Abstract
An efficient method for the rapid extraction, separation and purification of chlorogenic acid (CGA) from by-products of Eucommia Ulmoides Oliver (E. ulmoides) by microwave-assisted extraction (MAE) coupled with high-speed counter-current chromatography (HSCCC) was developed. The optimal MAE parameters were evaluated by response surface methodology (RSM), and they were extraction time of 12 min, microwave power of 420 W, ethanol concentration of 75 %, solvent/sample ratio of 30:1 (mL/g), yield of CGA reached 3.59 %. The crude extract was separated and purified directly by HSCCC using ethyl acetate-butyl alcohol-water (3:1:4, v/v) as the two-phase solvent system. The 14.5 mg of CGA with the purity of 98.7 % was obtained in one-step separation from 400 mg of crude extract. The chemical structure of CGA was verified with IR, ESI-MS analysis. Meanwhile, the purified CGA extract was evaluated by MTT assay and results indicate that CGA extract exhibited potential anti-tumor activity for AGS gastric cancer cell.
Keywords: Microwave-assisted, Eucommia ulmoides Oliver, Chlorogenic acid, Response surface methodology, High-speed counter-current chromatography
Introduction
Eucommia ulmoides Oliver (E. ulmoides) (Chinese name Du-Zhong) is a living-fossil plant, and it is commonly used for the treatment of hypertension, rheumatoid arthritis, lumbago and ischialgia in traditional Chinese medicine (Chen, Sang, Li, Zhang, and Bai 2010). People used to study the bark of E. ulmoides, but the bark resource is in short supply, and the time periods for obtaining high yield of chlorogenic acid (CGA) from the cortex are only in July and November, the conflict between supply and demand becomes a major problem (Takamura et al. 2007). Now, modern scientific research has shown that the leaves (a by-product of E. ulmoides) also have pharmacological effects which are similar to the bark. 22 constituents have been identified by previous investigation (Zhou, Zhang, Chen, and Liang 2009), and the principal components are geniposidic acid and CGA. Therefore, an effective method for the isolation and purification of CGA from by-products of E. ulmoides is needed.
Chlorogenic acid, an ester formed between caffeic acid and quinic acid, is a phenolic compound. The pharmacological functions of CGA have been studied extensively. Potentially beneficial properties to human such as antimicrobial, anti-inflammatory, antioxidant, anticancer (Ramalakshmi et al. 2009), antiviral and hepatoprotective (Farah and Donangelo 2006) activities have been attributed to CGA in vitro, in vivo and epidemiological studies. Mubarak et al. (Mubarak et al. 2012) reported that CGA could lower blood pressure acutely, which would benefit cardiovascular health. Because of these functions, CGA has received more and more attention and now been officially recorded in the National Pharmacopoeia of China. Gastric cancer, also called stomach cancer, ranks third as the most frequent cause of cancer death in China today (Epplein et al. 2010). In this study, we investigated the potential effect of CGA on anti-gastric cancer activity.
In the extraction process of CGA from E. ulmoides, conventional extraction techniques as ethanol extraction and acetone extraction usually require prolonged extraction time and large volume of solvents. MAE has already been widely applied in solvent extraction because it allows faster extraction and reduces solvent consumption (Rostagno, Palma, and Barroso 2007; Zhang, Yang, and Liu 2008). The principal of this method lies in the fact that microwave energy is absorbed by the extractant which in turn transfers it to the sample in the form of heat (Perino-Issartier, Zill-e-Huma, Abert-Vian, and Chemat 2011). Response surface methodology (RSM) is a combination of statistical and mathematical techniques, and it is less laborious and time-consuming than other approaches and has been successfully used in optimizing biochemical process (Liyana-Pathirana and Shahidi 2005) and extraction of effective substances (Zhang, Zhang, Yue, Fan, Li, and Chen 2009; Silva, Rogez, and Larondelle 2007). Although, recent research studies have shown the development of MAE methods for the extraction of biological compounds, the application of RSM in CGA extraction was little been reported.
To our best knowledge, little reports have been published on the use of high speed counter-current chromatography (HSCCC) for separation and purification of CGA from by-products of E. ulmoides, especially on MAE coupled with HSCCC. In the past, CGA was often separated and purified by some conventional methods including silica gel, sephadex, polyamide and preparative high-performance liquid chromatography (HPLC), which are tedious, time-consuming, bulking amount of organic solvents and requiring multiple chromatographic steps resulting lower recovery and higher cost. HSCCC is a unique liquid-liquid partition chromatography technique that uses no solid support matrix (Jin et al. 2005). HSCCC eliminates the irreversible adsorptive loss of sample onto the solid support matrix and can yield a highly efficient separation of multigram quantities of samples in several hours. This method has been widely used for the preparative separation and purification of natural products, such as Artemisia princeps (Yoon, Chin, Yang, and Kim 2011), Zingiber officinale Roscoe (Zhan, Xu, and Yin 2011) and Adinandra nitida (Yuan, Liu, Ning, and Chen 2009).
The present study was designed to employ RSM to purify CGA from by-products of E. ulmoides by MAE coupled with HSCCC and investigate its potential anti-tumor effect against gastric cancer. The structure of CGA was identified with IR and (+) ESI-MS. This study benefits the large-scale production of CGA from by-products of E. ulmoides, and it provides a new choice to extract CGA and expands the E. ulmoides market.
