Abstract
Phytosterols enriched products are innovative types of functional foods, in which dairy products, like low fat yogurt are ideal vehicles for this functional component. In this study, phytosterol dispersions were prepared using an oil/water (O/W) emulsion. The emulsion was added to yogurt milk. pH, titratable acidity (TA), syneresis, firmness and apparent viscosity of enriched yogurt were determined during storage. Moreover, phytosterols distribution in different parts of enriched yogurt was studied. Results indicated that in enriched yogurt, apparent viscosity and syneresis were lower and firmness was higher compared to the control. Addition of phytosterol to the yogurt had significant effect on acidity. Distribution of phytosterols in different parts of one sample was not uniform. Sensory results showed that there was no significant difference between enriched and control on texture, appearance, flavor and overall acceptance.
Keywords: Phytosterol, Enriched yogurt, Physicochemical characteristics
Introduction
Phytosterols (plant sterols), are naturally occurring components of vegetables, fruits and cereals. The quantity of phytosterols in a regular diet is not sufficient to enhance and show their health effects (Derakhshan-Honarparvar et al. 2010). They are white powders with mild characteristic odor, insoluble in water, soluble in alcohols and have melting point of 100 to 215 °C. The cholesterol-lowering properties of phytosterols were observed already in humans in the early 1950s (Pollak 1953). As a food ingredient or additive, phytosterols have been shown to reduce total cholesterol and low density lipoprotein (LDL) cholesterol in normo-cholesterolemic and hyperlipidemic populations (Micallef and Garg 2008). Phytosterols are thought to displace cholesterol from bile acid micelles and/or co-precipitate cholesterol in the intestinal lumen, thereby limiting its uptake (Yokoyama 2004). Intake of phytosterols and/or phytostanols at a level of 1.5-3.0 g/day has been documented to reduce blood LDL-cholesterol by 10 % (Katan et al. 2003; Demonty et al. 2009). Enriched products containing phytosterols/stanols are innovative types of functional foods and their presence in the market is increasing rapidly. The lipid profile can effectively be reduced to lower the incidence of cardiovascular disease using combinations of Lactobacillus-based probiotics and phytosterols in functional foods (Awaisheh et al. 2013).
Major problems related to enrichment of products with phytosterols are high melting temperature and chalky taste. Esterification of phytosterols and stanols with long chain fatty acids increases their lipid solubility and consequently facilitates their incorporation into foods (Noakes et al. 2005). However, this process adversely affects cholesterol reducing properties of phytosterols. In an efficacy study on phytosterols, a daily consumption of 24 g of spread containing 2–3 g of phytosterols esters, total serum cholesterol and LDL cholesterol were lowered up to 6.4 and 10.1 %, respectively. While the consumption of 1.6–2.0 g phytosterols or phytostanols per day resulted in reduction of total serum cholesterol and LDL cholesterol by 8–13 % (Nguyen 1999). Richelle et al. (2004) compared the effects of phytosterol esters and free phytosterols on bioavailability of β-carotene and α-tocopherol and they found that phytosterol esters reduce the bioavailability more than free plant sterols.
The objective of this study was dispersing phytosterol in an O/W emulsion, production of enriched yogurt with phytosterol and investigation of physicochemical properties of enriched yogurt. Moreover, because of high density of phytosterols, distribution state of phytosterol in different parts of enriched yogurt was studied using gas chromatography (GC).
Materials and methods
Materials
Phytosterols used in this study consisted of β-sitosterol, stigmasterol, campsterol and brassicasterol at ratio of (~40 %), (15 ~ 30 %), (15 ~ 30 %) and (<10 %), respectively. It was a gift from Zhejiang Medicine Co. (Zhejiang Medicine Co., Ltd Xinchang Pharma, China). Lactic acid esters of monoglycerides (LACTEM) was supplied by Poratus CO., (Poratus CO., Belgium). Milk was obtained from research farm of Isfahan University of Technology (Isfahan, Iran). Starter culture was obtained from Proquiga (Proquiga CO., Spain). Skim milk powder was purchased from Pegah Dairy Industry (Mashhad, Iran). The local market cold pressed vegetable oils and double-distilled water were used for preparation of O/W emulsions. All chemicals were of reagent grade and were used as such.
