Abstract
Outbreaks of food-borne diseases related to consumption of contaminated shellfish have been reported in many countries, but not in Brazil, possibly due to deficient reporting. Here we investigated the suitability of the clam Anomalocardia brasiliana as an animal sentinel for coliform monitoring in shellfish harvesting areas of Brazil’s northeast. Samples of shellfish meats (40 clams per sample; n = 8 per collection) were collected at random from April 2009 through March 2010 in the bay area of Mangue Seco (state of Pernambuco). The numbers of thermotolerant coliforms were analyzed through the most probable number technique, and these contamination levels were tentatively correlated with the precipitation recorded on the day of sampling or 24 to 48 h beforehand. A. brasiliana shellfish meats from local retail shops (250 g per sample/ n = 3 per market) sold frozen were also investigated from August 2010 through June 2011. We found that the highest coliform contamination levels were correlated with recent rainfall events, limited to 24 h before sampling. However, irrespective of the rainfall level, the mean contamination above the Brazilian legal threshold of < 3 × 102 MPN/ 100 g for shellfish harvesting areas ranged from 18.7 to 93.7 % of samples analyzed monthly. Additionally, a large number of samples obtained from retail shops were also highly contaminated by coliforms during rainy periods, and therefore were not proper for human consumption. We conclude that A. brasiliana can be successfully used to monitor the contamination levels of coliforms in shellfish harvesting areas in Brazil’s northeast coast.
Keywords: Food safety, Thermotolerant coliforms, Bivalve mollusks, Outbreaks
Introduction
The long Brazilian coastline favors natural occurrence of many species of marine shellfish with high economic value. However, the quality and quantity of contaminating microorganisms in shellfish meats can vary under the influence of extrinsic factors such as tides and rainfall regimes (Solic et al. 1999; Brands et al. 2005; Band and Salvesen 2009). Human consumption of raw or undercooked shellfish is often associated with gastroenteritis episodes and intoxication by enteric human viruses as well as bacterial pathogens such as Salmonella, Vibrio parahaemolyticus, V. cholerae and V. vulnificus (Bauer et al. 2006; Ueki et al. 2007; Nappier et al. 2008). Infectious outbreaks have been reported in the USA, UK, Australia, Japan, Spain, Italy, China, Canada, Malaysia, Singapore, France and New Zealand (Rippey 1994; Potasman et al. 2002), but not in Brazil, possibly due to deficient reporting.
Here we investigate the suitability of the clam Anomalocardia brasiliana as an animal sentinel for coliform monitoring in shellfish harvesting areas of Brazil’s northeast. According to the World Register of Marine Species (WoRMS), A. brasiliana (Gmelin 1971; Veneridae family) is a synonymized taxa for A. flexuosa (Gmelin 1791), also recognized with different scientific names: A. rugosa (Schumacher 1817), Cryptogramma flexuosa (Linnaeus 1767), Venus brasiliana (Gmelin 1791), Venus flexuosa (Linnaeus 1767), Venus punctifera (G.B. Sowerby II 1853) (WoRNS 2012). This shellfish and its synonymized taxa is distributed in countries on the Atlantic seaboard and Caribbean, including Belize, Colombia, Costa Rica, Cuba, Jamaica, Lesser Antilles, Puerto Rico and Venezuela, as well as in Madagascar (WoRNS 2012). Therefore, the potential of these shellfish as bioindicators has broad reach.
Rainfall levels are known to affect seafood quality. Due to the irregularity of precipitation in Brazil’s northeast, samples of shellfish meats of A. brasiliana were collected at random in the bay area of Mangue Seco (state of Pernambuco), and the population of thermotolerant coliforms was investigated. The contamination levels were tentatively correlated with the precipitation recorded on the day of sampling or 24s to 48 h beforehand, during a 1-year-study. Additionally, frozen samples of shellfish meats obtained from local retail shops were also investigated. According to the results, we recommend the use of the clam A. brasiliana as an animal sentinel in harvesting areas of Brazil’s northeast.