Experimental
Apparatus
Preparative HSCCC was carried out with a model TBE-300A high-speed counter-current chromatography (Shanghai Tauto Biotech Co., Ltd., Shanghai, China) with a PTFE (polytetrauoroethylene), three preparative coils (diameter of tube, 2.6 mm, total volume, 300 mL) and a 20 mL sample loop. A DC-2006 constant-temperature circulating implement (Hangzhou Dawei Education Equipment Co., Ltd. Hangzhou, China) was used to control the separation temperature. The HSCCC system was equipped with a TBP-50A constant-flow pump, an 8823A-UV detector and a BSZ-100 fraction collector. The data were collected with N2000 chromatography workstation (Zhejiang University, Hangzhou, China). The analytical HPLC system used throughout this study consisted of an e2695 separations module (America Waters Co., Ltd., America) and a 2996 photodiode array detector (America Waters Co., Ltd., America), and a symmetry shield RP18 (4.6 × 250 mm, 5 μm) analytical chromatography column. Nuclear magnetic resonance (NMR) spectrometer was Bruker Avancedmx 500 NMR (Switzerland Brook Company, Switzerland). For HPLC-MS analysis, a high performance liquid chromatography and a trap multiple mass spectrometer (Agilent Technologies 1200 Series) were used for identification and determination of the content of compounds. A microwave lab station (Shanghai New Instrument Microwave Chemistry Technology Co., Ltd., Shanghai, China) was used for obtaining CGA extract of E. ulmoides.
Reagents and materials
Methanol used for HPLC was of chromatographic grade (Burcick & Jackson, America), and water was Wahaha pure water. The standard sample of CGA was bought from Sigma Company. Dimethyl sulfoxide (DMSO), and Tetrazolium (MTT) were purchased from Sigma-Aldrich Chemicals (China). Culture medium RPMI 1640, Trypsin/ EDTA solution, and fetal calf serum were purchased from Gibco (Invitrogen, China). Other solvents used for preparation of crude extract and HSCCC separation were of analytical grade (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China). The leaves, a by-prouct of E. ulmoides were purchased from Kaihua Quzhou Eucommia Tea Research Institute (Quzhou, Zhejiang, China). Gastric cancer cell line was cultured in the Department of Surgery, Zhejiang Cancer Hospital (Hagnzhou, Zhejiang, China)
Optimization of extraction method
Microwave-assisted extraction (MAE)
The leaves of E. ulmoides were extracted with different volume fractions ethanol-pure water liquid, using a Microwave lab station in a closed system under different sets of extraction time, ethanol concentration, solvent/sample ratio, and microwave power. The selection of the four independent variables was based on the previous paper (Shao, He, Sun, and Zhao 2012). The slurry was filtered to yield a clear extract that was used for quantitative analysis (Peng, Jia, Wang, Zhu, and Chen 2010).
Determination method of CGA
CGA was dissolved in methanol and detected by HPLC. The HPLC determination was accomplished with a symmetry shield RP18 column (4.6 × 250 mm, 5 μm) at 30 °C. Methanol-phosphate (0.5 %) was used as the mobile phase in gradient elution mode as following: 0–5 min, 30 % methanol; 5–15 min, 30–40 % methanol. The flow-rate of the mobile phase was 0.6 mL/min. The effluents were monitored at 329 nm by a photodiode array detector.
Response surface experimental design and statistical analysis
A four-variable, three-level of Box-Behnken design (BBD) (Dong, Xie, Wang, Zhang, and Yao 2009) was applied to optimize the extraction condition in order to obtain the high yield of CGA from the leaves of E. ulmoides. The four independent variables studied were extraction time (min, X1), microwave power (W, X2), ethanol concentration ratio (%, X3), and solvent/sample ratio (mL/g, X4), and each variable was set at three levels: −1, 0, and 1. The choice of variable levels was based on our preliminary study. Totally, 29 experiments were designed (Table 1). Each experiment was performed in triplicate and the average CGA content (%) was taken as the observed value, Y1. Regression analysis was performed to establish an empirical second-order polynomial model, as shown in the following equation:
| 1 |
Table 1.
Observed and predicted valuesa of extraction yield from Box-Behnken design
| Run | Independent variable | Observed Value, Y1 (%) | Predicted Value, Y2 (%) | |||
|---|---|---|---|---|---|---|
| X1(time, min) | X2(power, W) | X3(ethanol concentration, %) | X4(solvent /sample, mL/g) | |||
| 1 | 10(0) | 300(−1) | 70(0) | 25(−1) | 1.73 | 1.724 |
| 2 | 8(−1) | 400(0) | 65(−1) | 30(0) | 2.57 | 2.583 |
| 3 | 10 (0) | 400(0) | 75(+1) | 25(−1) | 2.76 | 2.621 |
| 4 | 12 (+1) | 400(0) | 75(+1) | 30(0) | 3.43 | 3.577 |
| 5 | 12 (+1) | 300(−1) | 70(0) | 30(0) | 2.63 | 2.500 |
| 6 | 8(−1) | 500(+1) | 70(0) | 30(0) | 2.51 | 2.420 |
| 7 | 10 (0) | 500(+1) | 70(0) | 25(−1) | 2. 22 | 2.304 |
| 8 | 8(−1) | 400(0) | 70(0) | 35(+1) | 1.74 | 1.776 |
| 9 | 10 (0) | 400(0) | 70(0) | 30(0) | 3.41 | 3.410 |
| 10 | 10 (0) | 400(0) | 75(+1) | 35(+1) | 3.02 | 2.813 |
| 11 | 10 (0) | 300(−1) | 70(0) | 35(+1) | 1.56 | 1.636 |
| 12 | 10 (0) | 400(0) | 70(0) | 30(0) | 3.41 | 3.410 |
| 13 | 10 (0) | 400(0) | 70(0) | 30(0) | 3.41 | 3.41 |
| 14 | 12 (+1) | 500(+1) | 70(0) | 30(0) | 3.29 | 3.00 |
| 15 | 8(−1) | 400(0) | 75(+1) | 30(0) | 2.32 | 2.277 |
| 16 | 10 (0) | 500(+1) | 65(−1) | 30(0) | 2.85 | 2.853 |
| 17 | 12 (+1) | 400(0) | 70(0) | 25(−1) | 2.48 | 2.524 |
| 18 | 10 (0) | 400(0) | 70(0) | 30(0) | 3.41 | 3.410 |
| 19 | 10 (0) | 500(+1) | 75(+1) | 30(0) | 2.87 | 3.027 |
| 20 | 10 (0) | 300(−1) | 65(−1) | 30(0) | 2.19 | 2.113 |
| 21 | 10 (0) | 400(0) | 70(0) | 30(0) | 3.41 | 3.410 |
| 22 | 12(+1) | 400(0) | 65(−1) | 30(0) | 2.89 | 3.083 |
| 23 | 10 (0) | 400(0) | 65(−1) | 25(−1) | 2.60 | 2.567 |
| 24 | 10 (0) | 400(0) | 65(−1) | 35(+1) | 2.78 | 2.679 |
| 25 | 8(−1) | 300(−1) | 70(0) | 30(0) | 1.23 | 1.280 |
| 26 | 12(+1) | 400(0) | 70(0) | 35(+1) | 3.21 | 3.256 |
| 27 | 10 (0) | 500(+1) | 70(0) | 35(+1) | 2.55 | 2.696 |
| 28 | 10 (0) | 300(−1) | 75(+1) | 30(0) | 2.05 | 2.127 |
| 29 | 8(−1) | 400(0) | 70(0) | 25(−1) | 2.16 | 2.204 |
a Average value of triplicate experiments
Where A0 is constant; A1, A2, and A3 are linear coefficients; A4, A5, and A6 are cross-product coefficients; A7, A8, and A9 are quadratic coefficients.
