Abstract
Suitability assessment of amaranth sprouts as a new functional food was carried out. The optimisation of sprouting process and the influence of selenium supplementation, in doses 10, 15, and 30 mg/l of selenium as sodium selenite, on amaranth growth and fatty acid profile were examined. Methods such as FRAP, DPPH, polyphenols content and GPX activity were applied to characterize antioxidant potential of seeds and sprouts of four different edible amaranth genera. E. coli, S. aureus, C. albicans were used to evaluate amaranth sprouts antimicrobial properties. Interaction between amaranth sprouts and biological systems was assessed by analysing antibacterial and antifungal properties with a disc diffusion test. The studies proved amaranth sprouts to be potentially attractive as functional food. As confirmed by all the data amaranth sprouts are suitable as a moderate selenium accumulator and are rich in essential fatty acids, especially linoleic and alpha-linolenic acids, which are precursors of long chain polyunsaturated fatty acids. Thus, it opens dietary opportunities for amaranth sprouts. They can also serve as a moderate source of antioxidant compounds. Nevertheless, the experiments revealed neither antibacterial, nor antifungal properties of sprouts. In general, amaranth sprouts biological activity under evaluation has failed to prove to be significantly impacted by selenium fertilization.
Keywords: Amaranth sprouts, New functional food, Selenium supplementation, Fatty acid profile, Antioxidant activity, Antibacterial and antifungal properties
Introduction
Over the last decade, amaranth seeds were successfully introduced not only to the common diet, but also to the diet of celiac disease sufferers, or sufferers of allergies to typical cereals (Berti et al. 2005). Amaranth seeds have high nutritional and functional values, associated with the quality and quantity of their proteins, fats and their antioxidant potential (Gorinstein et al. 2007; Paśko et al. 2009). Other beneficial effects of amaranth seeds on human organisms, such as regulation of lipid profile and moderate protective activity against free radicals manifested via reducing lipid peroxidation and enhancing antioxidant capacity of blood plasma and selected organs, were also confirmed (Paśko et al. 2011a, b). In the recent years a new trend in nutrition were observed as various sprouts focused attention as functional food due to their nutritive value associated with high content of beneficial and readily available compounds, such as amino acids, trace elements and vitamins, as well as flavonoids and phenolic acids. It seems particularly important to note that the amount of antinutritive compounds in sprouts, namely enzyme inhibitors and phytic acid, decreases significantly as plants grow. Generally, sprouting processes can greatly increase digestibility and improve important nutrient compounds utilization by organisms. Having undergone such a process, many hard-shelled plant products become nutrient-packed and easily adaptive to use and consumption (Márton et al. 2010).
Consumption of sprouts has become increasingly popular among people interested in improving and maintaining their health status by adjusting their dietary habits. Sprouts provide an excellent example of ‘functional food’ defined as a type of food lowering the risk of various diseases, and/or exerting health promoting effects in addition to their nutritive value. Such attributes make sprouts a rich source of many compounds, whose intake in the population tends to be limited.
According to Lintschinger et al. (2000), almost all European countries are classified as low-selenium regions. It is well known that this trace element content in daily diet depends mainly on selenium concentration in soil. Large differences in selenium concentrations in soil between European countries cause significant differences in respective selenium daily intake to occur (Sirichakwal et al. 2005). In order to improve selenium related nutritional status, soil enrichment or supplementation are applied (Lintschinger et al. 2000). Several plants, such as Allium vegetables, i.e. garlic, onion, shallot, chives, and ramp, and Brassica vegetables, i.e. broccoli and radish, were reported to be able to accumulate and transform inorganic selenium forms into bioactive organic compounds (Pyrzynska 2009). For edible sprouts most of papers published recently have been focused on studying “typical” sprouts such as broccoli, mung bean, radish, sunflower, red cabbage, alfalfa and soybean. Though they are widely available in markets, still not all of them accumulate selenium. Guided by earlier research and in search for new plants/sprouts to which selenium is easily and highly incorporated, we decided to focus on edible amaranth sprouts. Their great potential to become “new vegetables”, combined with a view to likely inclusion into nutrition of vegans and vegetarians, or/and into the common diet, as a valuable source of selenium, made them seem a very interesting subject to study.
So far amaranth leaves have been typically eaten as raw vegetables, boiled or as soup additives (Maiyo et al. 2010). Sprouting at home has gained popularity as it is a very simple process, though not many people are aware of amaranth seeds to be a selenium source. The goal of the present study was to develop the most favourable conditions for amaranth cultivation and harvesting to be adapted as home procedure, and to indicate what selenium doses stimulate sprouts to grow. We also studied amaranth sprouts with respect to their selenium accumulating capability. Moreover, we examined the influence of selenium supplementation on fatty acid profile in amaranth sprouts under the study. Additionally, we researched amaranth sprouts nutritional value and whether they prove a good source of antioxidants. The antioxidant potential of seeds and sprouts for four different edible amaranths were evaluated with the Total Reducing Capacity (by Folin-Ciocalteu assay), FRAP and DPPH methods. We investigated also glutathione peroxidase (GPx)) activity in the obtained material. The final part of our work was to determine influence of the obtained amaranth sprouts extracts, both standard and selenium supplemented, on the growth of selected bacteria and yeast.
Materials and methods
Four types of edible amaranth seeds were used in sprout harvesting. Voucher specimens were deposited in the Department of Food Chemistry and Nutrition, Faculty of Pharmacy, Medical College, Jagiellonian University with referring number AMCRU/PP/PL 1039 to Amaranthus cruentus, AMCAU/PP/PL 1040 to Amaranthus caudatus, AMPAN/PP/PL 1041 to Amaranthus paniculatus, and AMTRI/PP/PL 1042 to Amaranthus tricolor; harvested in Poland, Netherlands, Poland, and China, respectively.
Amaranth seeds were immersed in tap water (HCO3− 131.06 mg/l; F− 0.07 mg/l; Mg2+ 5.62 mg/l; Ca2+ 41.69 mg/l; Na+ 9.65 mg/l) or tap water with various selenium concentrations, namely 10, 15 and 30 mg/l, with selenium as a sodium selenite, for 3 h and then placed in clay vessels. Sprouts were grown at a fixed temperature of 24 ± 2 °C for 6 days following their seeding. They were watered every day. All cultures were stored under natural light conditions.
Extract preparation
Samples of amaranth seeds and fresh sprouts, of 4 g, were extracted with 35 ml of methanol for 3 h. The obtained extracts were decanted, centrifuged and dark stored in a freezer at −20 °C. They served later as estimation of the total antioxidant activity. To evaluate antibacterial and antifungal effect, dry methanol extracts were obtained with solvent removed by evaporation under reduced pressure. Dry methanol extracts were then dissolved in DMSO (Mosovska and Birosova 2012). Selenium concentration was estimated in lyophilized amaranth seeds and sprouts.