Material and methods
Study area
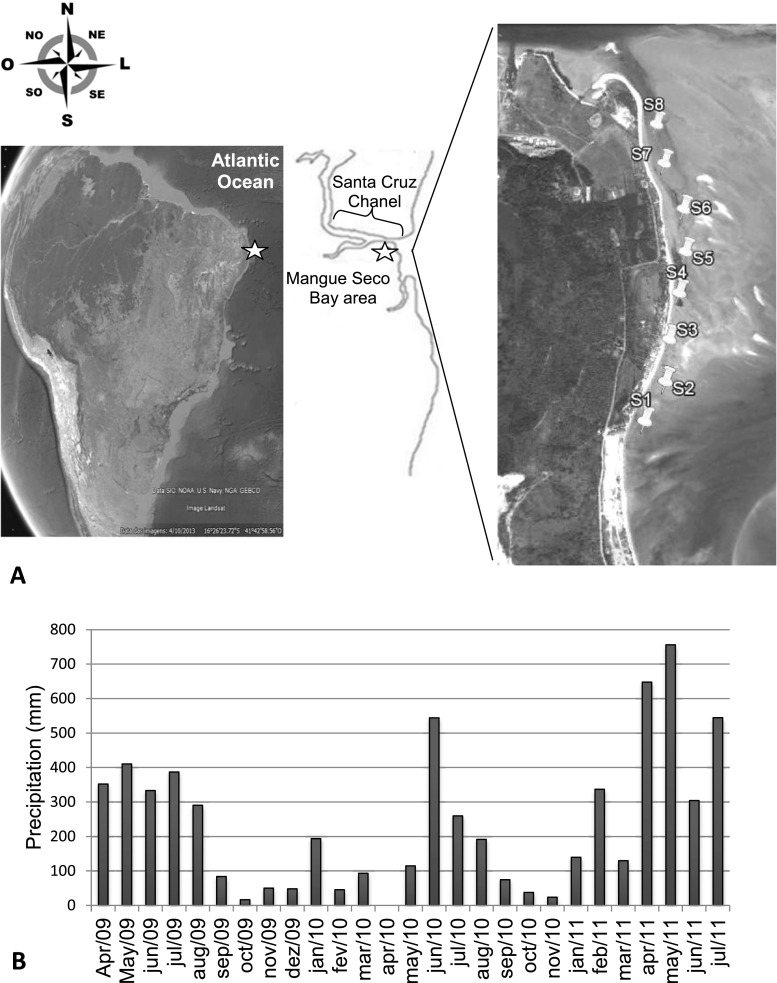
Clams were harvested along a 1600 m stretch of Mangue Seco Beach (7o 50′ 03.90″ S; 34o 50′ 39.52″ W), in the town of Igarassu, from April 2009 to March 2010 (Fig. 1a). This area is part of a highly productive ecosystem with extensive mangrove estuaries, segments of plains covered by coconut trees, coral reefs and small islands. The weather is usually hot and sunny, except for a period from April to June, when the rainfall reduces temperatures to approximately 24–26 °C, 3–6 °C degrees below those registered in the rest of the year. This beach is a natural breeding place for many species of mollusks, and particularly A. brasiliana. There are no structures near the shore, except for a group of small restaurants in the first 200 m of the beach (Fig. 1a, Section S1). At low tide, the beach is crowded with people from local coastal communities who harvest clams. The shellfish gathered are intended for personal consumption and to supply local restaurants and markets. Commercial shellfish were obtained at retail shops in the metropolitan area of Recife from August 2010 to June 2011. The precipitation throughout the study period is shown in Fig. 1b.
Fig. 1.
Study area (a) and mean precipitation recorded from April/ 2009 through July/ 2011 (b). Satellite images: Google Earth Software
Sampling procedures
Mature A. brasiliana specimens (40 clams per sample; n = 8 per collection) measuring 20 to 25 mm in length were harvested during low tide from eight sampling points along Mangue Seco Bay, separated by 200 m (Fig. 1a; sections S1 to S8). The samples were collected in isothermal plastic bags from April through August 2009 (rainy season), and from September 2009 through March 2010 (dry season). Random samples of frozen shelled clam meat (250 g per sample/ n = 3 per market) were obtained from retail shops from August through November 2010 (dry season), and from April through June 2011 (rainy season).