A software Design-Expert 8.0.6 was used to obtain the coefficients of the quadratic polynomial model. The quality of the fitted model was expressed with the coefficient of determination R2, and its statistical significance was checked by F-test.
Separation and purification methods
Selection of two-phase solvent system
The crude extract was determined by HPLC according to the 2.3.2 condition and the higher content CGA was chosen as target compound. The solvent system for HSCCC separation was selected according to the difference of partition coefficient (K) of target compound between the two-phase solvent systems. The solution was then determined by HPLC and the peak area was recorded as A1. Then equal volume of the upper phase was added to the solution and mixed thoroughly. After partition equilibration was reached, the lower phase solution was determined by HPLC again and the peak area was recorded as A2. The K values were calculated according to the following equation: K= (A1−A2)/A2 (Deng et al. 2009; Du, Chen, Jerz, and Winterhalter 2004).
Preparation of two-phase solvent system and sample solution
In the present study, the two-phase solvent system composed of ethyl acetate–butyl alcohol–water at volume ratio of 3:1:4 was used for HSCCC separation. Each solvent was added to a separatory funnel and thoroughly equilibrated at room temperature for a whole night. The upper phase and the lower phase were separated and degassed by sonication for 30 min before using. The sample solution for HSCCC separation was prepared by dissolving 400 mg of the dried powder of the crude extract in 10 mL lower phase and 10 mL upper phase of the two-phase-solvent system (Lee, Lee, Park, and Moon 2010).
HSCCC separation procedure
In each separation, the multi-layer coiled column was first entirely filled with the upper phase as the stationary phase. Then, the lower phase as the mobile phase was pumped into the column at a flow-rate of 2.0 mL/min, while the column was rotating at 900 rpm. Temperature of the apparatus was kept at 30 °C. After the mobile phase front emerged and hydrodynamic equilibrium was established in the column, sample solution containing 400 mg of the crude extract was injected through the injection valve by an AKTA prime system. The column effluent was continuously monitored with a UV detector and each peak fraction was collected according to the elution profile and evaporated under reduced pressure. The residual was dissolved in methanol for HPLC analysis. The retention of the stationary phase relative to the total column capacity was computed from the volume of the stationary phase collected from the column.
Analysis and identification of HSCCC peak fractions
The crude extract was separated and purified by HSCCC, and the fractions obtained were analyzed by HPLC in order to determine the purity of CGA after separation. The condition of HPLC analysis was as the same as 2.3.2.
Identification of CGA fractions was carried out by IR and (+) ESI-MS.
Anti-tumor activity
Cell culture and cell viability assay
The human gastric cancer cell line AGS was cultured in RPMI 1640 medium supplemented with 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin, at 37 °C in an incubator containing 5 % CO2. Cells were passaged every 2 days using Trypsin (0.25 %) / EDTA (0.02 %) solution. Exponentially growing cells were used for experimentation.
MTT assay was used to evaluate the effect of the test compound on cell growth, as described previously (Alley et al. 1988), with slight modification. Briefly, 6000 cells per well seeded in 96-well microtiter plate. Cells were treated with the crude extract by MAE and purified CGA extract by HSCCC under different concentrations for 24 and 48 h. Thereafter, the supernatant was removed and 20 μL MTT reagent (5 mg/mL) was added. After 4 h incubation at 37 °C, 200 μL of DMSO was added and plates were oscillated for 10 min in a balance oscillator. The extent of the MTT reduction was measured by a plate reader at a wavelength of 329 nm. The inhibitory rate of cell proliferation was calculated as: inhibitory rate (%) = (1 - experimental group A value / control group A value) ×100 %.
Statistical analysis
Data were shown as mean ± standard deviation of three independent experiments and evaluated by one-way analysis of variance (ANOVA). Significant differences were established at p < 0.05.
Results and discussion
Response surface optimization of MAE conditions
The MAE conditions of CGA from the leaves of E. Ulmoides were optimized according to the Box-Behnken design. Table 1 presents the experiment design and corresponding response date for the yield of CGA. The regression coefficients of the intercept, linear, quadratic, and interaction terms of the model were calculated using the least square technique and are presented in Table 2. It was evident that the model is significant. In this case X1, X2, X1X3, X1X4, X12, X22, X32, X42 were significant model terms and the result indicated tha the microwave power and extraction time were the major contributing factors to the yield of CGA among the four variables.
Table 2.
