Abstract
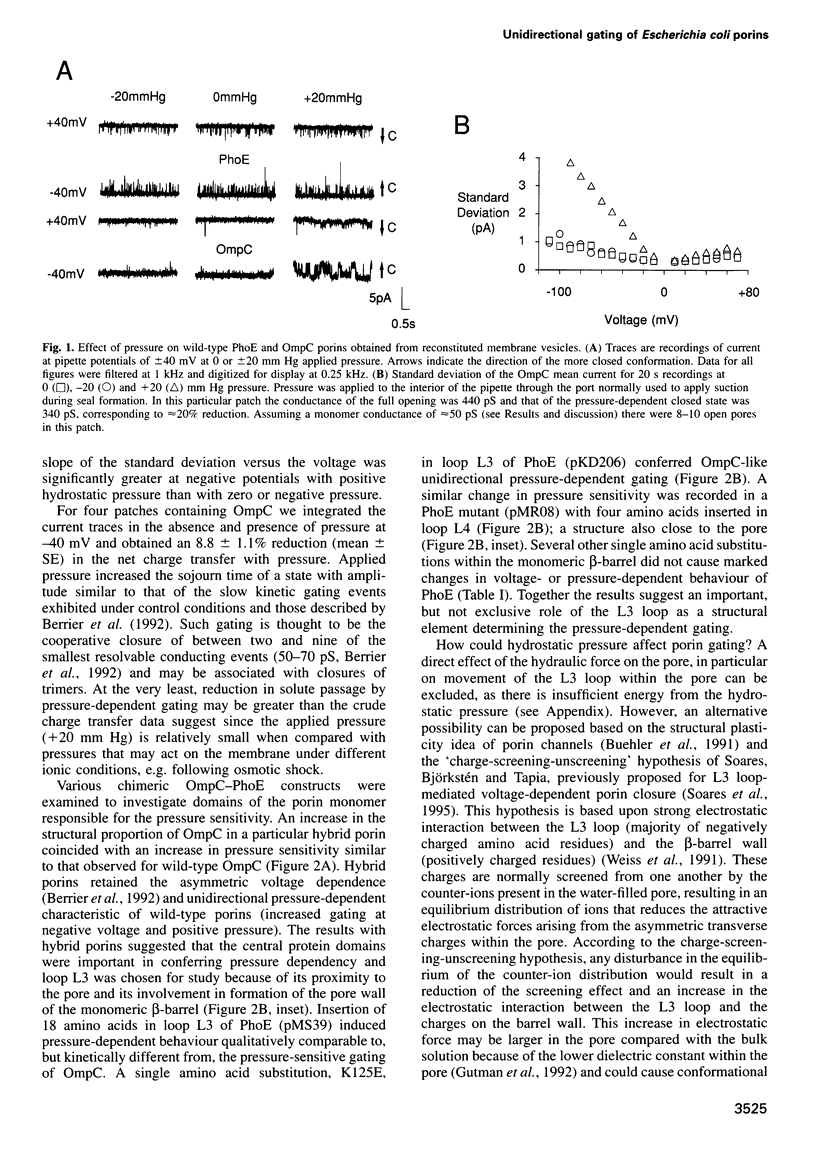
OmpC and PhoE porins of Escherichia coli were examined by the patch-clamp technique following reconstitution in liposomes, and were observed primarily in the open (conducting) state. With application of negative voltage and positive hydrostatic pressure, OmpC exhibited marked gating towards a more closed state whereas PhoE remained largely unaffected by pressure application. Hybrid chimeric OmpC-PhoE proteins showed an increased tendency for pressure-dependent gating as the OmpC proportion in the chimeric molecule increased. In addition, several PhoE mutants with amino acid substitutions and insertions in either the L3 or L4 loop of the monomer exhibited pressure sensitivity comparable with the wild-type OmpC porin. Our data support the structural plasticity model of porins and are consistent with the 'charge-screening-unscreening' hypothesis that describes how these proteins may exist in distinct conformations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agterberg M., Benz R., Tommassen J. Insertion mutagenesis on a cell-surface-exposed region of outer membrane protein PhoE of Escherichia coli K-12. Eur J Biochem. 1987 Nov 16;169(1):65–71. doi: 10.1111/j.1432-1033.1987.tb13581.x. [DOI] [PubMed] [Google Scholar]
- Bauer K., Struyvé M., Bosch D., Benz R., Tommassen J. One single lysine residue is responsible for the special interaction between polyphosphate and the outer membrane porin PhoE of Escherichia coli. J Biol Chem. 1989 Oct 5;264(28):16393–16398. [PubMed] [Google Scholar]
- Benz R., Bauer K. Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Review on bacterial porins. Eur J Biochem. 1988 Sep 1;176(1):1–19. doi: 10.1111/j.1432-1033.1988.tb14245.x. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Berrier C., Coulombe A., Houssin C., Ghazi A. Fast and slow kinetics of porin channels from Escherichia coli reconstituted into giant liposomes and studied by patch-clamp. FEBS Lett. 1992 Jul 20;306(2-3):251–256. doi: 10.1016/0014-5793(92)81011-a. [DOI] [PubMed] [Google Scholar]
- Bosch D., Leunissen J., Verbakel J., de Jong M., van Erp H., Tommassen J. Periplasmic accumulation of truncated forms of outer-membrane PhoE protein of Escherichia coli K-12. J Mol Biol. 1986 Jun 5;189(3):449–455. doi: 10.1016/0022-2836(86)90316-5. [DOI] [PubMed] [Google Scholar]
