Abstract
Menaquinone 7 (MK-7) is nutritionally important metabolite found by fermentation mainly using B. subtilis species. In this study, soybean medium was modified to improve the MK-7 production using Bacillus subtilis NCIM 2708 under solid state fermentation. The objective of this study was to produce large amount of MK-7 within a short period of time. Nine nutritional components viz. glycerol, mannitol, dextrose, sucrose, yeast extract, malt extract, K2HPO4, MgSO4.7H2O and CaCl2 were investigated to obtain the maximum MK-7 concentration. The highest MK-7 concentration 39.039 μg/g was obtained after 24 h of fermentation in the following optimised medium components: soybean 20 g, glycerol 40 ml/kg, mannitol 60 g/kg, yeast extract 4 g/kg, malt extract 8 g/kg and calcium chloride 4 g/kg. The maximum production of MK-7 56.757 μg/g was predicted by point prediction tool of Design Expert 7.1 software (Statease Inc. USA). This data shows 68.78 % validity of the predicted model.
Keywords: Soybean, Solid state fermentation, Menaquinone-7, Medium engineering
Introduction
Vitamin K is fat soluble and occurs naturally in two forms vitamin K1 and vitamin K2. Vitamin K1 is major dietary source of vitamin K, exist naturally in green plants, algae, and photosynthetic bacteria while vitamin K2 includes chicken egg yolk, butter, cow liver, certain cheeses and in fermented soybeans (Vermeer and Schurgers 2000; Fujita et al. 2011). Vitamin K3 (menadione) is a synthetic compound that can be converted to K1 in the intestinal tract. It is only used in animal nutrition. Vitamin K2 family has a methylated naphthoquinones ring structure with a variable side chain length of 4 to 13 isoprene units. The compounds in this series are referred to as menaquinone-n, where n denotes the number of isoprene units (Shearer 1990). The level of menaquinone-7 (MK-7) in different food products is very low except for fermented soybean foods such as natto (9.39 μg/g), Hikiwari natto (chopped natto, 8.27 μg/g) and black soybean natto (796 μg/g) (Kamao et al. 2007). Recently, research has demonstrated that MK-7 consumption significantly reduces the risk of bone fractures (Schurgers et al. 2007; Truong and Booth 2011) and cardiovascular disorders (Gast et al. 2009; Theuwissen et al. 2012). European experts suggested that Recommended Daily Intake of vitamin K, preferably in the form of vitamin K2 is 200–500 μg/day, which is required for optimal carboxylation of extrahepatic GLA proteins. Natto, a Japanese fermented food, has traditionally been obtained from cooked soybean fermented with Bacillus species under SSF (Sakano et al. 1988). In Japan, natto has received approval as a nutritional food containing high amount of MK-7. Natto is required in a large quantity to meet the therapeutic dose of Vitamin K2 for treatment of osteoporosis and cardiovascular disease. Hence, it is advantageous to develop vitamin K rich natto as a dietary supplement.
For fermentation medium engineering, the screening of important parameters influencing fermentation productivity is initially carried out by barrowing method or by Plackett-Burman design and then, RSM is employed to develop, improve and optimise the process using the screened parameters (Kennedy and Krouse 1999). Berenjian et al. (2011a, b) reported that nitrogen source (yeast extract, soy peptone mixture) and carbon source (glycerol) were the most effective nutrients for enhancing MK-7 production. The presence of mono, di and polysaccharides and glycerol in the fermented medium can be used in glycolysis process by B. subtilis strains. Menaquinone production is the very complex systematic process. The pathway for isoprene side chain and quinone skeleton (1, 4-naphthoquinone) production is dependent on the presence of carbon sources such as glucose, fructose and glycerol in the fermentation media (Sonenshein et al. 2002). Nutritional requirements of Bacillus species play an important role for synthesizing their metabolic products; therefore fermentation medium designing is an important process for production of any secondary metabolite. MK-7 is produced mainly by submerged fermentation using Bacillus subtilis species (Sato et al. 2001b; Beulens et al. 2009).
Researchers have reported that genetic manipulation of microbial strains can improve MK-7 productivity during fermentation process (Sato et al. 2001a, b; Tsukamoto et al. 2001). Several researchers have studied the mechanism of MK biosynthesis in Bacillus subtilis (Rowland et al. 1995; Rowland and Taber 1996; Seto et al. 2008). Other studies focused on increasing the MK-7 productivity by modifying the fermentation medium and process parameters. Nevertheless, there are only few examples in literature that involve response surface methodology (RSM), a strategic experimental tool, for enhancing the production of menaquinone-7 (Mahanama et al. 2011; Wu and Ahn 2011; Mahanama et al. 2012a; Berenjian et al. 2011b). Mahanama et al. (2012b) have reported the effect of enzymatic pre-treatment on MK-7 production in SSF. Fermentation media and operating conditions have a significant impact on increasing MK-7 production. No research till now conducted for optimization of fermentation medium and followed by process optimization for production of MK-7 rich soya nutraceutical.
Thus, the research was carried out to investigate the effect of different nutritional components and to optimise the significant medium parameters and further optimization of the solid state fermentation condition (process parameters) for maximum MK-7 production in soybeans.