Estimated regression coefficients for the quadratic polynomial model and the analysis of variance (ANOVA) for the experimental results
| Parametera | Estimated coefficients | Standard error | DFb | Sum of squares | F value | P value |
|---|---|---|---|---|---|---|
| Intercept | Model | |||||
| β0 | 3.41 | 0.068 | 1 | 0.73 | 31.71 | <0.0001 |
| β1 | 0.45 | 0.044 | 1 | 2.43 | 105.14 | <0.0001 |
| β2 | 0.41 | 0.044 | 1 | 2.00 | 86.57 | <0.0001 |
| β3 | 0.047 | 0.044 | 1 | 0.027 | 1.17 | 0.2974 |
| β4 | 0.076 | 0.044 | 1 | 0.069 | 2.99 | 0.1060 |
| β11 | −0.38 | 0.060 | 1 | 0.95 | 41.06 | <0.0001 |
| β22 | −0.73 | 0.060 | 1 | 3.46 | 149.57 | <0.0001 |
| β33 | −0.15 | 0.060 | 1 | 0.14 | 6.21 | 0.0259 |
| β44 | −0.59 | 0.060 | 1 | 2.25 | 97.29 | <0.0001 |
| β12 | −0.16 | 0.076 | 1 | 0.096 | 4.16 | 0.0608 |
| β13 | 0.20 | 0.076 | 1 | 0.16 | 6.75 | 0.0210 |
| β14 | 0.29 | 0.076 | 1 | 0.33 | 14.31 | 0.0020 |
| β23 | 0.040 | 0.076 | 1 | 6.40E-003 | 0.28 | 0.6070 |
| β24 | 0.12 | 0.076 | 1 | 0.063 | 2.70 | 0.1223 |
| β34 | 0.020 | 0.076 | 1 | 1.60E-003 | 0.069 | 0.7963 |
| Lack of fit | 7 | 0.032 | 1.35 | 0.2309 | ||
| Pure error | 0.000 | |||||
| R2 | 0.9694 | Adj R2 | 0.9389 | |||
| C.V.% | 4.75 | PRESS | 1.86 |
a Coefficients refer to the general model
b Degree of freedom
The analysis of variance for the experimental results of the Box-Behnken design is also shown in Table 2. The coefficient of determination (R2) of the model was 0.9694, indicating that the model adequately represented the real relationship between those parameters chosen. Furthermore, results of the error analysis indicated that the lack of fit was insignificant (p > 0.05). The coefficient of variation (C.V.) of less than 5 % indicated that the model was reproducible. The Predicted Residual Sum of Squares (PRESS) for the model, which is a measure of how a particular model fits each point in the design, was 1.86. The model F-value of 31.71 implies that the model was significant. The “Pred. R-Squared” of 0.8239 is in reasonable agreement with the “Adj-R-Squared” of 0.9389. The predicted second-order polynomial model was:
| 2 |
To determine optimal levels of the variables for the yield of CGA, the three-dimensional surface plots were constructed according to Eq. (2).
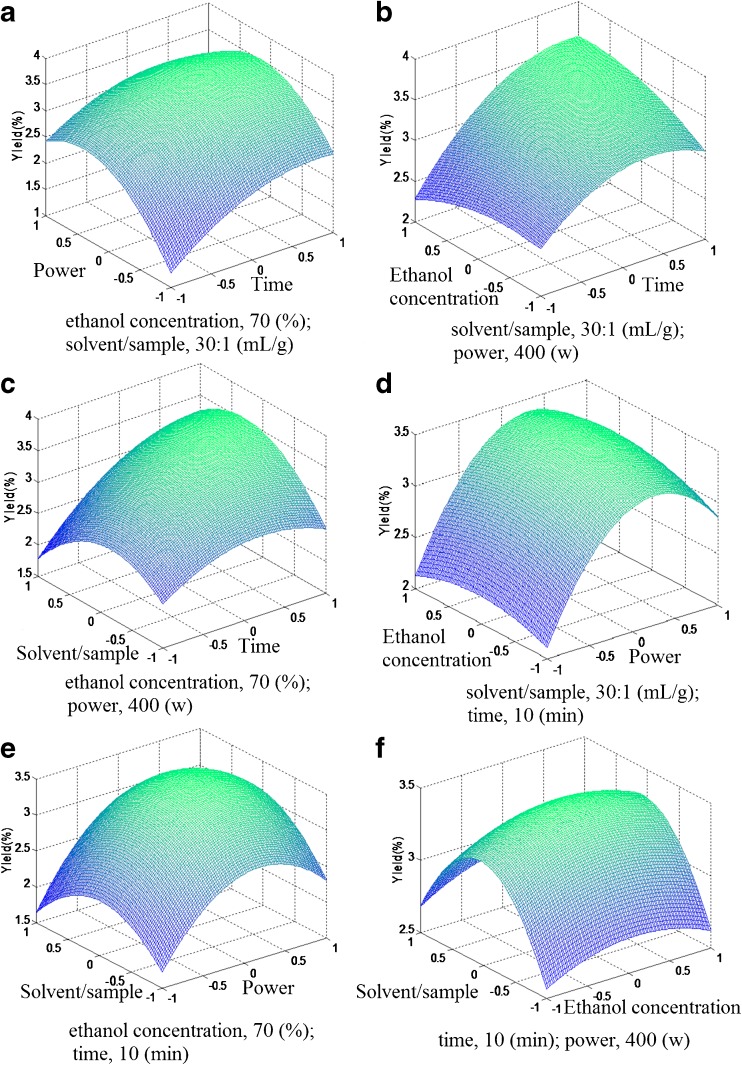
The best way to visualize the influence of independent variables on dependent one is to draw a surface response plot of the model. The response surfaces are shown in Fig. 1. The yield of CGA was mainly influenced by extraction time, microwave power and solvent/sample ratio, while the effect of ethanol concentration was insignificant. Figure 1a, d and e showed that CGA yield increased fast at first, but when microwave power more than 417.30 W, there was a decline of CGA yield. We knew that the temperature of the extraction medium increases with increasing microwave power and the higher extraction temperature is preferable for the extraction because it reduces the extraction time. But, if temperature becomes too high, it may destroy the sensible components for targeted in the extract (Liazid, Palma, Brigui, and Barroso 2007).