Determination of the most probable number (MPN) of thermotolerant coliforms
The presence of thermotolerant coliforms in A. brasiliana meat was evaluated through the most probable number technique (MPN), following the recommendations of the National Sanitary Surveillance Agency, ANVISA (Brasil 2011). Briefly, the samples were diluted to 10−1 to 10−3 and then 0.1 mL aliquots from each dilution were added to a set of nine tubes (triplicate) with lactose broth (LB) for presumptive identification of coliforms. Coliform bacteria were confirmed after incubation for 24–48 h at 35 °C in brilliant green bile broth (BGB). Then, 0.1 mL of material from the BGB-positive tubes was added to tubes with E. coli broth (EC) selective media, which were incubated for a further 24 h at 44.5 °C. After this time, bacterial growth plus gas production into Durham tubes was interpreted as indicative of thermotolerant coliforms. Results were expressed as mean geometric density of thermotolerant coliforms (MPN/ g).
Hypothesis and statistical analyses
The bacterial populations in shellfish meats of A. brasiliana were recorded during a 1-year-study to test the following null hypotheses:
No correlation between recent rainfall events and contamination levels by thermotolerant coliforms in shellfish meats of A. brasiliana gathered along Mangue Seco Beach.
No correlation between the local rainfall regime and contamination levels by thermotolerant coliforms in shellfish meats of A. brasiliana sold in retail shops.
For statistical evaluation of the contamination levels of thermotolerant coliforms, the values bellow < 3 MPN/ g were normalized as “2”, and those above > 1100 MPN/ g as “1200”. In this case, the mean geometric density of coliforms was calculated among samples collected on a single day, and then tested for Pearson’s correlation to the precipitation recorded on day of collection and 24 to 48 h beforehand. The contamination levels of coliforms in shellfish meat obtained in local retail shops discriminated among samples sold in the dry and rainy seasons. Simple comparisons between populations of bacteria of two different samples were made by the Student t-test followed by the Mann-Whitney test. In all situations, the level of significance was set at P < 0.05. The Prism GraphPad (version 5.1) program was used for calculations. Data on precipitation was provided by the local official agency (National Institute of Meteorology, Recife).
Results and discussion
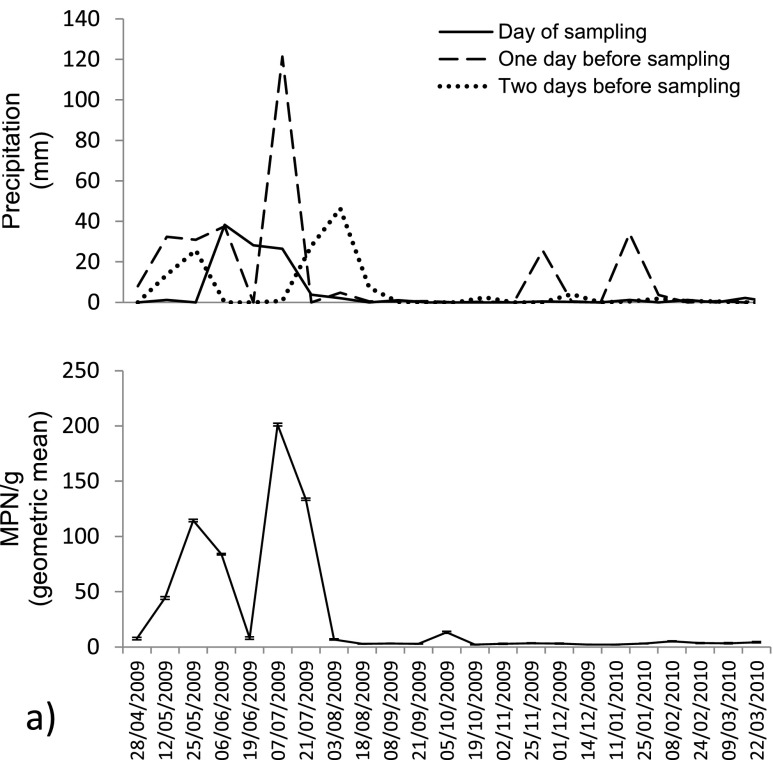
Previous data have shown the suitability of animal sentinels for monitoring seafood quality (Martinez and Oliveira 2010). Here, the presence of thermotolerant coliforms in shellfish meats of A. brasiliana was higher following rainfall events and lower in the dry season of the year (Fig. 2). The likelihood of the rainfall regimen affecting the quantity of coliforms in contaminated samples was positive (Table 1). This was especially clear when these contamination levels were compared to the precipitation data 1 day before sampling (P < 0.0001). Moreover, a large number of shellfish meat samples collected in rainy periods were above the Brazilian legal threshold of 3 × 102 MPN/ 100 g determined for production areas (Table 2).