Selenium concentration
A 12-positional microwave system MARS X (CEM, Matthews, USA), equipped with temperature (RTP-300) and pressure (ESP-1500 Plus) sensors, was used for sample preparation. The system allowed programmable control of the pressure and temperature up to 5,520 kPa (800 psi) and 220 °C, respectively. A Mini-vap device (Sigma Aldrich, Germany) was used to remove gaseous products with nitrogen.
Analysis: a double-channel atomic fluorescence spectrometer AFS-230 (Beijing Haiguang Instrument Co., China) with a flow hydride-generation system was used. The light source used was a cathode lamp (Se-HCL) designed for AFS measurements, operating at pulsed current of 100 mA. The argon-shielded gas flow and the carrier gas flow were 800 and 500 ml/min, respectively. The atomization process occurred in Ar-H2 flame at 200 °C. The signals corresponding to selenium content in plants and extracts were recorded and processed in the peak area mode with the use of an IBM 586 computer (Beijing Haiguang Instrument Co., China).
Analytical Procedure: a portion of 0.3 ml or 0.2 mg of sample in 7 ml of concentrated HNO3 was digested at the maximum temperature of 200 °C. The digestion conditions applied for the microwave system were 16 min/960 W and 8 min/1,080 W. Following digestion, the sample solution was cooled in the air to 25 °C and then purged under nitrogen flow for 10 min. Finally, samples were transferred into a 25 ml volumetric flask, mixed with 12.5 ml of 6 mol/L HCl and diluted to the mark with deionized water. For all the samples the mean value of three replicates was expressed as mg/kg dry weight.
Determination of fatty acids profile
Lipids from the amaranth seeds and sprouts were extracted with chloroform: methanol solution (2:1 v/v) according to the Folch method (Folch et al. 1957) with added BHT (0.005 %). Fatty acid methyl esters (FAME) were synthesized with 20 % BF3 in methanol at 60 °C. The analysis of FAME was performed with gas chromatography on the Agilent 6890 N gas chromatograph with a capillary column DB-23 (50 %-Cyanopropyl)-methylpolysiloxane, 60 m, ID 0.25 mm, film 0.25 μm) and a FID detector. Temperature and analytical conditions of chromatographic evaluation were as follows: FID 260 °C, injector 250 °C, split ratio 50:1; oven 140 °C for 5 min, 140–190 °C at 4 °C/min, 190 °C for 15 min, 190–240 °C at 2,75 °C/min, 240 °C for 4 min; carrier gas—helium, constant pressure mode. Injection 2 μl. To identify fatty acids, retention times of fatty acid methyl esters standard from Supelco (47801) were used. For all fatty acids the mean value of three replicates was expressed as the percentage of the total fatty acids pool. The total sums of saturated and unsaturated fatty acids SFA, MUFA, PUFA, as well as saturated fatty acids (SFA)/unsaturated fatty acids–MUFA + PUFA ratio were determined.
Total reducing capacity
The Folin–Ciocalteau assay
Everette et al. (2010) suggested the Folin–Ciocalteau assay to be regarded as a measure of total reducing capacity rather than phenolic content, as phenolics are the most abundant antioxidants in many plants. Total reducing capacity (TRC) was determined colorimetrically using the Folin–Ciocalteau reagent, as described elsewhere (Paśko et al. 2009). The assay was conducted by mixing 2.7 ml of deionized water, 0.3 ml of extracts, 0.3 ml 7 g/100 g Na2CO3 and 0.15 ml Folin–Ciocalteu reagent. Absorbance of the mixture was measured at 725 nm and 760 nm with Jasco UV-530 spectrophotometer. A standard curve was prepared with gallic acid. Final results are given as gallic acid equivalents (GAE). For all the samples the mean value of three replicates was expressed as mg GA/kg dry weight.
FRAP method
FRAP assay was carried out according to Benzie and Strain (1996), and modified to 48-well plates and an automatic reader (Synergy-2, BioTek/USA) with syringe rapid dispensers. Briefly, the oxidant in the FRAP assay (reagent mixture) consisted of ferric chloride solution (20 mmol/l), 2,4,6-tripyridyl-striazine (TPTZ) solution (10 mmol/l TPTZ in 40 mmol/l HCl) and acetate buffer (pH =3.6) in the proportion of 5:5:10, respectively, and was freshly prepared. To each plate 0.4 ml of acetate buffer (pH 3.6) was dispensed, followed by 50 μl of the sample (methanol extracts), standard or blank. The plate was conditioned at the temperature of 37 °C for 2 min, then 0.2 ml of reagent mixture was added and shaken for 30 s; subsequently, absorbance at 593 nm was measured in a kinetic mode for 15 min. For all the samples the mean value of three replicates was expressed as mmol Fe2+/kg of dry weight.
DPPH method
The radical scavenging activity of the amaranth seeds and sprouts samples against DPPH was measured according to Dávalos et al. (2003), with a slight modification. One millilitre of methanolic DPPH solution (25 mg/L) was mixed with 25 μL of the evaluated sample. The mixture was shaken and left in the dark at 30 °C for 1 h, and the absorbance was recorded at 517 nm. The antioxidant activity was measured with Jasco UV-530 spectrophotometer as the percentage inhibition of DPPH radical (% DPPH) by evaluated samples. For all the samples the mean value of three replicates was presented.
Glutathione peroxidase (GPx) activity
The fresh material was stored at −80 °C in order to investigate selenium supplementation on glutathione peroxidase (GPx) activity. GPx determination were essentially the same as in our earlier paper (Zagrodzki et al. 2007), using H2O2 as substrate. For all the samples the mean value of three replicates was expressed as U/L of the plant extract.
Determination of antibacterial and antifungal activity—preparation of organisms
Gram-positive Staphylococcus aureus (S.aureus-ATCC 6538), Gram-negative Eschericshia coli (E.coli-ATCC 25922) and yeast Candida albicans (C. albicans - ATCC 10231) were chosen to conduct the pure culture studies on agar plates. In order to prepare cultures for testing, sterile 10 ml aliquots of Nutrient Broth (Oxoid), Columbia Broth (Difco) and Malt Extract Broth (Difco) were inoculated with E. coli, S. aureus and C. albicans respectively. Prepared bacterial suspensions were incubated overnight at 37 °C in an orbital shaker. Next, cultures were centrifuged at 4,000 r.p.m. for 15 min, supernatants removed and formed pellets washed with sterile Phosphate Buffer Saline (PBS-Sigma-Aldrich). Concentration of bacterial and fungal cultures was adjusted accordingly, giving a cell suspension of approximately 106 CFU/ml.
Antimicrobial and antifungal activity of obtained DMSO extracts was evaluated by the agar diffusion test. Briefly cooled but still molten sterile Mueller-Hinton agar was aseptically poured into a sterile Petri dish and inoculated separately with E. coli, S. aureus or C. albicans (106 cells/plate) prior to solidification. Next, paper filters impregnated with the extracts were placed in a Petri dish on the solidified agar surface. The prepared systems were incubated at 37 °C for 20 h. Following the designated time, inhibition zones round the samples were measured in order to evaluate antimicrobial or antifungal properties of extracts. Inhibition zones size, interpreted by reference to CLSI standards, were determined as resistant, intermediate or susceptible to the tested amaranth extracts. Chloramhenicol and nystatin were used as a positive standards control for antibiotic and antifungal effects, respectively. All the specimens were tested in triplicate.