Fig. 1.
Response surface plots of the yield of chlorogenic acid affected by extraction time, microwave power, ethanol concentration ratio, and solvent/sample ratio
In the response surface plots, as seen in Fig. 1a, it could be noticed that CGA yield increased with the increase of extraction time. Figure 1b and c also indicated that when the extraction time more than 11.72 min, there was a slight drop of CGA yield. Generally it is considered that under proper conditions, extract yield increases with increasing extraction time (Wang, Sun, Cao, Tian, and Li 2008). However, considering both of the energy saving and time restraint, extraction for very a prolonged time is not preferable. Therefore, the extraction was carried out for a maximum of 12 min.
Figure 1c, e and f depicted the response surface of the similar effect of solvent/sample ratio on the CGA yield. When other extraction conditions were kept constant, the effect of solvent/sample ratio on the surface displayed a linear increase while the ratio ranged from 25:1 to 31.25:1, but it showed a quadratic effect when the ratio was higher than 31.25:1. One possible reason for the increased efficiency might be due to the presence of some solvent, resulting in the increase in swelling of the plant material, which increased the contact surface area between the plant matrix and the solvent.
The slight tortuose surface in Fig. 1b, d and f showed the interaction effects between ethanol concentration and extraction time, power and solvent/sample ratio. The CGA yield increased slowly with the increase of ethanol concentration until the ethanol concentration reached to 73.92 %. There was a decline with the further increase of the ethanol concentration. The result illustrated that higher ethanol concentration was not suitable for CGA extraction. Higher ethanol concentration may have an impact on the stability of CGA.
The superiority of MAE is clear. MAE allows for simplified designing, efficient and economical pilot scale studies, and also has a positive impact on future purification studies (Neme and Orsat 2012). In this study, the calculation of the optimal MAE conditions was further carried out according to the RSM model equation. The result was extraction time of 11.72 min, microwave power of 417.30 W, ethanol concentration of 73.92 %, and solvent/sample ratio of 31.25:1 (mL/g). For the convenience of experiment, the actual MAE conditions chosen were as follows: extraction time at 12 min, microwave power at 420 W, ethanol concentration at 75 % and solvent/sample ratio at 30:1 (mL/g). The mean value of 3.59 ± 0.08 % (n = 3), obtained from real experiments, demonstrated the validity of the RSM model, since there was no significant (p > 0.05) differences between the predicted result (3.68 %) and the practical value (3.59 ± 0.08 %). The strong correlation between the practical and the predicted results confirmed that the response model was adequate to reflect the expected optimization.
Separation and purification of CGA
Selection of two-phase solvent system
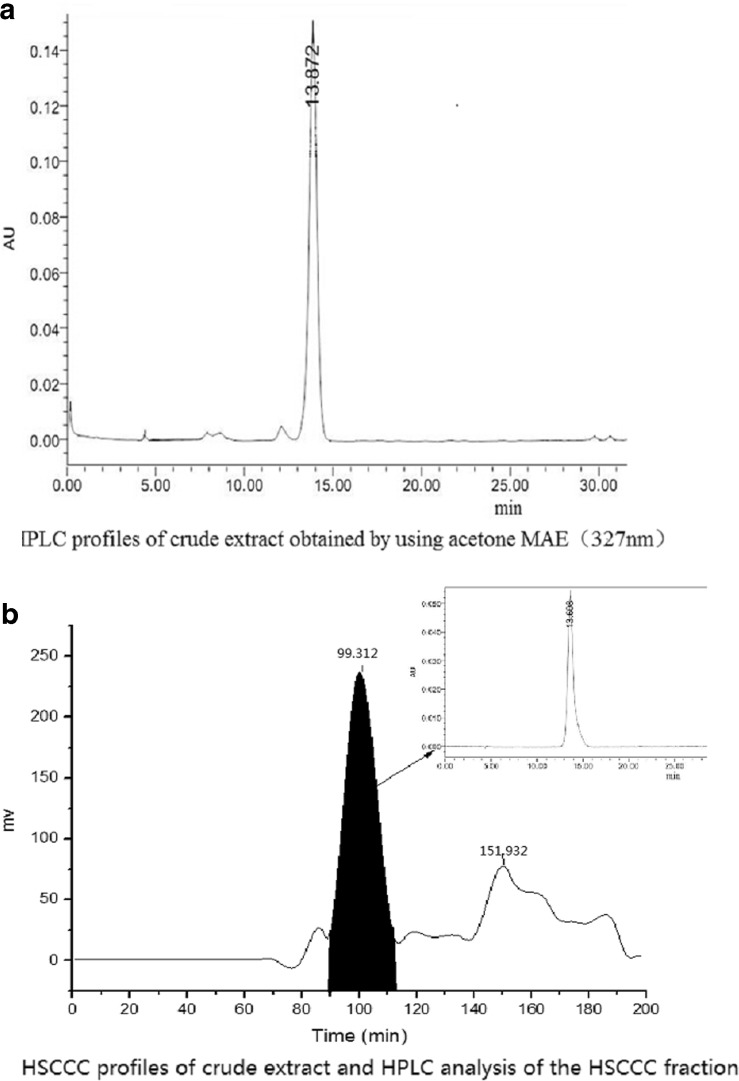
The crude extract was analyzed by HPLC and the target compound is showed in Figure 2a. In our experiment, different solvent systems such as ethyl acetate-n-butanol-methanol-water, chloroform-methanol-water, ethyl acetate-n-butanol-water, were used as the two-phase-solvent system to optimize the HSCCC separation condition. The K values of the target compound corresponded to peak fraction in different solvent systems were determined by HPLC as the procedure shown in Section 2.3.2. The results were given in Table 3 and indicated that the target compound in solvent systems composed of ethyl acetate-n-butanol-water at the volume ratios of 4:1:5 and 3:1:4 (v/v) had the best K values. At last, the solvent system composed of ethyl acetate-n-butanol-water at a volume ratio of 3:1:4 (v/v) was selected in the present study, which is good enough to perform a highly efficient separation method for the target compound. In some other reported studies, Researchers always attempted to obtain better resolution and more different target compounds by changing some operation conditions. For example, Xiao et al. used three different solvent systems and constant flow-rate to obtain five flavonoid glycosides (Peng, Yang, Fan, and Wu 2005). In our study, we used one solvent system and constant flow-rate, and the method is proved to be simple, good reproducibility and feasible.