Materials and methods
Materials
Chemicals and microbial strain
All the chemicals used were purchased from Hi-media Laboratories Limited (Mumbai, India) and were of analytical grade. Soybean variety (PUSA-9814) was used as substrate for MK-7 production under solid state fermentation. The soybean variety PUSA-9814 was collected from the Pulse Lab of Indian Agricultural Research Institute (IARI), PUSA, New Delhi. Culture of Bacillus subtilis NCIM 2708 was obtained from National Collection of Industrial Microorganisms, National Chemical Laboratory, Pune, Maharashtra, India. The culture was maintained on nutrient agar slants at 4 °C and sub cultured at every 30 days.
Methods
Preparation of seed culture
Spore suspension of Bacillus subtilis NCIM 2708 was prepared from actively growing slants using sterile water. Spore suspension was inoculated to sterilized medium: soybean powder 6 % (soybean variety, SL- 525), sodium chloride 0.5 % and distilled water adjusted to pH 7.0 with 0.1 N HCl or NaOH (Hu et al. 2000). These cultures were incubated at 37 °C for 16 h in a shaker incubator at 180 rpm.
Solid -state fermentation of soybean
Soybean seeds (20 g) were washed, soaked in water for 12 h, boiled and dehulled. This soybean-based medium was used directly for production of MK-7 after adjusting the pH to 7. Sterilized medium was inoculated with seed culture (1 ml/g of substrate) and incubated for 24 h at 37 °C. After 24 h, fermented soybeans were removed from incubator and kept at 4 °C. The samples were processed for extraction and analysed by HPLC (Shimadzu, Japan).
Optimization of medium
The optimization of medium components for maximization of MK-7 production by B. subtilis under solid state fermentation was evaluated in two stages. The first stage, screening of medium component by Plackett-Burman experimental design was done. The second stage was optimization of key nutrients concentration influencing MK-7 production under solid state fermentation by central composite design of response surface methodology (Kennedy and Krouse 1999). Percentage contribution was calculated with the help of design expert 7.1 software (Statease Inc. USA).
Screening of medium components influencing menaquinone-7 production
The Plackett-Burman experimental design (PBD) for eleven variables with nine nutritional components (X1 to X9) (independent variables) and two dummy variables (D1 and D2) were used to evaluate the relative importance of the nutrients to enhance the production of MK-7 under SSF condition. These variable represented at two- levels, high concentrations (+) and low concentrations (−) and two dummy variables in eleven trials as shown in Table 1. The incubation period, temperature, medium pH, Inoculum volume was maintained constant throughout the fermentation process (Kennedy and Krouse 1999).
Table 1.
Plackett-Burman Design of twelve experimental trials generated for nine different medium and two dummy components by software Design Expert 7.1.6 and Menaquinone-7 (MK-7) production
| Trial | X1 | X 2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | MK-7 (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | + | + | − | − | − | + | − | 26.4 |
| 2 | − | + | + | − | + | + | + | − | − | − | + | 7.08 |
| 3 | + | − | + | + | − | + | + | + | − | − | − | 13.14 |
| 4 | − | + | − | + | + | − | + | + | + | − | − | 2.88 |
| 5 | − | − | + | − | + | + | − | + | + | + | − | 2.91 |
| 6 | − | − | − | + | − | + | + | − | + | + | + | 2.82 |
| 7 | + | − | − | − | + | − | + | + | − | + | + | 2.85 |
| 8 | + | + | − | − | − | + | − | + | + | − | + | 2.94 |
| 9 | + | + | + | − | − | − | + | − | + | + | − | 2.85 |
| 10 | − | + | + | + | − | − | − | + | − | + | + | 2.97 |
| 11 | + | − | + | + | + | − | − | − | + | − | + | 2.85 |
| 12 | − | − | − | − | − | − | − | − | − | − | − | 2.88 |
Optimization of medium components
The optimum levels of screened medium components (Table 2) affecting MK-7 production were determined by the Central Composite Design (CCD) of RSM. The five independent variables contributing maximum to MK-7 production such as glycerol, mannitol, malt extract, yeast extract and CaCl2 were studied at five different levels (−1, − α, 0, +1, +α) with the 50 treatment combinations. The Experimental range and levels of each independent variable is presented in Table 3. Data collected for MK-7 concentrations in each run were analysed using the design expert 7.1.6 software.
Table 2.