Statistical approach
Where appropriate, the obtained data were tested with one-way ANOVA using STATISTICA-10 package (StatSoft Inc.,Tulsa, OK, USA), followed by Tukey post hoc test. Pearson correlation coefficients and p-values were applied to show correlations and their significance.
A Principal Component Analysis (PCA) model was used to reveal the correlation structure between the investigated parameters. A statistically significant PCA model was sought with a trial-and-error method, i.e. through interactive testing various sets of original parameters. The cross validation procedure was used for subsequent elimination of uninformative parameters with the smallest weights. The parameters with weights over 0.3 on the final PCA plot were assumed to be correlated. The associations between parameters were quantified by calculating their correlation weights, i.e. for each pair of the considered parameters the algebraic products of their corresponding weights and cosine of the corresponding angle were calculated. PCA model was constructed by means of SIMCA-P v. 9 program (Umetrics, Umeå, Sweden), and the PCA final plot was performed with STATISTICA-10 package (Tulsa, OK, USA). The correlation weights were calculated with software delivered by MP System Co. (Poland).
Results and discussion
Harvesting procedure and influence of various selenium doses on sprouts condition
Under this study selenium tolerance for various edible amaranth seeds was screened in a preliminary experiment to evaluate the most appropriate amaranth genus for selenium enrichment.
Initially the seeds were immersed either in tap water, or tap water with different selenium concentration (as described in the previous section) to stimulate dormant seeds. Due to small size of amaranth seeds, it was also important to mix them up well to assure good water-seed contact. As amaranth seeds are considered to be hard, water temperature was increased (40 °C) to shorten soaking time. For the consecutive 6 days, seeds were watered with adequate solutions. The optimal time for amaranth sprouting determined elsewhere (Paśko et al. 2009) was properly adjusted to the current experiment. As in our earlier study, we believe draining to be very important process when amaranth sprouts, thus sprouts shall be drained thoroughly after rinsing to prevent them from deteriorating. All the cultures were harvested in natural light conditions. No differences in germination capacity for the harvested sprouts and lengthwise growth of stalk and roots between the amaranth sprouts from tap water and those from selenium solutions were found (data not presented). All the harvested sprouts were similar in purple colour.
A. tricolor was found to be the most afflicted during sprouting and its sprouts growth was slightly reduced. The dose of 30 mg/l was determined too high for all the assessed amaranth sprouts; an apparent reduction in seedling development for such selenium dose was observed, and sprouts started dying on the third day after sowing. Similar observations were made by Lintschinger et al. (2000), still, the opposite results were also noted by Carlson et al. (1989) who determined the influence of various selenium (as a selenate or selenite (1, 2, 4, 8, 16, and 32 mg Se/l)) doses for 2–4 days on the growth in cabbage, lettuce, radish, sorghum, turnip and wheat. They also found selenium not to affect investigated seeds germination at concentrations up to 32 mg of Se /l.
Selenium concentration in amaranth seeds and sprouts
According to Dumont et al. (2006) selenium is accumulated in young leaves at the early vegetative growth stage. At the reproductive stage, high amounts of selenium are also found in seeds. Selenium content in the investigated amaranth seeds varied within the range 0.10 ± 0.00–0.80 ± 0.01 mg/kg dry weight. The lowest concentrations were observed in seeds of Amaranthus caudatus and Amaranthus paniculatus, namely 0.10 ± 0.00 mg/kg dry weight and 0.15 ± 0.01 mg/kg dry weight, respectively, while the highest in seeds of Amaranthus cruentus. Generally, the average selenium content in amaranth seeds in comparison to other pseudocereals, such as quinoa, was found similar (Szlósarczyk et al. 2011), whereas for buckwheat seeds it was much higher as Stibilj et al. (2004) reported selenium concentration in buckwheat seeds to be approximately 43 ng/g.
This trace element is well known to be toxic to most plants, although some plants accumulate selenium compounds. They belong to such families as Asteraceae (Oonopsis sp., Xylorhiza sp.), Fabaceae (Neptunia sp., Astragalus sp.), Rubiaceae (Morinda sp.) or Brassicaceae (Stanleya sp.) (Terry et al. 2000). None of these plants is eatable, though some occur in folk medicine. Another reason to search for new plants consumable by humans was to find a good dietary source, preferably accumulating selenium. It is highly required to look for such products because selenium plays an important role in human nutrition, as it is the key element of several enzymes such as deiodinases, thioredoxine reductases, or glutathione peroxidises.
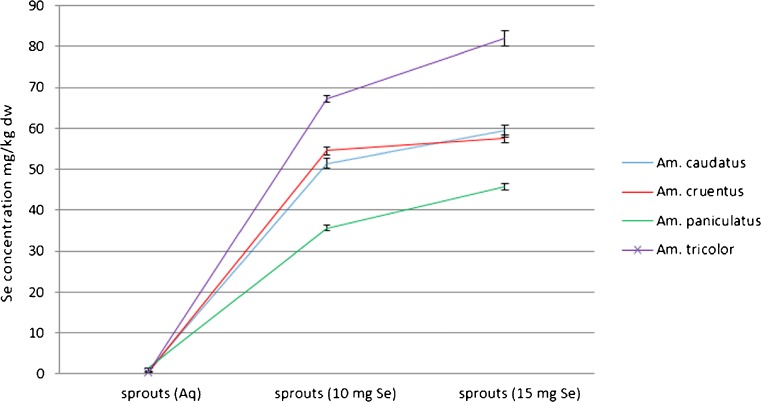
The effectiveness of selenium accumulation in amaranth sprouts was determined by measuring the total selenium concentration in plant material following 6 days from germination. Total selenium content in A. cruentus, A. caudatus, A. paniculatus and A. tricolor sprouts rinsed with tap water was equal to 0.37 ± 0.01, 0.54 ± 0.05, 1.36 ± 0.06, and 0.33 ± 0.03 mg Se/kg dry weight, respectively. In Fig. 1, selenium content for four types of amaranth sprouts as a function of the applied Se solution concentrations are presented. Sprouts of Amaranthus tricolor accumulated maximum of 82 mg Se/kg dry weight of sprouts. The level of selenium in this species was significantly higher (p < 0.05) than in the rest of the evaluated sprouts. Significant differences between all the evaluated amaranth sprouts watered with tap water, and with tap water containing selenium, were observed. Concentration of selenium and its chemical forms, as well as the presence of competing ions determine micronutrient uptake and accumulation in plants (Terry et al. 2000; Zayed et al. 1998). Both selenite and sulfate anions compete for uptake as they are probably taken up via a sulfate transporter in the plasma membrane (Terry et al. 2000). Subsequent formation of selenium derivatives that cannot be incorporated into proteins is a putative mechanism in order to prevent selenium toxicity in plant.