Methods
Preparation of O/W emulsion
LACTEM and soy oil were used for dissolving and dispersing phytosterols. The mixtures of oil, emulsifier and phytosterol were heated to 130–140 °C and mixed for 2 min at 700 rpm using a laboratory mixer (Heidolph, Germany) and then heated water (90 °C) was added to oil phase. In aprevious study, the mixture components was optimized at 10 % emulsifier, 10 % phytosterols, 68.39 % water and 11.61 % oil (Izadi et al. 2012). Therefore, the four component mixtures (emulsifier, phytosterol, water and oil) were mixed for 4 min at 3,000 rpm to achieve an emulsion with soft texture and white color. This homogenous mixture was added to skim milk based on 2 g phytosterol in each serving size yogurt before pasteurization and homogenized using piston homogenizer at 200 bars to disperse phytosterol in milk.
Starter culture preparation
The starter culture was prepared by addition of 1 g of direct vat starter freeze-dried yogurt culture which combined of Lactobacillus delbrueckii ssp. (YO-A) (0.3 g), Lactobacillus bulgaricus (YO-B) (0.3 g) and Streptococcus thermophilus (YO-S) (0.4 g) to 1 L of sterile skim milk. 2 ml of prepared mixture was inoculated to 100 ml of yogurt milk.
Yogurt manufacture
Solid not fat (SNF) of fresh skim milk was adjusted on 10.7 % using skim milk powder. O/W emulsion was added to skim milk based on 2 g phytosterol in each serving size yogurt before pasteurization. Milk was homogenized at 200 bar and 60 °C to obtain a homogenous emulsion. Then the mixture pasteurized at 85 °C for 30 min and rapidly cooled down to 45 °C was inoculated with 2 % (w/w) starter culture. The inoculated milk was dispensed in 150 cc plastic containers and incubated at 45 °C until pH reached to 4.6 and then samples were cooled down to 5 °C (Dave and Shah 1998). Nonfat yogurt control was prepared based on explained method without adding the phytosterol emulsion. Yogurt pH and titratable acidity (TA), syneresis, firmness and apparent viscosity were measured 24 h after production and each week during 4 weeks storage.
Comparative analysis of yogurt samples
Before the analyses, except for the gel firmness, the yogurt samples were gently stirred to ensure homogeneity. All measurements were carried out in triplicate.
pH and titratable acidity (TA)
The pH values of each yogurt sample were measured using a digital pH-meter (Hanna H18314, England). The amount of TA (as lactic acid percentage) was determined after mixing the sample with 10 ml of hot distilled water (∼90 °C) and titration was performed using 0.1 N NaOH in the presence of 0.5 % phenolphthalein indicator to an end point of faint pink color (Dave and Shah 1998).
Syneresis
The Susceptibility of yogurt to syneresis was determined using drainage method (Hassan et al. 1996). The test was performed at 6 °C. Yogurt was transferred into a funnel fitted with a 120 mesh stainless steel screen. The volume of the collected whey over 2 h was measured.
Firmness
An Instron Universal Testing Machine (Instron model 1440, UK) was used to determine textural property of yogurts. For textural analysis, samples were prepared in plastic containers of 7.5 cm diameter. The ratio of yogurt container diameter to diameter of probe was 3:1. Firmness was determined by performing a penetration test with a 24.5 mm cylindrical probe. Force distance curves were obtained operating at 6 °C and at a constant speed of 2 mm/s until 35 mm of sample was penetrated. The maximum required force was recorded in mN. Textural analyses were carried out at 1, 7, 14, 21 and 28 days after yogurt production.
Apparent viscosity
Apparent viscosity of the homogenized samples was measured using a Brookfield viscometer (Brookfield viscometer DVII, USA) with a spindle no.4 and 3 rpm rotation speed at 6 °C. Results recorded in centipoises (cP) after 50 s of shearing.
Determination of phytosterol and survey of its distribution state in enriched yogurt preparation of standard solution
Stock solutions of phytosterol (β-sitosterol, stigmasterol, campsterol, brassicasterol) were prepared in ethanol at concentration of 2 mg/ml. The temperature of solutions was maintained between 2 and 8 °C in a refrigerator.