Contribution of different carbon, nitrogen and ions on MK-7 production in experimental trials carried out by Plackett-Burman Design
| Designation | Variable | ΣH (+1) | ΣL (−1) | Mean Square | Main Effect | F-value | % Contribution |
|---|---|---|---|---|---|---|---|
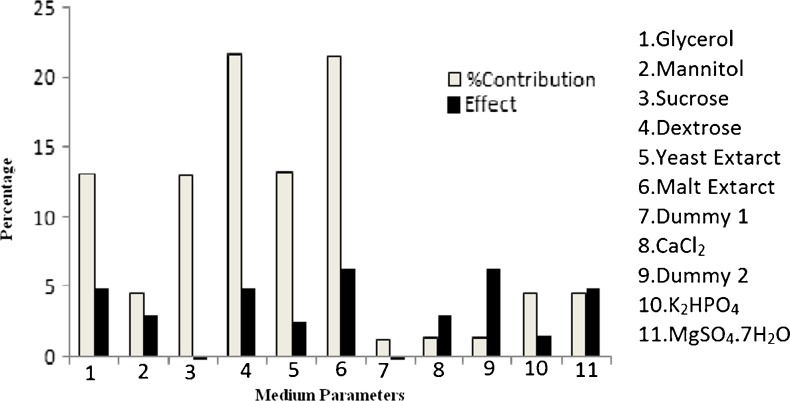
| X1 | Glycerol | 51.03 | 21.54 | 72.47 | 4.91 | 10.73 | 13.09 |
| X 2 | Mannitol | 45.12 | 27.45 | 25.49 | 2.94 | 3.77 | 4.59 |
| X3 | Sucrose | 31.8 | 40.77 | 71.88 | −1.49 | 10.64 | 12.94 |
| X4 | Dextrose | 51.06 | 21.51 | 120.78 | 4.92 | 17.89 | 21.73 |
| X5 | Yeast Extract | 44.97 | 27.6 | 73.66 | 2.56 | 10.91 | 13.25 |
| X6 | Malt Extract | 55.29 | 17.28 | 120.02 | 6.33 | 17.78 | 21.6 |
| X7 | Dummy 1 | 31.62 | 40.95 | 6.44 | −1.55 | 0.95 | 1.16 |
| X8 | CaCl2 | 27.69 | 44.88 | 7.07 | 2.86 | 1.04 | 1.27 |
| X9 | Dummy 2 | 17.25 | 55.32 | 7.16 | 6.34 | 1.06 | 1.29 |
| X10 | K2HPO4 | 40.8 | 31.77 | 25.14 | 1.5 | 3.72 | 4.52 |
| X11 | MgSO4.7H2O | 21.51 | 51.06 | 25.32 | 4.9 | 3.75 | 4.56 |
Table 3.
Experimental range and levels of the five significant independent variables used in RSM for optimization of fermentation medium parameters
| Variables (g/kg) | Levels | ||||
|---|---|---|---|---|---|
| -α | −1 | 0 | +1 | +α | |
| Glycerol | 40 | 30 | 50 | 60 | 70 |
| Mannitol | 40 | 30 | 50 | 60 | 70 |
| Malt Extract | 4 | 2 | 6 | 8 | 10 |
| Yeast Extract | 4 | 2 | 6 | 8 | 10 |
| CaCl2 | 4 | 2 | 6 | 8 | 10 |
Optimization of process parameters
Box-Behnken Design of RSM was used to determine the optimum level of key process variables to obtain the maximum MK-7 concentration. In this study, three independent variables effecting MK-7 production such as fermentation medium pH, fermentation temperature (°C) and Inoculum Volume (ml/100 g) were studied at different levels (−1, 0, +1) with 17 treatment combinations as presented in Table 6. Data collected for MK-7 concentrations in each run was analysed using the design expert 7.1.6 software.
Table 6.
Experimental range and levels of three independent fermentation process parameters used in Box-Behnken’s Design
| Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Medium pH | 4 | 7 | 10 |
| Temperature (°C) | 27 | 37 | 47 |
| Inoculum Volume (ml/100 g) | 5 | 20 | 35 |
Extraction of menaquinone-7
Menaquinone-7 was extracted from the fermented soybean samples using solvents propan-2-ol: n-hexane (1:2 v/v) as described by Berenjian et al. (2011b). Crushed samples (5 g) were dispersed in 15 mL of each solvent and vigorously mixed at room temperature for 10 min. This dispersion was centrifuged at 3,000 rpm for 5 min. The organic layers were separated and concentrated up to 1 mL and filtered through 0.45 μm membrane filter unit prior to HPLC analysis.
Analysis of menaquinone-7
Menaquinone-7 analysis was performed by HPLC (SHIMADZU, Japan) using a 250 mm x 4.6 mm ID Lichrosper® 100 C18 column containing 5 μm sized particles and a 20 ml loop injector. The optimum separation condition of HPLC was carried out with a multi-step gradient elution at a flow-rate of 1.2 ml/min with the following system: B: acetonitrile, C: Mixture of water and methanol (1:1) acidified to pH 3.0 by ortho-phosphoric acid. After sample injection (20 μL), the system was maintained at 80 % B for 3.5 min and then increased to 100 % in 4.5 min, held for 2 min and at the end of 10 min, the system was recycled back to 80 % B. The detection was carried out by UV detector at wavelength 254 nm.