Fig. 1.
Total selenium concentration for 4 edible amaranth sprouts (Amaranthus cruentus; Amaranthus caudatus; Amaranthus paniculatus; Amaranthus tricolor) following 6 sprouting days as a function of selenium concentration in applied water based solutions (10 mg Se/l and 15 mg Se/l) (n = 3, mean ± SD)
The eight edible seeds of alfalfa, barley, millet, oat, pea, rye, sunflower, and wheat were germinated by Lintschinger et al. (2000) in order to find the best food products, likely to be implementable into daily nutrition schemes. Unfortunately, barley, millet, oat, pea, rye sprouts showed a major reduction of germination rates with selenium doses present. Sprouts of wheat and alfalfa were less resistant and selenium content was enriched up to concentrations of 100 mg and 150 mg of Se/kg of dry mass, respectively (Lintschinger et al. 2000). Such results were obtained during sprouting with 150 mg Se/l and 200 mg Se/l dose added. In our experiment sprouts, in particular A. tricolor amaranth sprouts, exhibited much higher effectiveness in accumulating selenium. At concentration of 15 mg Se/l in water, selenium content in sprouts varied roughly from 45.0 ± 0.4 to 82.0 ± 0.7 mg/kg of dry weight, however, the best results were obtained for sunflower sprouts where selenium concentrations up to 900 mg of Se/kg of dry mass (Lintschinger et al. 2000) was recorded. Another good selenium source provide mung bean sprouts (Vigna radiate), whose practical application (contained 223.45 mg Se/kg) was evaluated by Chinrasri et al. (2009), who determined their effect on productivity, quality and Se concentrations in eggs. Se enriched mung bean whose sprouts were produced by cultivation of seeds rinsed with water containing 90 mg of sodium selenate/l (3 days in dark) remarkably enhanced selenium content in eggs. According to Lintschinger et al. (2000) sprouts containing designed amounts of selenium can be produced, and it may provide a substantial proportion of this element in daily intakes. Selenium content in plants is closely related to protein content. This relationship is partly due to plants capabilities to take up selenates and selenites from soil. Selenate is then reduced to selenite, which in turn is reduced to selenide. The selenide undergoes transformation to Se–Cys. It was postulated by Dumont et al. (2006) that Se–Cys is metabolized to Se–Met, the latter further metabolised to Se– adenosyl–Se–Met, Se–MeSeMet, which finally is converted to Se–MeSeCys and γ–glu–SeMeSeCys. At elevated Se levels, Se–MeSeCys becomes a predominant Se compound in plant (Dumont et al. 2006) and probably also in sprouts. Kápolina et al. (2007) indicated that for supplementation with Se (IV) and SeMet, metabolism of selenium in chive caused the formation of more organic forms of selenium. For radish (Raphanus sativus) harvested in Se (IV) media, in organic species almost 95 % of selenium content was transformed (Pedrero et al. 2006). In conclusion, edible amaranth sprouts may provide an interesting component in designing improved pseudocereal-based diets.
Fatty acid profile in investigated amaranth seeds and sprouts
Fatty acid profiles for the studied amaranth seeds and sprouts are presented in Table 1. A major fatty acid present in all seeds was linoleic acid (LA), with the highest content observed in seeds of A. tricolor (58.80 ± 3.00 %) and the lowest in seeds of A. cruentus (33.18 ± 3.10 %). The remaining MUFA and PUFA occurred in much lower amounts (0.25 ± 0.10–6.77 ± 1.40 %), except for alpha-linolenic acid (ALA) which ranged from 7.99 ± 1.90 to 12.36 ± 2.00 %. Only in A. tricolor seeds, ALA concentration (2.91 ± 0.45 %) was significantly lower than in the other investigated amaranth seeds. The obtained LA level for all amaranth seeds was similar to the results presented by He and Corke (2003), who investigated fatty acid profile in grains of 30 amaranth species. When referencing our results against other cereals, pseudocereals and legumes, the level of LA in amaranth was similar to quinoa, chick peas, barley, maize, rye, spelt, lentils and peas (Ryan et al. 2007). Interestingly enough, ALA content in amaranth seeds was higher than in pumpkin, sesame, buckwheat, millet and comparable to the one of quinoa (Ryan et al. 2007). Significant differences were found also in the total SFA contents for different seeds of the examined species. The total SFA ranged from 22.0 to 39.6 % and palmitic acid ranged from 8.55 ± 3.5 % to 13.82 ± 1.5 % as the prevailing fatty acid in all the evaluated amaranth seeds. The next abundant SFA was stearic acid. Our values are in accordance with those reported by He and Corke (2003) and Jahaniaval et al. (2000). The value of SFA/(MUFA + PUFA) ratio is very important in determination of nutritional impact of food products. For the evaluated amaranth grains this ratio ranged from 0.28 to 0.65 for A. caudatus and A. cruentus, respectively. A similar level of SFA/(MUFA + PUFA) ratio for different amaranth seeds was presented by He and Corke (2003).
Table 1.