Fig. 2.
Contamination levels by thermotolerant coliforms in shellfish meats of A. brasiliana during 1-year-study
Table 1.
Pearson correlation analyses between precipitation and the contamination levels of thermotolerant coliforms in shelfish meats of A. brasiliana during 1-year study
| Precipitation (mm) versus contamination by thermotolerant coliforms a | Day of sampling | One day before sampling | Two days before sampling |
|---|---|---|---|
| Number of XY Pairs | 23 | 23 | 23 |
| Pearson r | 0.5069 | 0.7641 | 0.2897 |
| 95 % confidence interval | 0.1197 to 0.7603 | 0.5136 to 0.8946 | −0.1392 to 0.6271 |
| P value (two-tailed) | 0.0136 | P < 0.0001 | 0.1799 |
| Is the correlation significant? (alpha = 0.05) | Yes | Yes | No |
| R squared | 0.2570 | 0.5838 | 0.08394 |
aGeometric mean of MPN/ g was calculated for eight samples per day of collection. Precipitation data was provided for each day of collection by the National Institute of Meteorology (INMET, Recife-PE)
Table 2.
Contamination levels of thermotolerant coliforms above the threshold determinate by the Brazilian official standards set forth for shellfish meats
| Apr/ 09 | Mai/ 09 | Jun/ 09 | Jul/ 09 | Aug/ 09 | Sep/ 09 | Oct/ 09 | Nov/ 09 | Dec/ 09 | Jan/ 10 | Feb/ 10 | Mar/ 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of samples of shellfish meats above 3 × 102 MPN/ 100 g of thermotolerant coliforms | ||||||||||||
| Number | 3 out of 8 | 9 out of 16 | 10 out of 16 | 15 out of 16 | 7 out of 16 | 7 out of 16 | 7 out of 16 | 5 out of 16 | 3 out of 16 | 4 out of 16 | 6 out of 16 | 6 out of 16 |
| % | 37.5 | 56.2 | 62.5 | 93.7 | 43.7 | 43.7 | 43.7 | 31.2 | 18.7 | 25.0 | 37.5 | 37.5 |
* The Brazilian official standards set forth establish a maximum of < 3 × 102 MPN/ 100 g of thermotolerant colifomrs for shellfish meats in harvesting areas (BRASIL 2011)
Rainfall causes suspension of bottom sediments and input of nutrients into seawater, favoring eutrophication and shellfish bioaccumulation. Therefore, seawater quality degradation in estuaries often increases from 1 to 2 days after rainfall events greater than 10 mm/day (Bougeard et al. 2011). Trials carried out in Hawaii also showed that fecal coliforms deposited in the soil can survive on substrates and eventually be detected in the water column (Fujioka et al. 1998). A. brasiliana can filter an average of 19 to 50 L of water per hour (Barnes 1984). The presence of mucus in the gill filament assists in capturing the phytoplankton used as food, and microorganisms that accumulate in the visceral mass hepatopancreas and intestinal lumen (Cook 1991). Our results show that A. brasiliana was very sensitive to detect contamination by coliforms in the water column after recent rainfall events.