Results and discussion
Effect of medium components
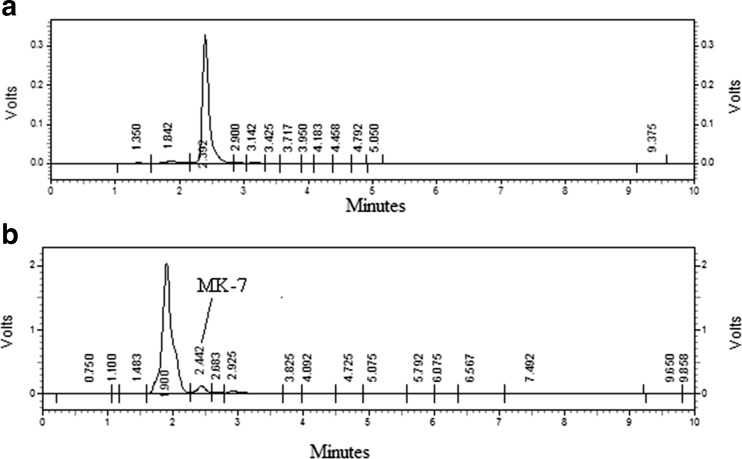
The effect of carbon source (glycerol, dextrose, mannitol, sucrose), nitrogen source (malt extract, yeast extract) was investigated in this experiment. Among the entire carbon sources, it was found that dextrose has high contribution, but dextrose was not selected for the RSM study because recommended dietary adequate intake (RDAI) of sugar may exceed above the normal level in patients suffering from diabetic disease. So the glycerol and mannitol was found to have contributing factor 13.09 %, 4.59 %, respectively. The malt extract is better nitrogen source than yeast extract and showed 21.6 % contribution in MK-7 production. Similarly out of all the micro and macro nutrients (K2HPO4, MgSO4, CaCl2) CaCl2 was found to be best nutrient for MK-7 production with 1.27 % contribution. All the results of Plackett-Burman experimental design with % contribution towards MK-7 production are shown in Table 2. Sucrose has negative effect on menaquinone-7 production while rest of nutrients including glycerol, mannitol, dextrose, malt extract, yeast extract, K2HPO4, CaCl2 and MgSO4 has significant positive effect on menaquinone-7 (MK-7) production from soybean by Bacillus subtilis NCIM 2708 (Fig. 1). The HPL chromatograms of standard MK-7 and extracted MK-7 is shown in Fig. 2.
Fig. 1.
Percentage contribution of different medium parameters and their effects on MK-7 productivity during solid state fermentation process of soybean obtained by Plackett-Burman Design
Fig. 2.
The HPLC chromatogram of standard MK-7 (a) and extracted MK-7 (b) from fermented soybean
The PBD is the preliminary technique for rapid illustration of the effects of various medium constituents. It tests each variable at two levels only; hence it cannot give exact idea regarding the optimum level of constituent required in the medium. Therefore, further optimization of selected nutrients for menaquinone-7 production is necessary.
Optimization of selected medium component
After screening the medium components viz. mannitol, glycerols, yeast extract, malt extract, CaCl2 were chosen as best C, N and ion sources for further optimization using RSM and CCD. The predicted and experimental MK-7 concentrations were obtained from CCD response surface design in each run are shown in Table 4. Results were analysed using the software Design Expert 7.1.6 and fitted into a multiple nonlinear regression model, resulting in the following equation for MK-7 production.
Table 4.
The predicted and experimental MK-7 concentrations of fermentation trials carried out by CCD of response surface methodology for optimization of fermentation medium parameters
| Run | Glycerol (ml/kg) |
Mannitol (g/kg) |
Yeast Extract (g/kg) |
Malt Extract (g/kg) |
CaCl2
(g/kg) |
Actual MK-7 (μg/g) |
Predicted MK-7 (μg/g) |
|---|---|---|---|---|---|---|---|
| 1 | 40 | 40 | 4 | 4 | 4 | 8.37 | 6.78 |
| 2 | 60 | 40 | 4 | 4 | 4 | 3.03 | 5.47 |
| 3 | 40 | 60 | 4 | 4 | 4 | 10.26 | 10.62 |
| 4 | 60 | 60 | 4 | 4 | 4 | 2.94 | 7.85 |
| 5 | 40 | 40 | 8 | 4 | 4 | 2.85 | 4.19 |
| 6 | 60 | 40 | 8 | 4 | 4 | 9.9 | 6.96 |
| 7 | 40 | 60 | 8 | 4 | 4 | 3.48 | 6.24 |
| 8 | 60 | 60 | 8 | 4 | 4 | 10.8 | 7.56 |
| 9 | 40 | 40 | 4 | 8 | 4 | 9.36 | 12.10 |
| 10 | 60 | 40 | 4 | 8 | 4 | 9.87 | 9.13 |
| 11 | 40 | 60 | 4 | 8 | 4 | 23.55 | 18.92 |
| 12 | 60 | 60 | 4 | 8 | 4 | 15.36 | 14.50 |