Average composition for fatty acids in amaranth grains and sprouts (n = 3, mean ± SD)
| FA [%] of total lipids | Seeds | Sprouts | Sprouts 10 mg Se | Sprouts 15 mg Se |
|---|---|---|---|---|
| Amaranthus cruentus | ||||
| SFA | ||||
| C 4:0 butyric acid | 3.45 ± 1.11 | 2.47 ± 0.91 | 2.10 ± 1.12 | 1.99 ± 0.20 |
| C 6:0 caproic acid | 4.84 ± 1.00 | 2.74 ± 0.63 | 2.67 ± 0.91 | 2.69 ± 0.62 |
| C 8:0 caprylic acid | 5.11 ± 1.20 | 0.68 ± 0.21 | 0.22 ± 0.02 | 0.33 ± 0.32 |
| C 10:0 capric acid | 0.85 ± 0.15 | 1.18 ± 0.90 | 0.32 ± 0.06 | 0.79 ± 0.21 |
| C 12:0 lauric acid | 2.27 ± 0.35 | 0.51 ± 0.09 | 1.24 ± 0.72 | 0.27 ± 0.04 |
| C 14:0 myristic acid | 1.52 ± 0.62 | 0.21 ± 0.03 | 0.36 ± 0.05 | 0.21 ± 0.02 |
| C 16:0 palmitic acid | 11.76 ± 1.32 | 18.06 ± 2.00 | 9.52 ± 1.61 | 9.47 ± 1.21 |
| C 18:0 stearic acid | 9.77 ± 1.13 | 10.31 ± 1.31 | 7.96 ± 0.91 | 8.60 ± 0.91 |
| Total SFA | 39.61,2,3 | 36.2A,B | 24.4A | 24.3B |
| MUFA | ||||
| C 14:1 myristoleic acid (n-5) | 2.35 ± 0.50 | 0.68 ± 0.09 | 0.28 ± 0.02 | 0.15 ± 0.02 |
| C 15:1 cis-10-pentadecenoic acid (n-5) | 3.54 ± 0.70 | 7.00 ± 0.40 | 4.24 ± 0.30 | 4.83 ± 0.20 |
| C 18:1 oleic acid (n-9) | 5.47 ± 0.62 | 4.30 ± 0.36 | 2.90 ± 0.09 | 2.15 ± 0.15 |
| Total MUFA | 11.36 | 11.98 | 7.42 | 7.13 |
| PUFA | ||||
| C 18:2 linoleic acid (n-6) | 33.18 ± 3.10 | 1.81 ± 0.03 | 2.96 ± 0.03 | 3.00 ± 0.05 |
| C 18:3 gamma-linolenic acid (n-6) | 1.63 ± 0.02 | 1.01 ± 0.02 | 2.69 ± 0.02 | 2.58 ± 0.03 |
| C 18:3 alpha-linolenic acid (n-3) | 11.10 ± 2.00* | 45.79 ± 4.70 | 48.02 ± 2.70 | 60.78 ± 7.0 |
| C 20:4 arachidonic acid (n-6) | 1.42 ± 0.03 | 1.78 ± 0.02 | 5.03 ± 0.60 | 1.07 ± 0.02 |
| C 22:2 docosadienoic acid (n-6) | 1.72 ± 0.05 | 1.46 ± 0.02 | 9.50 ± 0.80 | 1.08 ± 0.02 |
| Total PUFA | 49.05 | 51.85 | 68.2 | 68.51 |
| (SFA)/(MUFA + PUFA) ratio | 0.65 | 0.57 | 0.32 | 0.32 |
| Amaranthus caudatus | ||||
| SFA | ||||
| C 4:0 butyric acid | 0.32 ± 0.03 | 1.16 ± 0.02 | 1.09 ± 0.02 | 2.76 ± 0.02 |
| C 6:0 caproic acid | 0.56 ± 0.02 | 0.96 ± 0.10 | 0.72 ± 0.09 | 2.23 ± 0.03 |
| C 8:0 caprylic acid | 1.05 ± 0.09 | 0.61 ± 0.06 | 0.95 ± 0.07 | 1.88 ± 0.02 |
| C 10:0 capric acid | 0.47 ± 0.04 | 0.83 ± 0.30 | 0.58 ± 0.03 | 1.20 ± 0.02 |
| C 12:0 lauric acid | 0.77 ± 0.05 | 0.75 ± 0.20 | 0.45 ± 0.03 | 0.99 ± 0.05 |
| C 14:0 myristic acid | 2.69 ± 0.07 | 1.95 ± 0.20 | 1.78 ± 0.09 | 4.21 ± 0.90 |
| C 16:0 palmitic acid | 10.02 ± 1.00 | 8.19 ± 0.80 | 7.98 ± 1.00 | 5.39 ± 0.95 |
| C 18:0 stearic acid | 6.12 ± 0.90 | 5.23 ± 0.60 | 5.10 ± 0.90 | 2.79 ± 0.06 |
| Total SFA | 221,4,5 | 19.68 | 18.65 | 21.45 |
| MUFA | ||||
| C 14:1 myristoleic acid (n-5) | 2.54 ± 0.20 | 2.08 ± 0.06 | 2.01 ± 0.03 | 4.00 ± 0.30 |
| C 15:1 cis-10-pentadecenoic acid (n-5) | 3.27 ± 0.25 | 3.03 ± 0.08 | 4.78 ± 0.60 | 5.40 ± 0.60 |
| C 18:1 oleic acid (n-9) | 5.62 ± 0.30 | 2.08 ± 0.03 | 1.59 ± 0.09 | 2.48 ± 0.09 |
| Total MUFA | 11.43 | 7.19 | 8.38 | 11.88 |
| PUFA | ||||
| C 18:2 linoleic acid (n-6) | 50.25 ± 2.80 | 4.97 ± 0.60 | 4.07 ± 0.50 | 2.45 ± 0.09 |
| C 18:3 gamma-linolenic acid (n-6) | 1.09 ± 0.09 | 11.04 ± 1.70 | 11.67 ± 2.20 | 5.44 ± 0.90 |
| C 18:3 alpha-linolenic acid (n-3) | 12.36 ± 2.00# | 51.10 ± 6.50 | 51.94 ± 5.40 | 55.50 ± 6.10 |
| C 20:4 arachidonic acid (n-6) | 1.45 ± 0.05 | 2.79 ± 0.10 | 2.28 ± 0.20 | 1.16 ± 0.03 |
| C 22:2 docosadienoic acid (n-6) | 1.41 ± 0.03 | 3.24 ± 0.15 | 3.02 ± 0.20 | 2.12 ± 0.20 |
| Total PUFA | 66.56 | 73.14 | 72.98 | 66.67 |
| (SFA)/(MUFA + PUFA) ratio | 0.28 | 0.24 | 0.23 | 0.27 |
| Amaranthus paniculatus | ||||
| SFA | ||||
| C 4:0 butyric acid | 0.97 ± 0.03 | 0.66 ± 0.04 | 2.08 ± 0.10 | 2.11 ± 0.30 |
| C 6:0 caproic acid | 0.80 ± 0.05 | 0.84 ± 0.03 | 0.59 ± 0.05 | 0.96 ± 0.10 |
| C 8:0 caprylic acid | 0.34 ± 0.06 | 0.95 ± 0.05 | 1.53 ± 0.20 | 0.35 ± 0.04 |
| C 10:0 capric acid | 0.17 ± 0.04 | 0.25 ± 0.05 | 0.61 ± 0.02 | 1.78 ± 0.15 |
| C 12:0 lauric acid | 0.46 ± 0.02 | 0.49 ± 0.04 | 1.24 ± 0.03 | 1.54 ± 0.07 |
| C 14:0 myristic acid | 1.00 ± 0.03 | 1.12 ± 0.09 | 1.01 ± 0.03 | 1.22 ± 0.04 |
| C 16:0 palmitic acid | 13.82 ± 1.50 | 7.31 ± 1.20 | 9.07 ± 0.80 | 15.93 ± 3.20 |
| C 18:0 stearic acid | 12.18 ± 1.90 | 6.20 ± 0.90 | 6.84 ± 0.60 | 14.62 ± 3.60 |
| Total SFA | 29.742,4 | 17.82C | 22.97 | 38.51C |
| MUFA | ||||
| C 14:1 myristoleic acid (n-5) | 1.01 ± 0.10 | 1.02 ± 0.20 | 0.76 ± 0.20 | 0.92 ± 0.30 |
| C 15:1 cis-10-pentadecenoic acid (n-5) | 2.45 ± 0.90 | 2.36 ± 0.60 | 2.27 ± 0.50 | 3.34 ± 0.70 |
| C 18:1 oleic acid (n-9) | 6.77 ± 1.40 | 1.35 ± 0.20 | 2.14 ± 0.40 | 2.50 ± 0.30 |
| Total MUFA | 10.23 | 4.73 | 5.17 | 6.76 |
| PUFA | ||||
| C 18:2 linoleic acid (n-6) | 47.98 ± 4.00 | 3.37 ± 1.00 | 6.94 ± 2.00 | 9.97 ± 2.70 |
| C 18:3 gamma-linolenic acid (n-6) | 1.47 ± 0.20 | 5.00 ± 1.20 | 7.31 ± 1.40 | 13.47 ± 1.90 |
| C 18:3 alpha-linolenic acid (n-3) | 7.99 ± 1.90☼ | 67.19 ± 8.20 | 55.68 ± 6.30 | 29.16 ± 3.60 |
| C 20:4 arachidonic acid (n-6) | 1.65 ± 0.10 | 1.61 ± 0.20 | 1.57 ± 0.10 | 1.46 ± 0.20 |