Samples were prepared as described by Santos et al. (2007). Enriched yogurt samples were homogenized using a manual homogenizer. Ten ml of each sample were centrifuged at 4,000 rpm for 5 min and 100 μl of supernatant was used as the sample aliquots. Subsequently, 1.5 ml KOH solution (2.5 M in 90 % ethanol) was added and the saponification procedure occurred at 60 °C for 90 min. After saponification, the unsaponifiable fraction (at top of vessel) was extracted with 1 ml of n-hexane. Then, 250 μl of n-hexane was transferred to a derivatization vial and was evaporated through a nitrogen stream at 60 °C. The dry residue was derivatized with 50 μl of N, O-Bis (trimethylsilyl) trifluoroacetamide with 1 % Trimethylchlorosilane [BSTFA: TMCS (99:1)] at 60 °C for 30 min.
Gas chromatography
In this study phytosterols was determined using an HP-5MS column (30 m × 0.25 mm internal diameter (i.d.) × 0.25 μm film thickness), (6890N, USA) via injection in split mode with 1/20 ratio, in 1 min, with 1.0 μl of the derivatized sample at an injection temperature of 250 °C. Total flow was 63.5 ml/min in which columns flow was 1.5 ml/min. Nitrogen was used as mobile phase at a pressure of 3.240 bars at the head of the column. The column initial temperature (200 °C) was kept for 1 min and then increased gradually 20 °C/min up to 300 °C. This temperature was maintained for 10 min. The detector temperature was set at 280 °C. In this study, 3 samples from different parts of yogurt (bottom, middle and surface) were prepared for injection to GC. Stock solution of phytosterol (2 mg/mL) was used as standard. Results were evaluated comparing between the obtained ratios of the standard curve and peak areas of each chromatogram.
Sensory evaluation
Consumer acceptability of yogurts was studied after 14 days of storage at 4 °C. A panel consisting of 12 members (6 female, 6 male) ages 25 to 45-year old, evaluated the yogurt samples presented in coded cups in individual booths at room temperature. They had to score texture, appearance, flavor and overall acceptability on a 9-point hedonic scale, 1 = the least, the lowest; 9 = the most, the highest (Tamjidi et al. 2012).
Statistical analysis
All statistical analyses were performed using SAS version 9.0 (SAS Institute Inc., Cary, NC). An ANOVA was applied using the general linear models procedure to determine significant differences among the samples. Mean values were compared using Fisher’s Least Significant Difference (LSD) procedure. All experiments were performed in triplicates.
Results and discussion
Physicochemical properties of yogurt samples
Titratable acidity and pH
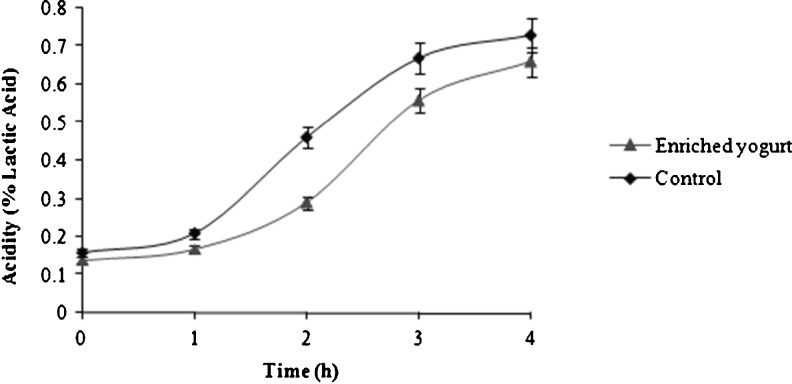
Table 1 shows, pH values of enriched and control yogurt during storage. Result indicated a significant difference between the pH values of enriched and control yogurt during storage. pH of samples was significantly affected by the addition of 1.5 % (w/v) phytosterol compared to the control during storage. However, the pH yogurt decreased significantly during storage time, and the TA significantly increased (Table 1). TA of both enriched and control yogurt samples following 4 h of fermentation (Fig. 1) increased from 0.14 to 0.66 and from 0.16 to 0.73 % lactic acid, respectively. TA of enriched yogurt was increased significantly until day 21 after production, but in the case of control yogurt, TA was increased significantly until day 14 and between 14th to 21th days the differences in TA was not significant. Nevertheless, TA of enriched yogurt was significantly (P < 0.05) lower than the control (Table 1). Lower TA in enriched yogurt, may be due to addition of O/W emulsion (fat) in enriched yogurt. Bonczar et al. (2002) reported that fat content of milk affected TA, pH and content of free fatty acids of plain and probiotic yogurts. TA of samples which containing high percentage of fat was lower than those samples containing lower percentage of fat and vice versa. Shaker et al. (2000) surveyed the rheological properties of yogurts at four levels of fat content during fermentation and they concluded that by increasing of fat, acid production by starter cultures is decreased.