| 13 | 40 | 40 | 8 | 8 | 4 | 3.66 | 5.11 |
| 14 | 60 | 40 | 8 | 8 | 4 | 3.84 | 6.23 |
| 15 | 40 | 60 | 8 | 8 | 4 | 9.00 | 10.16 |
| 16 | 60 | 60 | 8 | 8 | 4 | 7.77 | 9.82 |
| 17 | 40 | 40 | 4 | 4 | 8 | 8.49 | 8.53 |
| 18 | 60 | 40 | 4 | 4 | 8 | 8.01 | 7.64 |
| 19 | 40 | 60 | 4 | 4 | 8 | 9.03 | 8.63 |
| 20 | 60 | 60 | 4 | 4 | 8 | 9.78 | 6.29 |
| 21 | 40 | 40 | 8 | 4 | 8 | 8.79 | 10.25 |
| 22 | 60 | 40 | 8 | 4 | 8 | 9.18 | 13.46 |
| 23 | 40 | 60 | 8 | 4 | 8 | 9.39 | 8.58 |
| 24 | 60 | 60 | 8 | 4 | 8 | 8.64 | 10.33 |
| 25 | 40 | 40 | 4 | 8 | 8 | 9.24 | 10.48 |
| 26 | 60 | 40 | 4 | 8 | 8 | 8.46 | 7.94 |
| 27 | 40 | 60 | 4 | 8 | 8 | 9.60 | 13.58 |
| 28 | 60 | 60 | 4 | 8 | 8 | 9.09 | 9.58 |
| 29 | 40 | 40 | 8 | 8 | 8 | 10.29 | 7.81 |
| 30 | 60 | 40 | 8 | 8 | 8 | 9.27 | 9.36 |
| 31 | 40 | 60 | 8 | 8 | 8 | 9.92 | 9.13 |
| 32 | 60 | 60 | 8 | 8 | 8 | 9.03 | 9.22 |
| 33 | 30 | 50 | 6 | 6 | 6 | 10.32 | 9.75 |
| 34 | 70 | 50 | 6 | 6 | 6 | 9.21 | 8.53 |
| 35 | 50 | 30 | 6 | 6 | 6 | 8.85 | 7.29 |
| 36 | 50 | 70 | 6 | 6 | 6 | 9.69 | 10.99 |
| 37 | 50 | 50 | 2 | 6 | 6 | 9.66 | 10.62 |
| 38 | 50 | 50 | 10 | 6 | 6 | 9.36 | 7.66 |
| 39 | 50 | 50 | 6 | 2 | 6 | 9.03 | 7.03 |
| 40 | 50 | 50 | 6 | 10 | 6 | 13.05 | 11.25 |
| 41 | 50 | 50 | 6 | 6 | 2 | 9.90 | 8.56 |
| 42 | 50 | 50 | 6 | 6 | 10 | 9.42 | 9.72 |
| 43 | 50 | 50 | 6 | 6 | 6 | 9.81 | 9.14 |
| 44 | 50 | 50 | 6 | 6 | 6 | 9.24 | 9.14 |
| 45 | 50 | 50 | 6 | 6 | 6 | 9.72 | 9.14 |
| 46 | 50 | 50 | 6 | 6 | 6 | 10.32 | 9.14 |
| 47 | 50 | 50 | 6 | 6 | 6 | 10.08 | 9.14 |
| 48 | 50 | 50 | 6 | 6 | 6 | 10.26 | 9.14 |
| 49 | 50 | 50 | 6 | 6 | 6 | 9.87 | 9.14 |
| 50 | 50 | 50 | 6 | 6 | 6 | 9.24 | 9.14 |
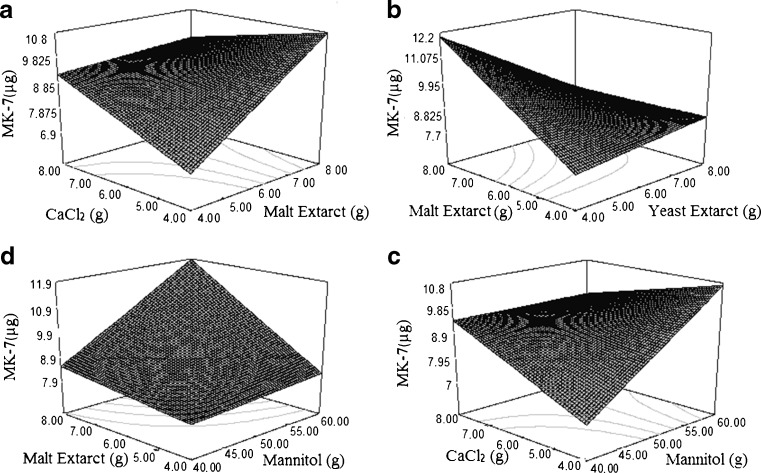
MK-7(μg/g) =9.14–0.31 A + 0.93 B-0.74 C + 1.05 D + 0.29 E-0.36 AB + 1.02 AC-.41 AD + 0.11AE - 0.44BC + 0.75BD-0.93BE-1.10CD + 1.08CE-0.84DE
Where A, B, C, D and E represents glycerol, mannitol, yeast extract, malt extract and calcium chloride respectively in g/kg. The effects of all nutrient parameters on MK-7 production can be compared with the help of response surface plots. Nonlinear regression models resulted in ten response graphs for MK-7 production, shown in Fig. 3. The result adequacies were analysed using the analysis of variance (Table 5) and determination of coefficient R2. In this study, the value R2 is 0.6141 that indicates 61 % variability in the response could be explained by the model. According to ANOVA of nonlinear regression model, F-value” of 3.61 implies the model is significant and a very low probability value (P < 0.0500). There is only a 0.09 % chance that a “model F-value” this large could occur due to noise. “Adequate precision” measures the signal to noise ratio and ratio greater than 4 is desirable. The Adequate precision value 11.035 indicates an adequate signal which implies that this model can be used to navigate the design space. A negative “Pred R-Squared” implies that the overall mean is a better predictor of your response than the current model ”. The “Lack of Fit F-value” of 40.79 implies the Lack of Fit is significant. There is only a 0.01 % chance that a “Lack of Fit F-value” this large could occur due to noise.
Fig. 3.
Response surface plots showing the effect of different nutrient parameters (glycerol, mannitol, yeast extract, malt extract and CaCl2) on MK-7 production during Solid state fermentation of soybean
Table 5.