| C 22:2 docosadienoic acid (n-6) | 0.92 ± 0.10 | 0.31 ± 0.02 | 0.37 ± 0.03 | 0.66 ± 0.06 |
| Total PUFA | 60.01 | 77.48 | 71.87 | 54.72 |
| (SFA)/(MUFA + PUFA) ratio | 0.42 | 0.21 | 0.3 | 0.62 |
| Amaranthus tricolor | ||||
| SFA | ||||
| C 4:0 butyric acid | 6.78 ± 1.90 | 0.46 ± 0.06 | 1.60 ± 0.30 | 0.91 ± 0.20 |
| C 6:0 caproic acid | 2.39 ± 0.60 | 0.35 ± 0.03 | 1.05 ± 0.20 | 1.10 ± 0.20 |
| C 8:0 caprylic acid | 1.31 ± 0.20 | 0.26 ± 0.02 | 0.64 ± 0.30 | 0.76 ± 0.07 |
| C 10:0 capric acid | 1.35 ± 0.20 | 0.35 ± 0.04 | 0.93 ± 0.20 | 0.92 ± 0.09 |
| C 12:0 lauric acid | 0.70 ± 0.06 | 0.24 ± 0.03 | 0.76 ± 0.15 | 0.72 ± 0.08 |
| C 14:0 myristic acid | 0.86 ± 0.08 | 0.89 ± 0.40 | 1.12 ± 0.60 | 0.79 ± 0.07 |
| C 16:0 palmitic acid | 8.55 ± 3.50 | 11.28 ± 2.30 | 15.92 ± 4.10 | 11.42 ± 3.00 |
| C 18:0 stearic acid | 7.85 ± 1.40 | 8.44 ± 1.60 | 9.42 ± 2.70 | 13.75 ± 3.30 |
| Total SFA | 29.793,5 | 22.27 | 31.44 | 30.37 |
| MUFA | ||||
| C 14:1 myristoleic acid (n-5) | 0.92 ± 0.20 | 1.01 ± 0.20 | 1.24 ± 0.20 | 0.86 ± 0.20 |
| C 15:1 cis-10-pentadecenoic acid (n-5) | 1.89 ± 0.30 | 3.24 ± 0.90 | 4.15 ± 0.95 | 3.43 ± 0.90 |
| C 18:1 oleic acid (n-9) | 2.44 ± 0.90 | 2.82 ± 0.70 | 4.52 ± 1.10 | 1.53 ± 0.60 |
| Total MUFA | 5.25 | 7.07 | 9.91 | 5.82 |
| PUFA | ||||
| C 18:2 linoleic acid (n-6) | 58.80 ± 3.00 | 3.82 ± 1.00 | 5.25 ± 1.60 | 3.68 ± 0.90 |
| C 18:3 gamma-linolenic acid (n-6) | 2.11 ± 0.30 | 8.06 ± 1.20 | 6.21 ± 1.50 | 5.93 ± 1.80 |
| C 18:3 alpha-linolenic acid (n-3) | 2.91 ± 0.45*#☼ | 56.21 ± 10.20 | 43.84 ± 3.50 | 51.20 ± 5.60 |
| C 20:4 arachidonic acid (n-6) | 0.91 ± 0.20 | 1.42 ± 0.20 | 1.79 ± 0.60 | 1.31 ± 0.60 |
| C 22:2 docosadienoic acid (n-6) | 0.25 ± 0.10 | 1.14 ± 0.10 | 1.56 ± 0.40 | 1.69 ± 0.50 |
| Total PUFA | 64.98 | 70.65 | 58.65 | 63.81 |
| (SFA)/(MUFA + PUFA) ratio | 0.42 | 0.28 | 0.45 | 0.43 |
SFA, saturated fatty acid
MUFA, monounsaturated fatty acids
PUFA, poliunsaturated fatty acids
Means of triplicate determinations ± SD; selected, important differences (p < 0.05) were indicate in table, results marked with the same letters in upper index within each row differ significantly for fatty acids; results marked with the same symbols in upper index within each column differ significantly for evaluated plant material
The fatty acid compositions of the studied edible amaranth sprouts were essentially not the same. For MUFA and PUFA—ALA the highest amounts (more than 40 %) were recorded with smaller amount of LA, oleic acid (OA) and gamma – linolenic acid (GLA). Palmitic and stearic acids were the dominating saturated fatty acids in all the sprouts. To the best of our knowledge, none report on fatty acid profile in amaranth sprouts has been published. On the other hand, amaranth leaves receive massive attention as vegetable not only in developing countries, and thus to compare the obtained results with those for amaranth leaves that already provide a daily nutrition element, seems worthwhile. According to Fernando and Bean (1985), who analyzed six weedy and vegetables species of amaranth, LA was also the main unsaturated fatty acid (about 20 %), with oleic acid and ALA further in line. He and Corke (2003) investigated amaranth leaves and obtained similar results to those presented in the current work –ALA to be the major fatty acid, with further LA and OA. Similar observations were made by Liu et al. (2002) who showed ALA to be the major (59.5 %) unsaturated fatty acid in A. viridis leaves. So as to the fatty acid profile in various sprouts, Chung et al. (1989) presented results for barley and canola. In barley sprouts, the major unsaturated fatty acids were LA, OA and ALA, listed down with amounts. In canola sprouts the highest amount of OA, followed by LA and ALA were found. For soy sprouts the main unsaturated fatty acids were respectively ALA, LA and OA (Orhan et al. 2007). Amaranth sprouts showed a more favourable SFA/(MUFA + PUFA) ratio than seeds which resulted from PUFA, particularly ALA, high content. Differences between fatty acids profile for sprouts, when compared to leaves, could be attributed to different growth stages; previously such a relation was observed in leaves, where the amount of lipids was lower in mature leaves than in young ones (He and Corke 2003). Nevertheless, selenium influence on the saturated fatty acids profile was not clear. For total saturated fatty acids, a significant decrease was observed between A. cruentus sprouts harvested in normal condition and those rinsed with selenium solution. Particularly high increase in the total SFA for normal and Se-enriched A. paniculatus sprouts were revealed. The content of unsaturated fatty acids, as well as changes in the amount of such compounds, remain ambiguous. To summarise, the interaction between selenium and fatty acids showed no significant correlations between the selenium concentration and the quantity of single fatty acid/a group of saturated/ unsaturated, or all fatty acids, were observed.