- Buehler L. K., Kusumoto S., Zhang H., Rosenbusch J. P. Plasticity of Escherichia coli porin channels. Dependence of their conductance on strain and lipid environment. J Biol Chem. 1991 Dec 25;266(36):24446–24450. [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Dahan D., Vachon V., Laprade R., Coulton J. W. Voltage gating of porins from Haemophilus influenzae type b. Biochim Biophys Acta. 1994 Jan 19;1189(2):204–211. doi: 10.1016/0005-2736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Delcour A. H., Adler J., Kung C. A single amino acid substitution alters conductance and gating of OmpC porin of Escherichia coli. J Membr Biol. 1991 Feb;119(3):267–275. doi: 10.1007/BF01868731. [DOI] [PubMed] [Google Scholar]
- Delcour A. H., Adler J., Kung C., Martinac B. Membrane-derived oligosaccharides (MDO's) promote closing of an E. coli porin channel. FEBS Lett. 1992 Jun 15;304(2-3):216–220. doi: 10.1016/0014-5793(92)80622-n. [DOI] [PubMed] [Google Scholar]
- Delcour A. H., Martinac B., Adler J., Kung C. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys J. 1989 Sep;56(3):631–636. doi: 10.1016/S0006-3495(89)82710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman M., Tsfadia Y., Masad A., Nachliel E. Quantitation of physical-chemical properties of the aqueous phase inside the phoE ionic channel. Biochim Biophys Acta. 1992 Aug 24;1109(2):141–148. doi: 10.1016/0005-2736(92)90077-y. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Schmidt A., Bauer K., Benz R. Role of lysines in ion selectivity of bacterial outer membrane porins. Biochim Biophys Acta. 1986 Aug 21;860(2):263–267. doi: 10.1016/0005-2736(86)90522-5. [DOI] [PubMed] [Google Scholar]
- Häse C. C., Le Dain A. C., Martinac B. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J Biol Chem. 1995 Aug 4;270(31):18329–18334. doi: 10.1074/jbc.270.31.18329. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Slauch J. M., Silhavy T. J. Signal transduction in bacteria: kinases that control gene expression. New Biol. 1990 Jan;2(1):5–9. [PubMed] [Google Scholar]
- Jap B. K., Walian P. J. Biophysics of the structure and function of porins. Q Rev Biophys. 1990 Nov;23(4):367–403. doi: 10.1017/s003358350000559x. [DOI] [PubMed] [Google Scholar]
- Jeanteur D., Lakey J. H., Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991 Sep;5(9):2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Korteland J., Overbeeke N., de Graaff P., Overduin P., Lugtenberg B. Role of the Arg158 residue of the outer membrane PhoE pore protein of Escherichia coli K 12 in bacteriophage TC45 recognition and in channel characteristics. Eur J Biochem. 1985 Nov 4;152(3):691–697. doi: 10.1111/j.1432-1033.1985.tb09249.x. [DOI] [PubMed] [Google Scholar]
- Lakey J. H., Lea E. J., Pattus F. ompC mutants which allow growth on maltodextrins show increased channel size and greater voltage sensitivity. FEBS Lett. 1991 Jan 14;278(1):31–34. doi: 10.1016/0014-5793(91)80076-f. [DOI] [PubMed] [Google Scholar]