Analysis of variance values for model of MK-7 production by Bacillus subtilis NCIM 2708
| Source | Sum of Squares | DF | Mean Square | F value | p-value | |
|---|---|---|---|---|---|---|
| Model | 301.53 | 15 | 20.1 | 3.61 | 0.0009 | Significant |
| A-Glycerol | 3.74 | 1 | 3.74 | 0.67 | 0.4184 | |
| B-Mannitol | 34.24 | 1 | 34.24 | 6.14 | 0.0183 | |
| C-Yeast Extract | 21.8 | 1 | 21.8 | 3.91 | 0.0561 | |
| D-Malt Extract | 44.33 | 1 | 44.33 | 7.96 | 0.0079 | |
| E-CaCl2 | 3.31 | 1 | 3.31 | 0.59 | 0.4461 | |
| AB | 4.23 | 1 | 4.23 | 0.76 | 0.3899 | |
| AC | 33.44 | 1 | 33.44 | 6 | 0.0196 | |
| AD | 5.49 | 1 | 5.49 | 0.98 | 0.3281 | |
| AE | 0.37 | 1 | 0.37 | 0.066 | 0.7988 | |
| BC | 6.33 | 1 | 6.33 | 1.14 | 0.2941 | |
| BD | 17.9 | 1 | 17.9 | 3.21 | 0.082 | |
| BE | 27.81 | 1 | 27.81 | 4.99 | 0.0322 | |
| CD | 38.65 | 1 | 38.65 | 6.94 | 0.0126 | |
| CE | 37.3 | 1 | 37.3 | 6.69 | 0.0141 | |
| DE | 22.6 | 1 | 22.6 | 4.05 | 0.052 | |
| Residual | 189.47 | 34 | 5.57 | |||
| Adequate precision | 11.035 | |||||
| Lack of Fit | 40.79 | Significant | ||||
R2 = 0.61, Adj R2 = 0.4439
Bacillus subtilis NCIM 2708 produce higher amount of MK-7 in glycerol containing media which is in agreement with Berenjian et al. 2011a, b. Yeast extract and malt extract act as undefined nitrogen source results higher MK-7 production since complex nitrogen source contain amino acids, vitamins and provide better genetic requirement for protein & enzyme synthesis.
Optimization of fermentation process
In the study of process parameters, three independent variables (e.g. Temperature, pH, Inoculum volume) were tested. The results of experimental run with predicted and actual MK-7 level in fermentation medium is presented in Table 7, calculation for the analysis of variance is presented in Table 8. The Model F-value of 4.05 and very low probability value (P < 0.0500) implies the model is significant. There is only a 2.55 % chance that a “Model F-Value” this large could occur due to noise. The “Lack of Fit F-value” of 4.25 implies there is a 9.15 % chance that a “Lack of Fit F-value” this large could occur due to noise. “ Adequate precision” measures the signal to noise ratio. Adequate precision grater than 4 is desirable and it is 7.759 indicates an adequate signal. Results were analysed using the software Design Expert 7.1.6 and fitted into a multiple nonlinear regression model, resulting in the following equation for MK-7 production.
Table 7.
The experimental MK-7 concentrations of fermentation trials carried out by Box-Behnken’s Design of response surface methodology for optimization of fermentation process parameters
| Run | Mediun pH | Fermentation Temperature (°C) | Inoculum Volume (% v/w) | Menaquinone-7 (μg/g) |
|---|---|---|---|---|
| 1 | 4 | 27 | 20 | 7.35 |
| 2 | 10 | 27 | 20 | 0 |
| 3 | 4 | 47 | 20 | 5.76 |
| 4 | 10 | 47 | 20 | 6.27 |
| 5 | 4 | 37 | 5 | 5.28 |
| 6 | 10 | 37 | 5 | 0 |
| 7 | 4 | 37 | 35 | 5.79 |
| 8 | 10 | 37 | 35 | 5.67 |
| 9 | 7 | 27 | 5 | 4.65 |
| 10 | 7 | 47 | 5 | 0 |
| 11 | 7 | 27 | 35 | 4.44 |
| 12 | 7 | 47 | 35 | 3.78 |
| 13 | 7 | 37 | 20 | 3.69 |
| 14 | 7 | 37 | 20 | 5.4 |
| 15 | 7 | 37 | 20 | 5.49 |
| 16 | 7 | 37 | 20 | 3.75 |
| 17 | 7 | 37 | 20 | 5.07 |
Table 8.