Total antioxidant activity (TRC, FRAP method, DPPH method) and GPx activity of amaranth seeds and the evaluated sprouts
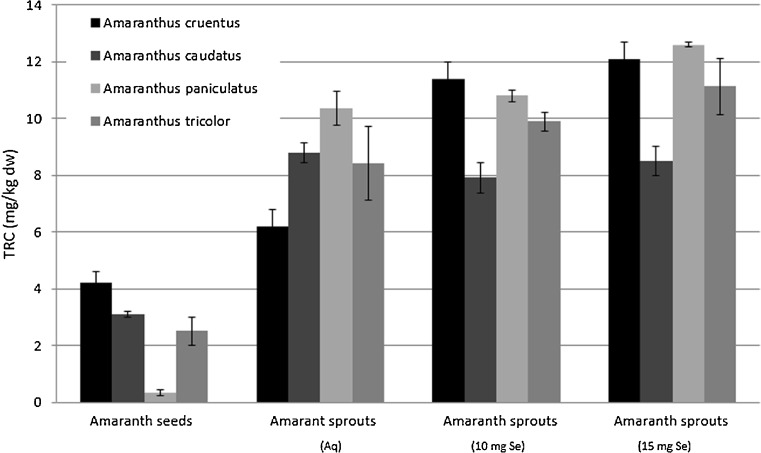
Polyphenol compounds have long been recognised as a major contributor to the overall antioxidant activity of plants. Amaranth seeds and sprouts were found elsewhere (Paśko et al. 2008) to contain antioxidant properties compounds such as gallic acid, p-hydroxybenzoic acid, vanillic acid and rutin. In the evaluated seeds and sprouts TRC ranged from 0.32 to 12.6 mg GA/kg dry weight. Obviously, amaranth sprouts showed significantly higher (p < 0.001) TRC than seeds. A positive impact of low dose selenium added to water on TRC was selective and found only for A. cruentus and A. tricolor sprouts, whereas for A. caudatus sprouts TRC decreased in response to added selenium. No significant differences were noted for A. paniculatus. A higher dose of selenium, namely 15 mg Se/l, caused only a slight, insignificant increase in amaranth sprouts TRC in comparison to sprouts harvested with 10 mg Se/l (see Fig. 2). A strong correlation between TRC results vs. FRAP (r = 0.971; p < 0.05) was observed in all the sprouts.
Fig. 2.
Total reducing capacity (TRC) in amaranth seeds and evaluated sprouts (n = 3, mean ± SD)
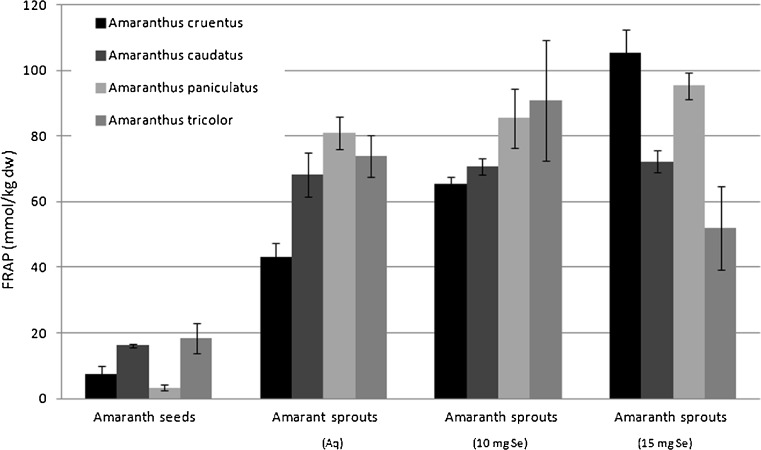
Edible amaranth seeds extracts under the experiment showed antioxidant activity measured by the FRAP method (mmol Fe2+/kg dry weight) to increase from 0.32 ± 0.1 for A. paniculatus, via 2.5 ± 0.5 for A. tricolor and 3.09 ± 0.1 for A. caudatus, to reach 4.22 ± 0.4 for A. cruentus (see Fig. 3). These data are similar to earlier evaluation by Paśko et al. (2009) for A. cruentus seeds, as well as reported by Nsimba et al. (2008) for A. cruentus and A. hypochondriacus. However, the latter total antioxidant activity was evaluated with different types of extracts. FRAP values for amaranth, recalculated into adequate units, were lower than those for oat, and higher than for rice or quinoa seeds (Paśko et al. 2009; Halvorsen et al. 2002).
Fig. 3.
Total antioxidant activity (FRAP) in amaranth seeds and evaluated sprouts (n = 3, mean ± SD)
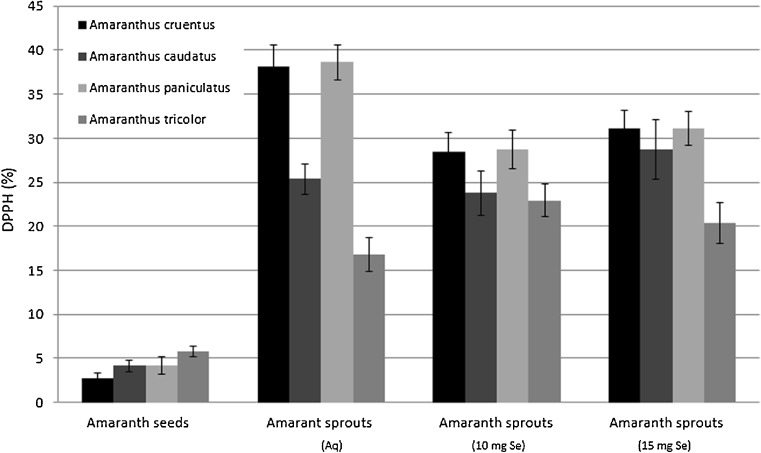
The total antioxidant capacity evaluated by DPPH method (%) varied within the range 2.76- 38.62, with the highest value observed in edible amaranth sprouts, and the lowest scavenging activity in Amaranthus cruentus seeds, though the value was similar for all the evaluated amaranth seeds (Fig. 4).
Fig. 4.