To prevent outbreaks and ensure the quality of shellfish for human consumption, classification of bivalve mollusk production areas in Europe establishes a maximum of 2.3 × 102 MPN of E. coli per 100 g of shellfish meat (CEFAS 2010). In this case, depuration processes are not necessary. In Brazil, current policies establish a threshold 2.3 × 102 MPN of E. coli or 3 × 102 MPN of thermotolerant coliforms per 100 g of shellfish meat in harvesting areas (BRASIL 2011), and 1 × 102 MPN of thermotolerant coliforms per g of shellfish meat processed for human consumption (BRASIL 2001). Since the level of thermotolerant coliforms in shellfish meats of A. brasiliana increased with rainfall, we also investigated clam meat sold at retail shops. We confirmed that the risk to human health was higher for frozen meat sold in the rainy season than in the dry season of the year (P < 0.0001) (Table 3).
Table 3.
Most probable number (MPN) of thermotolerant coliforms in shellfish meats of A. brasiliana traded in retail shops
| MPN/g (geometric mean; n = 3 per market) | ||||
|---|---|---|---|---|
| Markets | Frozen samples | Markets | Frozen samples | |
| A | 3.32 | I | 1200 | |
| B | 5.53 | J | 24.01 | |
| C | 5.53 | L | 9.18 | |
| D | 9.13 | M | 1200 | |
| E | 4.76 | N | 1200 | |
| F | 2.0 | O | 7.5 | |
| G | 0.3 | |||
| Overall mean contamination: | 4.37 ± 2.85 | 606.8 ± 649.86 | ||
| Mean precipitation (mm): | 96. 3 a | 569b | ||
aSamples were collected at random from August through November 2010
bApril through June 2011
Bacterial contamination of commercial shellfish is commonly affected by the season and tidal cycle (Lee and Morgan 2003). As a measure to diminish the risk to public health, closure of shellfish harvesting areas has been adopted after heavy rainfall events in Canada (Canadian Food Inspection Agency 2011). Recently we showed that several coagulase-negative staphylococcal species detected in shellfish meat of A. brasiliana were multi-drug resistant and positive for the presence of the mecA gene (Batista et al. 2013). Moreover, the enterotoxin-encoding genes seg and seh were detected among isolates from both environmental samples and those from retail shops (Batista et al. 2013). Taken together, our data provide further evidence that shellfish collected at Mangue Seco Bay area are prospective reservoirs of bacterial pathogens.
The results indicate the suitability of A. brasiliana as an animal sentinel for shellfish harvesting areas of Brazil’s northeast. Although a previous study suggested A. brasiliana for use as a bioindicator (Barros 2009), until the present work this was not tested in a long-term study. We conclude that depuration of shellfish collected in the bay area of Mangue Seco is crucial to prevent outbreaks, regardless of the rainfall regimen. Also, closure of shellfish harvesting areas for at least 24 h following heavy rainfall events is recommended.
Acknowledgments
The study was supported by grants from the Brazilian Council for Scientific and Technological Research (Grant number 578030/2008-0).
Contributor Information
José Vitor Lima-Filho, Phone: + 55 31 81 3320.6312, Email: jvitor@db.ufrpe.br.