Analysis of variance values for fermentation process parameters of MK-7 production by Bacillus subtilis NCIM 2708
| Source | Sum of Squares | DF | Mean Square | F Value | p-value | |
|---|---|---|---|---|---|---|
| Model | 58.74 | 6 | 9.46 | 4.05 | 0.0255 | Significant |
| Medium pH | 18.73 | 1 | 18.73 | 8.01 | 0.0178 | |
| Temperature | 0.05 | 1 | 0.05 | 0.021 | 0.8871 | |
| Inc. Volume | 11.88 | 1 | 11.88 | 5.08 | 0.0478 | |
| AB | 15.44 | 1 | 15.44 | 5.08 | 0.0279 | |
| AC | 6.66 | 1 | 6.66 | 6.61 | 0.1224 | |
| BC | 3.98 | 1 | 3.98 | 2.85 | 0.2212 | |
| Residual | 23.38 | 10 | 2.34 | 1.7 | ||
| Lack of Fit | 4.25 | Significant | ||||
| Adequate precision | 7.759 |
R2 = 0.708, Adj R2 = 0.5331
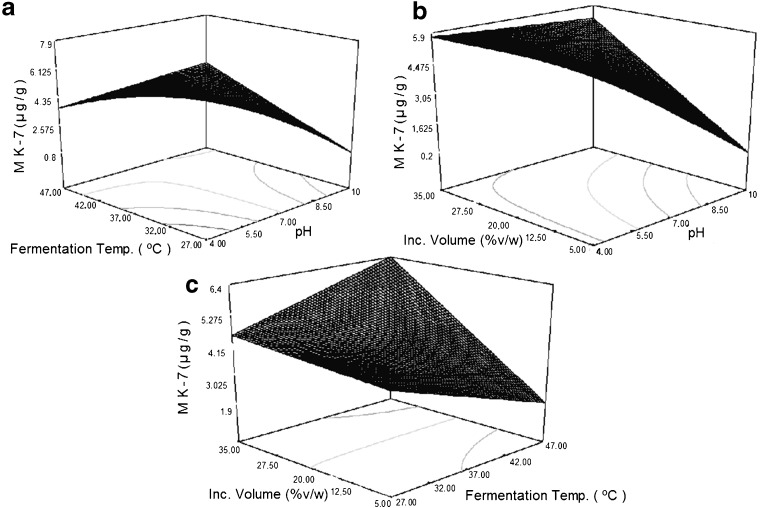
MK-7 (μg/g) =4.26–1.53 A - 0.079 B +1.22 C +1.97 AB +1.29 AC +1.00 BC
The effects of all process parameters on MK-7 production can be compared with the help of response plots. Nonlinear regression models resulted in three response graphs for MK-7 production, shown in Fig. 4.
Fig. 4.
Response surface plots showing the effect of process parameters (pH, temperature and inoculum volume) on MK-7 production during solid state fermentation of soybean
It was found that low pH of medium has positive effect on MK-7 production. The results agree with Hill et al. 1990. Fermentation temperature is inversely proportional to MK-7 production. Optimum temperature for Bacillus subtilis was 37 °C, but MK-7 production was found to be decreased with increase in temperature. No measurable effects of Inoculum volume was found on MK-7 production. There was only slightly increase in MK-7 production with increase in Inoculum volume. Several research reports maximum MK-7 production as high as 62.32 mg/L under submerged fermentation at 6th day (Berenjian et al. 2011b). However for The present research reports a new optimized fermentation medium and process condition which yield higher MK-7 production (39.039 μg/g) under solid state fermentation condition only after 24 h.
Conclusions
The Bacillus subtilis NCIM 2708 was used for production of MK-7 by solid state fermentation. Statistical experimental design proved to be valuable tools in optimizing a solid state fermentation medium for MK-7 production by Bacillus subtilis NCIM 2708. Five medium component viz. glycerol 40 ml/kg, mannitol 60 g/kg, yeast extract 4 g/kg, malt extract 8 g/kg, and calcium chloride 4 g/kg were identified and optimised by RSM. The predicted MK-7 was 56.75 μg/g by using above optimised medium combination. Practically MK-7 concentration 39.039 μg/g was achieved in the optimized fermented media. This shows 68.78 % validity of the predicted model.
Acknowledgments
The authors gratefully Professor S. K. Lal, IARI, PUSA, New Delhi for providing soybeans variety. All the authors acknowledge the Department of Science and Technology (DST), Govt. of India for providing fellowship for scholar under INSPIRE Programme.
Footnotes
Rishipal Singh, Alka Puri and Bibhu Prasad Panda have contributed equally to this paper
References
- Berenjian A, Mahanama R, Talbot A, Biffin R, Regtop H, Kavanagh J, Dehghani F. The effect of amino-acids and glycerol addition on MK-7 production. Proc World Congr Eng Comput Sci. 2011;11:19–21. [Google Scholar]
- Berenjian A, Mahanama R, Talbot A, Biffin R, Regtop H, Valtchev P, Kavanagh J, Dehghani F. Efficient media for high menaquinone-7 production: response surface methodology approach. New Biotechnol. 2011;28(6):665–672. doi: 10.1016/j.nbt.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Beulens JWJ, Bots ML, Atsma F, Bartelink MLEL, Prokop M, Geleijnse JM, Witteman JCM, Grobbee DE, Van Der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203(2):489–493. doi: 10.1016/j.atherosclerosis.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Iki M, Tamaki J, Kouda K, Youra A, Kadowaki E, Sato Y, Moon JS, Tomiaka N, Okamoto N, Kurumatani N. Association between vitamin K intake from fermented soybeans, natto, and bone mineral density in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study. Osteoporosis Int. 2011;23(2):705–714. doi: 10.1007/s00198-011-1594-1. [DOI] [PubMed] [Google Scholar]
- Gast GCM, De Roos NM, Sluijs I, Bots ML, Beulens JWJ, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PHM, Van Der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovas. 2009;19(7):504–510. doi: 10.1016/j.numecd.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Hill KF, Mueller JP, Taber HW. The Bacillus subtilis men CD promoter is responsive to extracellular pH. Arch Microbiol. 1990;153:355–359. doi: 10.1007/BF00249005. [DOI] [PubMed] [Google Scholar]