Table 1.
Rheological and physical characteristics of yogurt during 28 days of storage at 4 °C
| Yogurt sample | Storage time (day) | pH | TA (Lactic acid %) | Syneresis (ml) | Firmness (mN) | Apparent viscosity (cP) | Fat (%) |
|---|---|---|---|---|---|---|---|
| Enriched | 1 | 4.69 ± 0.01a | 0.71 ± 0.01g | 24.12f | 52.5f | 27,400d | |
| 7 | 4.58 ± 0.02c | 0.78 ± 0.01f | 32.52c | 55c | 30,200bc | ||
| 14 | 4.47 ± 0.01d | 0.81 ± 0.01e | 25.32e | 60b | 31,400bc | 0.93a | |
| 21 | 4.38 ± 0.01e | 0.87 ± 0.00cd | 23.41f | 75a | 30,900c | ||
| 28 | 4.29 ± 0.02f | 0.90 ± 0.01c | 21.65g | 52.5e | 29,500d | ||
| Control | 1 | 4.59 ± 0.01b | 0.79 ± 0.01f | 35b | 49.5e | 31,200bc | |
| 7 | 4.52 ± 0.02d | 0.86 ± 0.01d | 44a | 53.5d | 34,500a | ||
| 14 | 4.40 ± 0.01e | 0.92 ± 0.01b | 35b | 60b | 33,000b | 0.43b | |
| 21 | 4.32 ± 0.00f | 0.95 ± 0.01b | 32.5c | 55c | 32,500bc | ||
| 28 | 4.18 ± 0.01g | 0.97 ± 0.01a | 30.88d | 44g | 32,200bc |
Different superscripts in the same column are significantly different (P < 0.05) with LSD test
Data given in the table is Mean ± SD of three replicates
Fig. 1.
Change of acidity of enriched (black triangle) and control yogurt (black diamond) during fermentation. Each observation is a mean ± SD of three replicates
Syneresis
Syneresis is an important physical test of yogurt quality. Syneresis is related to the instability of the gel network and its poor ability to entrap all the serum phase (Lucey and Singh 1998). Results of syneresis of yogurts during 28 days storage at 4 °C are shown in Table 1. Syneresis of control and enriched yogurts decreased during storage.
Enriched yogurt had lower syneresis comparing to the control yogurt during storage (P < 0.05). Lower syneresis in enriched yogurt was because of higher total solids (TS) and interactions between fat globules and gel network. Lucey and Singh (1998) reported that syneresis can be prevented by increasing the TS of milk in set yogurt. The change of syneresis in control and enriched yogurt almost was similar. Our results show that syneresis increased until day 7 and then decreased for both enriched and control samples. It has been reported by Supavititpatana et al. (2010) that the syneresis of set yogurt increases during 21 days storage, but Sahan et al. (2008) reported that addition of β-glucan to non-fat yogurt decreases syneresis and this factor value for both plain and β-glucan-added yogurt samples decreases during 15 days storage. Barrantes et al. (1996) also reported that syneresis in set-type natural yogurt containing different oils decreased during storage time.
Firmness
The firmness of yogurts is critical in establishing consumer preference. The most important textural characteristics of yogurt are firmness and the ability to retain water. These two properties are closely related to gel microstructure. Yogurt firmness was measured during storage and the results are presented in Table 1. Firmness of enriched yogurt was higher than control that indicates adding of O/W emulsion positively affects texture of yogurt. Higher TS content of enriched yogurts forms strong casein-casein bonds therefore, homogenized fat globules are covered with casein, facilitating protein-protein interactions. Fat becomes trapped within this protein network that increased firmness of enriched yogurts. Aziznia et al. (2008) reported that reduction of firmness was due to the reduction of fat content. Interactions among emulsifier, soy oil droplets and casein micelles help participation of the components in gel network. Therefore, firmness of enriched yogurt was higher compared to the control. Tamime et al. (1991) reported that the difference in the firmness of yogurts could be attributed to the protein matrix structure of the gel.