Fig. 2.
HPLC profiles of crude extract obtained by MAE (329 nm); HSCCC profiles of crude extract and HPLC analysis of the HSCCC fraction
Table 3.
The partition coefficients (K) of the target compound in different solvent systems
| Slovent system | Ratio(v/v) | K-values |
|---|---|---|
| ethyl acetate– n-butanol–meth anol–water | 1:1:1:1 | Inf. |
| chloroform–methanol–water | 4:3:2 | 1.00 |
| ethyl acetate–n-butanol–wate | 4:1:5 | 1.14 |
| ethyl acetate–n-butanol–wate | 1:1:2 | 0.28 |
| ethyl acetate–n-butanol–wate | 2:1:3 | 2.13 |
| ethyl acetate–n-butanol–wate | 3:2:5 | 2.31 |
| ethyl acetate–n-butanol–wate | 3:1:4 | 0.82 |
Inf. Means the partition coefficient is too large that can’t be evaluated
The result of HSCCC separation
Under the optimized MAE conditions, 14.5 mg of CGA compound separated and purified by HSCCC, was obtained from the 400 mg crude extract of E. Ulmoides leaves in one-step elution. The HSCCC chromatogram is shown in Fig. 2b, which gave two peaks and the first one marked as component Icorresponded to the CGA peak showed in Fig. 2a in HPLC analysis of the crude extract sample. The HSCCC peak fraction was analyzed by HPLC and the result showed the purity of CGA was 98.7 % shown in Fig. 2b.
When the upper phase and the lower phase were balanced in the HSCCC system, the solution of the stationary phase (the upper phase) flowed out 90 mL. Because of the volume of whole pipeline is 300 mL, the retention ratio was 70 %, which is high level and easy for separation of the target compound.
Validation of the separated CGA peak
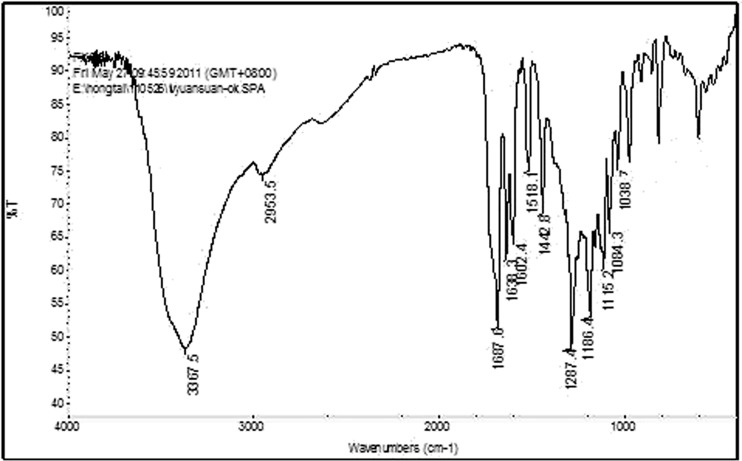
The IR-spectrogram analysis
The characteristic peaks of CGA are showed in Fig. 3 as follows: 3,367.5 cm−1 is the stretching vibration peak of –OH (alcohol); 2,953.5 cm−1 is the feature absorption peak of six-membered ring; 1,687.6 cm−1 is the feature absorption peak of –OH (carboxylic acid); 1,638.3 cm−1 is the feature absorption peak of -C=C-; 1,602.4 and 1,518.1 cm−1 are the feature absorption peak of benzene ring; 1,442.8, 1,287.4, 1,186.4, 1,115.2, 1,084.3 and 1038.7 cm−1 are the feature absorption peak of -OH and -CO (phenol and \ary alcohols).
Fig. 3.
The IR-spectrogram of component
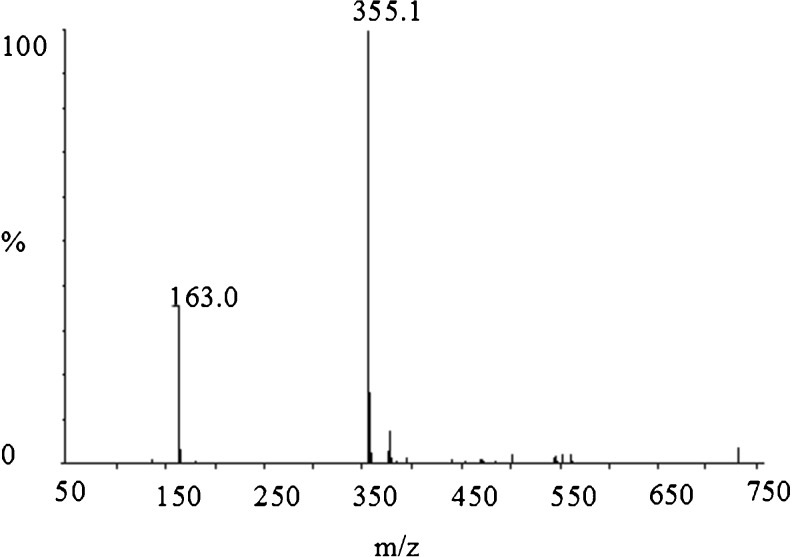
The (+) ESI-MS analysis
CGA fraction is a kind of light green powders. ESI-MS profile in Fig. 4 shows that its quasi-molecular ion peak [M+H]+ is m/z= 355.1; its fragment is m/z= 163.0. According to the result of 1H NMR, the fragment of m/z= 163.0([M+H-192] +) is the quasi-molecular ion peak (m/z= 355.1) subtracted a fragment (m/z= 192.1) that mostly maybe is 1, 3, 3, 4-tetrahydroxy cyclohexane carboxylic acid.