- Hu H, Yao S, Mei L, Zhu Z, Hur B. Partial purification of nattokinase from Bacillus subtilis by expanded bed adsorption. Biotechnol Lett. 2000;22(17):1383–1387. doi: 10.1023/A:1005652700650. [DOI] [Google Scholar]
- Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, Ishida H, Saski S, Okano T. Vitamin K content of foods and dietary vitamin K intake in japanese young women. J Nutr Sci Vitaminol. 2007;53(6):464–470. doi: 10.3177/jnsv.53.464. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Krouse D. Strategies for improving fermentation medium performance: a review. J Ind Microbiol Biot. 1999;23(6):456–475. doi: 10.1038/sj.jim.2900755. [DOI] [Google Scholar]
- Mahanama R, Berenjian A, Valtchev P, Talbot A, Biffin R, Regtop H, Dehghani F, Kavanagh JM (2011) Enhanced production of menaquinone 7 via solid substrate fermentation from Bacillus subtilis. Int J Food Eng 7(5): DOI:10.2202/1556-3758.2314
- Mahanama R, Boyce D, Berenjian A, Dehghani F, Kavanagh J. The effect of enzymatic pre- treatment on solid state fermentation of vitamin K2. J Am Sci. 2012;8(1):648–654. [Google Scholar]
- Mahanama R, Berenjian A, Dehghani F, Kavanagh J. Modelling the effect of bed height and particle size for vitamin K2 production in a static bed fermenter. Eng Lett. 2012;20(1):16–17. [Google Scholar]
- Rowland B, Taber H. Duplicate isochorismate synthase genes of Bacillus subtilis: regulation and involvement in the biosyntheses of menaquinone and 2,3-dihydroxybenzoate. J Bacteriol. 1996;178(3):854–861. doi: 10.1128/jb.178.3.854-861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland B, Hill K, Miller P, Driscoll J, Taber H. Structural organization of a Bacillus subtilis operon encoding menaquinone biosynthetic enzymes. Gene. 1995;167(1–2):105–109. doi: 10.1016/0378-1119(95)00662-1. [DOI] [PubMed] [Google Scholar]
- Sakano T, Notsumoto N, Nagaoka N. Measurement of K vitamins in food by high performance liquid chromatography with fluorometric detection. Vitam (Jpn) 1988;62(8):393–398. [Google Scholar]
- Sato T, Yamada Y, Ohtani Y, Mitsui N, Murasawa H, Araki S. Efficient production of menaquinone (Vitamin K2) by minadione-resistant mutant of Bacillus subtilis. J Ind Microbiol Biot. 2001;26(3):115–120. doi: 10.1038/sj.jim.7000089. [DOI] [PubMed] [Google Scholar]
- Sato T, Yamadaa Y, Ohtania Y, Mitsuib N, Murasawab H, Araki S. Production of Menaquinone (vitamin K2)-7 by Bacillus subtilis. J Biosci Bioeng. 2001;91(1):16–20. doi: 10.1016/S1389-1723(01)80104-3. [DOI] [PubMed] [Google Scholar]
- Schurgers LJ, Teunissen KJF, Hamulyák K, Knapen MHJ, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109(8):3279–3283. doi: 10.1182/blood-2006-08-040709. [DOI] [PubMed] [Google Scholar]
- Seto H, Jinnai Y, Hiratsuka T, Fukawa M, Furhata K, Itoh N, Dairi T. Studies on a new biosynthetic pathway for menaquinone. J Am Chem Soc. 2008;130(17):5614–5615. doi: 10.1021/ja710207s. [DOI] [PubMed] [Google Scholar]
- Shearer MJ. Vitamin K and vitamin K-dependent proteins. Brit J Haematol. 1990;75(2):156–162. doi: 10.1111/j.1365-2141.1990.tb02642.x. [DOI] [PubMed] [Google Scholar]
- Sonenshein AL, Hoch JA, Losick R (eds) (2002) Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington DC
- Theuwissen E, Smit E, Vermeer C. The role of vitamin K in soft-tissue calcification. Adv Nutr. 2012;3:166–173. doi: 10.3945/an.111.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong JT, Booth SL. Emerging issues in vitamin K research. J Evid Based Complement Altern Med. 2011;16(2):73–79. doi: 10.1177/1533210110392953. [DOI] [Google Scholar]
- Tsukamoto Y, Kasai M, Kakuda H. Construction of a Bacillus subtilis (natto) with high productivity of vitamin K2 (menaquinone-7) by analog resistance. Biosci Biotechnol Biochem. 2001;65(9):2007–2015. doi: 10.1271/bbb.65.2007. [DOI] [PubMed] [Google Scholar]
- Vermeer C, Schurgers LJ. A comprehensive review of vitamin K and vitamin K antagonists. Hematol Oncol Clin North Am. 2000;14(2):339–353. doi: 10.1016/S0889-8588(05)70137-4. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Ahn BY. Improved menaquinone (vitamin K2) production in cheonggukjang by optimization of the fermentation conditions. Food Sci Biotechnol. 2011;20(6):1585–1591. doi: 10.1007/s10068-011-0219-y. [DOI] [Google Scholar]