Firmness of control yogurt increased until day 14 of storage and then it decreased (P < 0.05). Although, in the case of enriched yogurt, the firmness increased until day 21 of storage to its maximum amount (75 mN). It has been reported by Torre et al. (2003) that in set-style yogurt, firmness decreases during storage, but Sahan et al. (2008) and Serra et al. (2009) reported that yogurt firmness increases during storage. In addition, Barrantes et al. (1996) reported that the firmness in set-type natural yogurt containing different oils content increased until day 8, but remained relatively constant thereafter.
Apparent viscosity
Apparent viscosity is affected by the strength and number of bonds between casein micelles in yogurt, as well as their structure and spatial distribution (Lucey and Singh 1998). Table 1 shows apparent viscosity of yogurt samples during 28 days storage at 4 °C. As it is seen in this table, the apparent viscosity of enriched yogurts is lower than the control. This is because yogurt is a gel/matrix of casein micelles with entrapped water. Adding phytosterol emulsion may interrupt the gel structure of the enriched sample. Apparent viscosity of enriched yogurt increased until day 14 and then decreased but apparent viscosity of control yogurt increased until day 7 and then decreased. It was reported that the apparent viscosity of yogurt during storage time decreases (Lee et al. 2007; Supavititpatana et al. 2010), while Sahan et al. (2008) reported that apparent viscosity can increase over time due to the rearrangement of protein and protein-protein contacts.
Distribution state of phytosterol in enriched yogurt
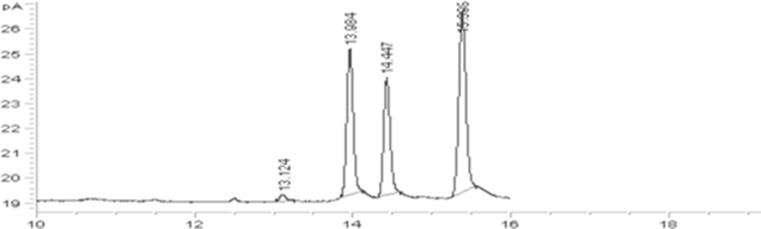
Figure 2 shows chromatogram of standard solution, peak identification left to right: Brasicasterol, stigmasterol, campesterol and β-sitosterol. Results of phytosterols distribution indicates that the distribution of phytosterol at different parts of one sample was not homogenous. It was found that the maximum content of phytosterols was at the lower part of samples and on the surface layer phytosterols content was lower comparing the middle and bottom layer (Table 2). The cause of sedimentation of phytosterol related to high density of these components that move down during early stage of incubation. At this step, viscosity of inoculated milk was low and based on Stokes’ law the separation rate is inversely related to the viscosity of samples.
Fig. 2.
Chromatograms of standard solution (Peak identification right to left: Brasicasterol, stigmasterol, campesterol and β- sitosterol)
Table 2.
Phytosterol content and distribution of enriched yogurt (surface, middle and bottom of enriched yogurt) injected to GC
| Enriched yogurt sample | Area % |
|---|---|
| Surface | 25.005 ± 0.07 |
| Middle | 26.96 ± 0.02 |
| Bottom | 26.98 ± 0.05 |
Data given in the table is Mean ± SD of three replicates
Sensory evaluation
The average sensory scores of all panelists are shown in Table 3. The sensory results showed that there were no statistically significant difference in the texture, appearance, flavor and overall acceptability of the yogurt samples (P > 0.05). The panelists could not identify the differences in these characteristics between yogurt with phytosterol from the control.
Table 3.