Fig. 4.
(+)ESI-MS profiles of component
Effect of CGA extract on the growth of gastric cancer cells
Cell viability was assayed in MCF7 cell cultures exposed to purified CGA extract (0–2 mg /mL) and the crude extract (0–20 mg/mL) under different concentrations for 24 or 48 h. The purified CGA and crude extract of CGA by MAE both showed dose- and time-dependent inhibitory effects on the growth of MCF7 gastric cell (Table 4). The concentration of crude extract inhibiting 50 % of MCF7 cell viability (IC50) at 48 h and 24 h were about 8.2 and 11.2 mg/mL, respectively. Obviously, purified CGA demonstrated a profound anticancer effect. The IC50 for purified CGA was only 0.31 mg /mL at 48 h and 0.73 mg /mL at 24 h, much less than that for crude extract.
Table 4.
Cell viability of purified CGA and crude extract under different concentrations for 24 or 48 h
| Concentration | Cell viability (%)a | ||
|---|---|---|---|
| 24 h | 48 h | ||
| Purified CGA (mg/mL) | 0 | 100 ± 1.7 | 100 ± 1.4 |
| 0.25 | 82.3 ± 1.8 | 56.7 ± 1.3 | |
| 0.5 | 61.7 ± 1.9 | 35.1 ± 1.5 | |
| 1.0 | 43.1 ± 1.5 | 23.4 ± 0.8 | |
| 1.5 | 25.3 ± 0.8 | 14.9 ± 0.5 | |
| 2.0 | 18.9 ± 0.6 | 8.7 ± 0.4 | |
| Crude extract (mg/mL) | 0 | 100 ± 1.6 | 100 ± 1.6 |
| 2.5 | 96.5 ± 1.8 | 93.2 ± 1.7 | |
| 5 | 89.6 ± 0.7 | 82.7 ± 1.4 | |
| 10 | 57.8 ± 0.9 | 43.5 ± 1.2 | |
| 15 | 37.9 ± 0.6 | 21.4 ± 0.5 | |
| 20 | 26.5 ± 0.6 | 9.6 ± 0.4 | |
a Values are expressed as the means ± standard deviation (n = 3)
Pharmacological experiments showed that E. ulmoides possess anti-inflammatory and immunological activities [Deyama, Nishibe & Nakazawa, 2002], which are closely related to the activation of complement. Therefore, in this study, the purified CGA isolated from E. ulmoides effectively inhibited the proliferation of MCF7 gastric cells in vitro, and our data suggested that the anti-tumor effect of crude extract may mostly due to the existence of CGA.
Conclusions
This study demonstrates that RSM was successfully applied to the optimization of MAE parameters. The experiment results show that the extraction time and microwave power are the major contributing factors to the yield. The crude extract of MAE was successfully isolated and separated with acetate-n-butanol-water (3:1:4) as the two-phase-solvent system of HSCCC in one-step separation. The results of IR and (+) ESI-MS confirmed that the separated fraction (component I) extracted from the leaves of E. ulmoides is CGA. Moreover, the purity of CGA reached up to 98.7 %. In addition, the purified CGA isolated from E. ulmoides effectively inhibited the proliferation of gastric cancer cell AGS in vitro. The results indicated that CGA exhibited anti-tumor activity for AGS gastric cancer cell (IC50 = 0.73 mg/mL, at 24 h; IC50 = 0.31 mg/mL, at 48 h) and the high anti-tumor activity of E. ulmoides might be related to CGA.
It could be concluded that the present method of MAE coupled with HSCCC was suitable for the preparative isolation and purification of CGA from the leaves of E. ulmoides. This method benefits the large-scale production of CGA from by-products of E. ulmoides, and it provides a new choice to extract CGA and expands the E. ulmoides market. This study also suggested that CGA might be a potential, natural apoptosis-inducing antitumor agent.
Acknowledgments
This work was supported by Zhejiang Provincial Natural Science Foundation of China (Y3110370). The authors have no conflicts of interest to declare.