- Lakey J. H., Pattus F. The voltage-dependent activity of Escherichia coli porins in different planar bilayer reconstitutions. Eur J Biochem. 1989 Dec 8;186(1-2):303–308. doi: 10.1111/j.1432-1033.1989.tb15209.x. [DOI] [PubMed] [Google Scholar]
- Lakey J. H. Voltage gating in porin channels. FEBS Lett. 1987 Jan 19;211(1):1–4. doi: 10.1016/0014-5793(87)81262-0. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H., Lonsdale J. T., Alder G. Polarity-dependent voltage-gated porin channels from Escherichia coli in lipid bilayer membranes. Biochim Biophys Acta. 1990 Jan 29;1021(2):175–181. doi: 10.1016/0005-2736(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer-membrane permeability of bacteria. Crit Rev Microbiol. 1986;13(1):1–62. doi: 10.3109/10408418609108734. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Mechanical transduction in biological systems. Crit Rev Biomed Eng. 1988;16(2):141–169. [PubMed] [Google Scholar]
- Sen K., Hellman J., Nikaido H. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J Biol Chem. 1988 Jan 25;263(3):1182–1187. [PubMed] [Google Scholar]
- Soares C. M., Björkstén J., Tapia O. L3 loop-mediated mechanisms of pore closing in porin: a molecular dynamics perturbation approach. Protein Eng. 1995 Jan;8(1):5–12. doi: 10.1093/protein/8.1.5. [DOI] [PubMed] [Google Scholar]
- Struyvé M., Visser J., Adriaanse H., Benz R., Tommassen J. Topology of PhoE porin: the 'eyelet' region. Mol Microbiol. 1993 Jan;7(1):131–140. doi: 10.1111/j.1365-2958.1993.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Tommassen J., van Tol H., Lugtenberg B. The ultimate localization of an outer membrane protein of Escherichia coli K-12 is not determined by the signal sequence. EMBO J. 1983;2(8):1275–1279. doi: 10.1002/j.1460-2075.1983.tb01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., van der Ley P., van Zeijl M., Agterberg M. Localization of functional domains in E. coli K-12 outer membrane porins. EMBO J. 1985 Jun;4(6):1583–1587. doi: 10.1002/j.1460-2075.1985.tb03820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- Xu G. Z., Shi B., McGroarty E. J., Tien H. T. Channel-closing activity of porins from Escherichia coli in bilayer lipid membranes. Biochim Biophys Acta. 1986 Nov 6;862(1):57–64. doi: 10.1016/0005-2736(86)90468-2. [DOI] [PubMed] [Google Scholar]
- Zizi M., Thomas L., Blachly-Dyson E., Forte M., Colombini M. Oriented channel insertion reveals the motion of a transmembrane beta strand during voltage gating of VDAC. J Membr Biol. 1995 Mar;144(2):121–129. doi: 10.1007/BF00232798. [DOI] [PubMed] [Google Scholar]
- delaVega A. L., Delcour A. H. Cadaverine induces closing of E. coli porins. EMBO J. 1995 Dec 1;14(23):6058–6065. doi: 10.1002/j.1460-2075.1995.tb00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]