Sensory scores of enriched and control yogurt samples
| Yogurt sample | Texture | Appearance | Flavor | Overall liking |
|---|---|---|---|---|
| Enriched | 7.25 ± 1.05a | 6.92 ± 1.00a | 6.33 ± 1.23a | 7.08 ± 1.50a |
| Control | 6.83 ± 0.94a | 6.50 ± 1.17a | 6.58 ± 1.24a | 6.67 ± 1.07a |
Means (n = 12) followed by different letters in the same column are significantly different (P < 0:05; LSD test). (9-most desirable, 1-least desirable)
Data given in the table is Mean ± SD of three replicates
Conclusion
The current study was designed to develop enriched yogurt with phytosterol and to evaluate the effects of adding phytosterol on rheological and physicochemical characteristics of the final product during storage. Results obtained from physicochemical properties show that enriched yogurt had lower syneresis, higher firmness and lower apparent viscosity compare to control yogurt. Distribution of phytosterols in the samples was not homogenous and phytosterols tends to be sedimented or be in the lower layer of samples. Sensory analyses indicated that there was no significant difference between control and enriched yogurt (P > 0.05).
Acknowledgments
The authors wish to acknowledge Mr. Bahman Bahrami for his good technical guidance and Yas Sepid Vash Co. for supplying phytosterols.
References
- Awaisheh SS, Khalifeh MS, Al-Ruwaili MA, Khalil OM, Al-Ameri OH, Al-Groom R. Effect of supplementation of probiotics and phytosterols alone or in combination on serum and hepatic lipid profiles and thyroid hormones of hypercholesterolemic rats. J Dairy Sci. 2013;96(1):9–15. doi: 10.3168/jds.2012-5442. [DOI] [PubMed] [Google Scholar]
- Aziznia S, Khosrowshahi A, Madadlou A, Rahimi J. Whey protein concentrate and gum tragacanth as fat replacers in nonfat yogurt: chemical, physical, and microstructural properties. J Dairy Sci. 2008;91:2545–2552. doi: 10.3168/jds.2007-0875. [DOI] [PubMed] [Google Scholar]
- Barrantes E, Tamime AY, Sword AM, Muir DD, Kaláb M. The manufacture of set-type natural yoghurt containing different oils 2: rheological properties and microstructure. Int Dairy J. 1996;6:827–837. doi: 10.1016/0958-6946(96)00010-6. [DOI] [Google Scholar]
- Bonczar G, Wszolek M, Siuta A. The effect of certain factors on properties of yoghurt made from ewe, s milk. Food Chem. 2002;79:58–91. doi: 10.1016/S0308-8146(02)00182-6. [DOI] [Google Scholar]
- Dave RI, Shah NP. Ingredient supplementation effects on viability of probiotic bacteria in yogurt. J Dairy Sci. 1998;81:2804–2816. doi: 10.3168/jds.S0022-0302(98)75839-4. [DOI] [PubMed] [Google Scholar]
- Demonty I, Ras RT, van der Knaap HCM, Duchateau GSMJE, Meijer L, Zock PL, Geleijnse JM, Trautwein EA. Continuous dose–response relationship of the LDL cholesterol lowering effect of phytosterol intake. J Nutr. 2009;139:271–284. doi: 10.3945/jn.108.095125. [DOI] [PubMed] [Google Scholar]
- Derakhshan-Honarparvar M, Hamedi MM, Pirouzifard MK. Rice bran phytosterols of three widespread iranian cultivars. J Agric Sci Technol. 2010;12:167–172. [Google Scholar]
- Hassan AN, Frank JF, Schmidt KA, Shalabi SI. Textural properties of yogurt made with encapsulated nonropylactic cultures. J Dairy Sci. 1996;79:2098–2103. doi: 10.3168/jds.S0022-0302(96)76583-9. [DOI] [Google Scholar]
- Izadi Z, Nasirpour A, Garoosi GA. Optimization of phytosterols dispersion in an oil/water emulsion using mixture design approach. JDST. 2012;33:1715–1722. [Google Scholar]
- Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–978. doi: 10.1016/S0025-6196(11)63144-3. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Hwang JH, Lee S, Ahn J, Kwak HS. Property changes and cholesterol-lowering effects in evening primrose oil enriched and cholesterol reduced yogurt. Int J Dairy Technol. 2007;60:22–30. doi: 10.1111/j.1471-0307.2007.00294.x. [DOI] [Google Scholar]