Sílvio M. Peixoto, Phone: + 55 31 81 3320.6312, Email: silvio.peixoto@gmail.com
References
- Band L, Salvesen D (2009) Climate Change Committee Report. Institute for the Environment University of North Carolina at Chapel Hill. Available at: www.ie.unc.edu/PDF/Climate_Change_Report.pdf. Accessed in 9 July 2012
- Barnes RD (1984) Zoology of invertebrates. Rocca, S. Paulo
- Barros CN. Enumeration of coliforms in Anomalocardia brasiliana (GMELIM, 1791) and Tagelus plebeius (LIGHTFOOT, 1786) in the estuary of Pina. Recife: Monograph, Pernambuco Federal Rural University; 2009. [Google Scholar]
- Batista JEC, Ferreira EL, Nascimento DCO, et al. Antimicrobial resistance and detection of the mecA gene besides enterotoxin-encoding genes among coagulase-negative staphylococci isolated from clam meat of Anomalocardia brasiliana. Food Pathog Dis. 2013;10:1–7. doi: 10.1089/fpd.2012.1234. [DOI] [PubMed] [Google Scholar]
- Bauer A, Ostensvik O, Florvag M. Occurrence of Vibrio parahaemolyticus, V. cholerae, and V. vulnificus in Norwegian Blue mussels (Mytilus edulis) Appl Environ Microbiol. 2006;72:3058–3061. doi: 10.1128/AEM.72.4.3058-3061.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougeard M, Le Saux JCL, Pérenne N. Modeling of Escherichia coli fluxes on a catchment and the impact on coastal water and shellfish quality. J Am Water Resour Assoc. 2011;47:350–366. doi: 10.1111/j.1752-1688.2010.00520.x. [DOI] [Google Scholar]
- Brands DA, Inman AE, Gerba CP. Prevalence of Salmonella spp. in oysters in the United States. Appl Environ Microbiol. 2005;71:893–897. doi: 10.1128/AEM.71.2.893-897.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRASIL, Brazilian Sanitary Agency – ANVISA. Resolution – RDC n. 12, 02/01/2001. Technical regulations for the microbiological patterns of foods. Diário Oficial da República
- BRASIL, Ministry of Fishring and Aquaculture. Portaria n. 122, 03/05/2011. Diário Oficial da União. Brasília, DF, Seção 1 em 04/05/2011
- Canadian food inspection agency. Canadian Shellfish Sanitation Program (2011) Available at: www.inspection.gc.ca/DAM/DAM-food-aliments/STAGING/text-stexte/fish_man_shellfish_cssppccsme_1351699265137_eng.pdf. Accessed in 20 March 2011
- CEFAS: Microbiological monitoring of bivalve mollusk harvesting areas - Guide for good practice: technical application. European Union Reference Laboratory for monitoring bacteriological and viral contamination of bivalve mollusks, 2010. Available at: http://www.crlcefas.org/Content/GPG_Issue4_Aug2010.pdf. Accessed in 20 March 2011
- Cook DW. Microbiology of bivalves molluscan shellfish. In: Ward DR, Hackney C, editors. Microbiology of marine food products. New York: Van Nostrand Reinhold; 1991. [Google Scholar]
- Fujioka R, Sian-Denton C, Borja M, Castro J, Morphew K. Soil: the environmental source of Escherichia coli and enterococci in Hawaii’s streams. J Appl Microbiol. 1998;85:83S–89S. doi: 10.1111/j.1365-2672.1998.tb05286.x. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Morgan OC. Environmental factors influencing the microbiological contamination of commercially harvested shellfish. Water Sci Technol. 2003;47:65–70. [PubMed] [Google Scholar]
- Martinez DI, Oliveira AJFC. Faecal bacteria in Perna perna (Linnaeus, 1758, mollusca bivalvia) for biomonitoring coastal waters and seafood quality. Braz J Oceanogr. 2010;58:29–35. doi: 10.1590/S1679-87592010000700005. [DOI] [Google Scholar]
- Nappier SP, Thaddeus KG, Kellogg JS. Bioaccumulation, retention, and depuration of enteric viruses by Crassostrea virginica and Crassostrea ariakensis oysters. Appl Environ Microbiol. 2008;74:6825–6831. doi: 10.1128/AEM.01000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potasman I, Paz A, Odeh M. Infectious outbreaks associated with bivalve shellfish consumption: a worldwide perspective. Clin Infect Dis. 2002;35:921–928. doi: 10.1086/342330. [DOI] [PubMed] [Google Scholar]
- Rippey SR. Infectious disease associated with molluscan shellfish consumption. Rev Clin Microbiol. 1994;7:419–425. doi: 10.1128/cmr.7.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solic MKN, Jozic S, Curac D. The rate of concentration of faecal coliforms in shellfish underdifferent environmental conditions. Environ Int. 1999;25:991–1000. doi: 10.1016/S0160-4120(99)00067-7. [DOI] [Google Scholar]
- Ueki Y, Shoji M, Suto A. Persistence of caliciviruses in artificially contaminated oysters during depuration. Appl Environ Microbiol. 2007;73:5698–5701. doi: 10.1128/AEM.00290-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WoRMS, World Register of Marine Species. Available at: http://www.marinespecies.org/. Accessed in 3 Dec 2012