties of full fat fluted pumpkin seed flour. (Telfairia occidentalis) Nig Food J. 1991;9:26–32. [Google Scholar]
- Fagbemi TN. Effect of blanching and ripening on functional properties of plantain flour. Plant Food Hum Nutr. 1999;54(3):261–269. doi: 10.1023/A:1008153404357. [DOI] [PubMed] [Google Scholar]
- Fagbemi TN. Effects of processing on the nutritional composition of fluted pumpkin (Telfairia occidentalis) seed flour. Nig Food J. 2007;25(1):1–22. [Google Scholar]
- Fagbemi TN. The influence of processing techniques on the energy, ash properties and elemental composition of cashew nut. (Anacardium occidentale Linn) Nutr Food Sci. 2008;38(2):136–145. doi: 10.1108/00346650810863019. [DOI] [Google Scholar]
- Fagbemi TN, Adeoya AS, Badejo AA. Effect of sulphiting on the physical and functional properties of acetylated cassava (Manihot esculenta) starch. Food. 2012;6(1):38–43. [Google Scholar]
- Fokou E, Achu MB, Tchounguep FM. Preliminary nutritional evaluation of five species of egusi seeds in Cameroon. Afr J Food Agric Nutr Dev. 2004;4(1):1–7. [Google Scholar]
- Giami SY, Bekebain DA. Proximate composition and processed full fat fluted pumpkin (Telfaria occidentalis) seed flour. J Sci Food Agric. 1992;59(3):321–325. doi: 10.1002/jsfa.2740590308. [DOI] [Google Scholar]
- Ige MM, Ogunsua AO, Oke OL. Functional properties of the proteins of some Nigerian oilseeds: conophor seeds and three varieties of melon seeds. J Agric Food Chem. 1984;32(4):822–825. doi: 10.1021/jf00124a031. [DOI] [Google Scholar]
- Koffi KK, Gbotto AA, Malice M, Dje Y, Bertin P, Baudoin J-P, Zoro Bi IA. Morphological and allozyme variation in a collection of Cucumeropsis mannii Naudin (Cucurbitaceae) from Cote d’Ivoire. Biochem Syst Ecol. 2008;36(10):777–789. doi: 10.1016/j.bse.2008.07.008. [DOI] [Google Scholar]
- Ku CS, Mun SP. Optimization of the extraction of anthocyanin from Bokbunja (Rubus coreanus Miq.) marc produced during traditional wine processing and characterization of the extracts. Bioresour Technol. 2008;99:8325–8330. doi: 10.1016/j.biortech.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Loukou AL, Gnakri D, Dje Y, Kippre AV, Malice M, Baudoin JP, Zoro Bi IA. Macronutrient composition of three cucurbit species cultivated for seed consumption in Cote d’Ivoire. Afr J Biotechnol. 2007;6:529–533. [Google Scholar]
- Odibo FJC, Nwabunnia E, Osuigwe DI. Biochemical changes during fermentation of Telfairia seeds for ogiri production. World J Microbiol Biotechnol. 1990;6:425–427. doi: 10.1007/BF01202127. [DOI] [PubMed] [Google Scholar]
- Odibo FJC, Nwabunnia E, Ezekweghi CC, Uzoeghe E. Fermentation of Cucumeropsis seeds, an uncommon substrate for ogiri production. Afr J Microbiol Res. 2012;6(24):5095–5099. [Google Scholar]
- Ogunbusola EM, Fagbemi TN, Osundahunsi OF. Chemical and functional properties of full fat and defatted white melon (Cucumeropsis mannii) seed flours. J Food Sci Eng. 2012;2:691–696. doi: 10.1007/s13197-014-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogungbenle HN. Chemical composition, functional properties and amino acid composition of some edible seeds. Riv Ital Sostanze Grasse. 2006;83(2):81–86. [Google Scholar]
- Olaofe O, Adeyemi FO, Adediran GO. Amino acid and mineral compositions and functional properties of some oilseeds. J Agric Food Chem. 1994;42(4):879–881. doi: 10.1021/jf00040a007. [DOI] [Google Scholar]
- Olaofe O, Faleye FJ, Adeniji AA, Akinsola AF. Amino acid, mineral composition and proximate analysis of Chinese bottle, Lagenaria sciceraria. Electr J Environ Agric Food Chem. 2009;8(7):534–543. [Google Scholar]
- Oshodi AA, Fagbemi TN. Functional properties of defatted and protein isolate of fluted pumpkin (Telfairia occidentalis) seed flours. Ghana J Chem. 1992;1:216–226. [Google Scholar]
- Padmashree TS, Vijayalakshmi L, Puttaraj S. Effect of traditional processing on the functional properties of cowpea (Vigna catjang) flour. J Food Sci Technol. 1987;24(5):221–225. [Google Scholar]
- Sanjur OI, Piperno DR, Andres TC, Wessel-Beaver L. Phylogenetic relationships among domesticated and wild species of Cucurbita (Cucurbitaceae) inferred from a mitochondrial gene: implications for crop plant evolution and areas of origin. Proc Natl Acad Sci U S A. 2002;99:535–540. doi: 10.1073/pnas.012577299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe SK, Desphande SS, Salunkhe DK. Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. J Food Sci. 1982;47(2):491–497. doi: 10.1111/j.1365-2621.1982.tb10110.x. [DOI] [Google Scholar]
- Vasconceios IM, Siebra EA, Maia AAB, Moreira RA, Neto AF, Campelo GJA, Oliveira JTA. Composition, toxic and antinutritional factors of newly developed cultivars of Brazilian soybean (Glycine max) J Sci Food Agric. 1997;75(4):419–426. doi: 10.1002/(SICI)1097-0010(199712)75:4<419::AID-JSFA886>3.0.CO;2-D. [DOI] [Google Scholar]