- Lucey JA, Singh H. Formation and physical properties of acid milk gels: a review. Food Rev Int. 1998;7:529–542. [Google Scholar]
- Micallef MA, Garg ML. The lipid lowering effects of phytosterols and (n-3) polyunsaturated fatty acids are synergistic and complementary in hyperlipidemic men and women. J Nutr. 2008;138:1086–1090. doi: 10.1093/jn/138.6.1086. [DOI] [PubMed] [Google Scholar]
- Nguyen TT. The cholesterol-lowering action of plant stanol esters. J Nutr. 1999;129:2109–2112. doi: 10.1093/jn/129.12.2109. [DOI] [PubMed] [Google Scholar]
- Noakes M, Clifton PM, Doornbos AME, Trautwein EA. Plant sterol ester enriched milk and yogurt effectively reduce serum cholesterol in modestly hypercholerolemic subjects. Eur J Nutr. 2005;44:214–222. doi: 10.1007/s00394-004-0513-z. [DOI] [PubMed] [Google Scholar]
- Pollak OJ. Reduction of blood cholesterol in man. Circulation. 1953;7:702–706. doi: 10.1161/01.CIR.7.5.702. [DOI] [PubMed] [Google Scholar]
- Richelle M, Enslen M, Hager C, Groux M, Tavazzi I, Godin JP, Berger A, Metairon S, Quaile S, Piguet-Welsch C, Sagalowicz L, Green H, Fay LB. Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of beta-carotene and alpha-tocopherol in normocholesterolemic humans. Am J Clin Nutr. 2004;80:171–177. doi: 10.1093/ajcn/80.1.171. [DOI] [PubMed] [Google Scholar]
- Sahan N, Yasar K, Hayaloglu AA. Physical, chemical and flavour quality of non-fat yogurt as affected by a β-glucan hydrocolloidal composite during storage. Food Hydrocoll. 2008;22:1291–1297. doi: 10.1016/j.foodhyd.2007.06.010. [DOI] [Google Scholar]
- Santos R, Limas E, Sousa M, da Conceicão CM, Ramos F, da Silveira MIN. Optimization of analytical procedures for GC-MS determination of phytosterols and phytostanols in enriched milk and yoghurt. Food Chem. 2007;102:113–117. doi: 10.1016/j.foodchem.2006.05.001. [DOI] [Google Scholar]
- Serra M, Trujillo AJ, Guamis B, Ferragut V. Evaluation of physical properties during storage of set and stirred yogurts made from ultra-high pressure homogenization-treated milk. Food Hydrocoll. 2009;23:82–91. doi: 10.1016/j.foodhyd.2007.11.015. [DOI] [Google Scholar]
- Shaker RR, Jumah RY, Abu-Jdayil B. Reological properties of plain yoghurt during coagulation process: impact of fat content and preheat treatment of milk. J Food Eng. 2000;44:175–180. doi: 10.1016/S0260-8774(00)00022-4. [DOI] [Google Scholar]
- Supavititpatana P, Wirjantoro TI, Raviyan P. Characteristics and shelf-life of corn milk yogurt. Chiang Mai University J Nat Sci. 2010;9:133–149. [Google Scholar]
- Tamime AY, Kaláb M, Davies G. The effect of processing temperature on the microstructure and firmness of labneh made from cow’s milk by traditional method or by ultrafiltration. Food Struct. 1991;10:345–352. [Google Scholar]
- Tamjidi F, Nasirpour A, Shahedi M. Physicochemical and sensory properties of yogurt enriched with microencapsulated fish oil. Food Sci Technol Int. 2012;18(4):381–390. doi: 10.1177/1082013211428212. [DOI] [PubMed] [Google Scholar]
- Torre LA, Tamime AY, Muir DD. Rheology and sensory profiling of set-type fermented milks made with different commercial probiotic and yoghurt starter cultures. Int J Dairy Technol. 2003;56:163–170. doi: 10.1046/j.1471-0307.2003.00098.x. [DOI] [Google Scholar]
- Yokoyama WH. Plasma LDL cholesterol lowering by plant phytosterols in a hamster model. Trends Food Sci Technol. 2004;15:528–531. doi: 10.1016/j.tifs.2004.03.006. [DOI] [Google Scholar]