References
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- Chen XM, Sang XX, Li SH, Zhang SJ, Bai LH. Study on a chlorogenic acid-producing endophytic fungi isolated from Eucommia ulmoides Oliver. J Ind Microbiol Biotechnol. 2010;37:447–454. doi: 10.1007/s10295-010-0690-0. [DOI] [PubMed] [Google Scholar]
- Deng SG, Deng ZY, Fan YW, Peng Y, Li J, Xiong DM, et al. Isolation and purification of three flavonoid glycosides from the leaves of Nelumbo nucifera (Lotus) by high-speed counter-current chromatography. J Chromatogr B. 2009;877:2487–2492. doi: 10.1016/j.jchromb.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Deyama T, Nishibe S, Nakazawa Y (2002) Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol Sin 22(12):1057–1070 [PubMed]
- Dong CH, Xie XQ, Wang XL, Zhang Y, Yao YJ. Application of Box-Behnken design in optimization for polysaccharides extraction from cultured mycelium of Cordyceps Sinensis. Food Bioprod Process. 2009;87:139–144. doi: 10.1016/j.fbp.2008.06.004. [DOI] [Google Scholar]
- Du QZ, Chen P, Jerz G, Winterhalter P. Preparative separation of flavonoid glycosides in leaves extract of Ampelopsis grossedentata using high-speed counter-current chromatography. J Chromatogr A. 2004;1040:147–149. doi: 10.1016/j.chroma.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Epplein M, Shu XO, Xiang YB, Chow WH, Yang G, Li HL, et al. Fruit and vegetable consumption and risk of distal gastric cancer in the shanghai women’s and men’s health studies. Am J Epidemiol. 2010;172(4):397–406. doi: 10.1093/aje/kwq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah A, Donangelo CM. Phenolic compounds in coffee. Braz J Plant Physiol. 2006;18:23–36. doi: 10.1590/S1677-04202006000100003. [DOI] [Google Scholar]
- Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, et al. Aphenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a newtype and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77(22):2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee KH, Park KH, Moon JH. Large scale isolation and purification of salvianolic acid B in high purity from roots of Dansham (Salvia miltiorrhiza Bunge) Food Sci Biotechnol. 2010;19:497–502. doi: 10.1007/s10068-010-0069-z. [DOI] [Google Scholar]
- Liazid A, Palma M, Brigui J, Barroso CG. Investigation on phenolic compounds stability during microwave-assisted extraction. J Chromatogr A. 2007;1140(1–2):29–34. doi: 10.1016/j.chroma.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Liyana-Pathirana C, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93(1):47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- Mubarak A, Bondonno CP, Liu AH, Considine MJ, Rich L, Mas E, et al. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: a randomized trial. J Agric Food Chem. 2012;60(36):9130–9136. doi: 10.1021/jf303440j. [DOI] [PubMed] [Google Scholar]
- Neme SM, Orsat V. Modeling the recovery patterns from solid phase extraction purification of secoisolariciresinol diglucoside, p-coumaric acid glucoside, and ferulic acid glucoside from microwave-assisted flaxseed extracts. Food Bioprod Process. 2012;90:453–465. doi: 10.1016/j.fbp.2011.11.003. [DOI] [Google Scholar]
- Peng JY, Yang GJ, Fan GR, Wu YT. Preparative isolation and separation of a novel and two known flavonoids from Patrinia villosa Juss by high-speed counter-current chromatography. J Chromatogr A. 2005;1092:235–240. doi: 10.1016/j.chroma.2005.07.073. [DOI] [PubMed] [Google Scholar]
- Peng L, Jia XP, Wang YZ, Zhu HB, Chen QM. Ultrasonically assisted extraction of rutin from Artemisia selengensis Turcz: comparison with conventional extraction techniques. Food Anal Methods. 2010;3:261–268. doi: 10.1007/s12161-009-9113-0. [DOI] [Google Scholar]
- Perino-Issartier S, Zill-e-Huma, Abert-Vian M, Chemat F. Solvent free microwave-assisted extraction of antioxidants from Sea Buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Tech. 2011;4:1020–1028. doi: 10.1007/s11947-010-0438-x. [DOI] [Google Scholar]
- Ramalakshmi K, Rao LJM, Takano-Ishikawa Y, Goto M. Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems. Food Chem. 2009;115(1):79–85. doi: 10.1016/j.foodchem.2008.11.063. [DOI] [Google Scholar]
- Rostagno MA, Palma M, Barroso CG. Microwave assisted extraction of soyisoflavones. Anal Chim Acta. 2007;588:274–282. doi: 10.1016/j.aca.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Shao P, He JZ, Sun PL, Zhao PC. Analysis of conditions for microwave-assisted extraction of total water-soluble flavonoids from Perilla Frutescens leaves. J Food Sci Tech Mys. 2012;49(1):66–73. doi: 10.1007/s13197-011-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55(3):381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- Takamura C, Hirata T, Yamaguchi Y, Ono M, Miyashita H, Ikeda T, Nohara T. Studies on the chemical constituents of green leaves of Eucommia ulmoides Oliver. J Nat Med. 2007;61:220–221. doi: 10.1007/s11418-006-0027-5. [DOI] [Google Scholar]
- Wang J, Sun BG, Cao YP, Tian YA, Li XH. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106(2):804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- Yoon KD, Chin YW, Yang MH, Kim J. Separation of anti-ulcer flavonoids from Artemisia extracts by high-speed countercurrent chromatography. Food Chem. 2011;129(2):679–683. doi: 10.1016/j.foodchem.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Yuan ED, Liu BG, Ning ZX, Chen CG. Preparative separation of flavonoids in Adinandra nitida leaves by high-speed counter-current chromatography and their effects on human epidermal carcinoma cancer cells. Food Chem. 2009;115(3):1158–1163. doi: 10.1016/j.foodchem.2009.01.009. [DOI] [Google Scholar]
- Zhan KY, Xu K, Yin HZ. Preparative separation and purification of gingerols from ginger (Zingiber officinale Roscoe) by high-speed counter-current chromatography. Food Chem. 2011;126(4):1959–1963. doi: 10.1016/j.foodchem.2010.12.052. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yang RY, Liu CZ. Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep Purif Technol. 2008;62(2):480–483. doi: 10.1016/j.seppur.2008.02.013. [DOI] [Google Scholar]
- Zhang QA, Zhang ZQ, Yue XF, Fan XH, Li T, Chen SF. Response surface optimization of ultrasound assisted oil extraction from autoclaved almond powder. Food Chem. 2009;116(2):513–518. doi: 10.1016/j.foodchem.2009.02.071. [DOI] [Google Scholar]
- Zhou JF, Zhang TM, Chen WA, Liang YZ. Comparative analysis of chemical components between barks and leaves of Eucommia ulmoides Oliver. J Cent South Univ Technol. 2009;16:0371–0379. doi: 10.1007/s11771-009-0063-x. [DOI] [Google Scholar]