Total antioxidant activity (DPPH) in amaranth seeds and evaluated sprouts (n = 3, mean ± SD)
For the amaranth sprouts watered with tap water, the highest antioxidant activity was determined for A. paniculatus sprouts, and all the obtained results were comparable both with antioxidant activity of sprouts of other amaranth varieties and with quinoa sprouts (Paśko et al. 2009). With respect to selenium influence on the increased antioxidant activity, the most interesting and significant results were obtained for A. cruentus. For the remaining sprouts, namely A. caudatus and A. paniculatus, antioxidant activity either did not change, or decreased strongly in response to 15 mg Se/l water dose (A. tricolor). Other authors (Xue et al. 2001; Djanaguiraman et al. 2005) evaluated selenium influence on young and senescing plants, i.e. lettuce or soybean. The increase in antioxidant activity was positively correlated with selenium concentration in plants (Djanaguiraman et al. 2005). Despite such evidence, we observed no clear relation between selenium concentration and antioxidant activity (FRAP, DPPH). Xue et al. (2001) suggested that the antioxidant effect of selenium was associated with increased activities of glutathione peroxidase (GPx) and superoxide dismutase (SOD), as well as with preventing the reduction of vitamin E level. The effect of applying various doses of selenium to water is presented in Table 2. 10 mg Se/L dose increased significantly GPx activity of amaranth sprouts, though a higher dose of 15 mg Se/L proved toxic and caused antioxidant enzyme activity to decrease. Seppänen et al. (2003) confirmed that Se altered transcript accumulation of GPx in potato, while higher selenium dosage depressed this enzyme activity for lettuce (Xue and Hartikainen 2000) and provoked stress response in wheat plants (Nowak et al. 2004). No correlation between selenium concentration and GPx activity was found in our experiment, which is in line with observations reported by Cartes et al. (2005).
Table 2.
GPx activity for edible amaranth seeds and sprouts (n = 3, mean ± SD)
| Sample | Seeds | Sprouts (Aq) | Sprouts (10 mg Se/L) | Sprouts (15 mg Se/L) |
|---|---|---|---|---|
| A. cruentus | 334.2 ± 5.9 | 494.2 ± 5.5 | 604.7 ± 8.9 | 585.2 ± 7.8 |
| A. caudatus | 323.8 ± 6.4 | 422.3 ± 6.4 | 466.5 ± 7.2 | 448.7 ± 5.2 |
| A. paniculatus | 331.6 ± 9.7 | 516.9 ± 7.3 | 718.5 ± 9.1 | 340.4 ± 6.8 |
| A. tricolor | 370.6 ± 5.7 | 432.7 ± 4.4 | 475.5 ± 9.4 | 432.4 ± 8.5 |
Finley et al. (2005) found that selenium fertilization caused significant decrease in phenolic acid concentration and moderately decreased the total amount of glucosinolates. It was suggested that accumulation of selenium in plants, namely broccoli, may not bring positive effects on overall antioxidant capacities, as maximizing the concentration of some bioactive compounds and – subsequently – their interference with other plant components, may cause a concomitant decrease in the activity of the latter ones.
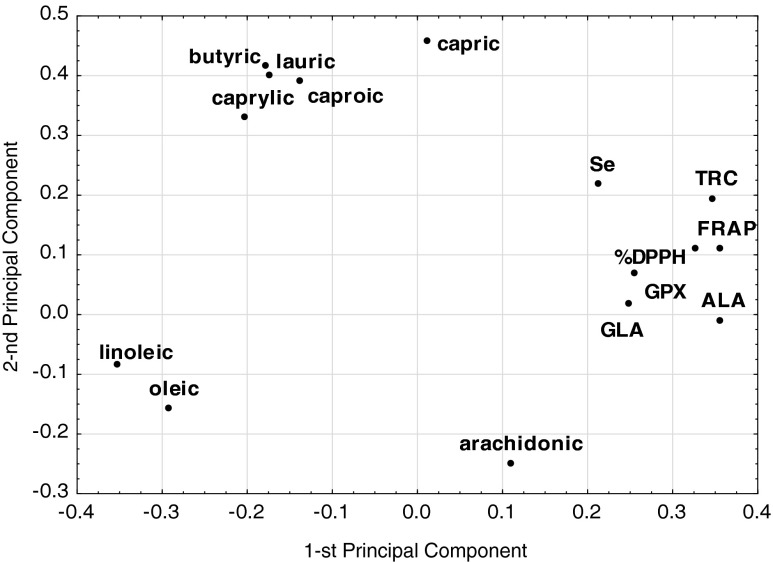
Principal component analysis is one of multivariate statistical methods used for exploratory data analysis to uncover hidden relationships between various parameters taken at once, which is the primary advantage of PCA over univariate methods. Another advantage is that PCA model retains most relevant information of an underlying systematic data structure, while the part of information unexplained by PCA model consists mainly of noise. All this is achieved by a reduction of original dimensionality to 2 or 3-dimentional subspace which can be shown on a PCA plot. Such plots make it easy to visually comprehend the correlation structure of complex data. The apparent associations between parameters are then quantified and stratified by calculating respective correlation weights.
The final PCA model with two significant components, according to cross-validation, explained 63 %, of the variance in the original parameters with eigenvalues equal to 6.64 and 2.81 for the first and second component of PCA model, respectively (Fig. 5). Three parameters, namely concentrations of myristic, palmitic, and stearic acids, were excluded from the model as being non-informative. The first component was highly correlated with FRAP, TRC, ALA and linoleic acid, while the second one was mainly correlated with capric, lauric and butyric acids. Therefore, the first component can be interpreted in terms of describing antioxidant features and some polyunsaturated fatty acids, whereas the second component was mainly determined by saturated fatty acids group.
Fig. 5.
The weights of the two first components of PCA model
The correlation weights obtained from PCA model, are presented in Table 3. The highest positive correlation weights were found among the concentrations of capric, lauric, caproic, and butyric acids. The antioxidant status parameters FRAP and TRC were also strongly positively correlated. The correlations between the other pairs of fatty acids, or between FRAP/TRC with fatty acids were moderate. Linoleic acid was the only fatty acid negatively correlated with FRAP. Neither selenium concentration, nor GPX activity correlated significantly with any of the other parameter.
Table 3.
PCA model based correlation weights
| Pairs of correlated parameters | Correlation weight (PCA) |
|---|---|
| Caproic acid and capric acid | 0.175 |
| Butyric acid and capric acid | 0.168 |
| Capric acid and lauric acid | 0.168 |
| Butyric acid and caproic acid | 0.164 |
| Caproic acid and lauric acid | 0.160 |
| Butyric acid and lauric acid | 0.154 |
| Caproic acid and caprylic acid | 0.134 |
| Butyric acid and caprylic acid | 0.129 |
| Caprylic acid and capric acid | 0.129 |
| Caprylic acid and lauric acid | 0.124 |
| FRAP and TRC | 0.123 |
| Linoleic acid and alpha-linolenic acid | 0.122 |
| FRAP and alpha-linolenic acid | 0.121 |
| FRAP and DPPH | 0.118 |
| TRC and DPPH | 0.113 |
| FRAP and linoleic acid | 0.108 |
| TRC and alpha-linolenic acid | 0.107 |
To date no systematic analysis of a correlation structure for a similar set of parameters in sprouts, with PCA method employed, has been undertaken. The correlations between fatty acids can be ascribed as an intrinsic feature of the specific sprout species. The correlation between FRAP and TRC revealed by PCA method is in accordance with standard Pearson correlation analysis results, whereas the negative correlation between FRAP and linoleic acid needs further investigations to be confirmed.
Antibacterial and antifungal activity
The folk medicine data on application of various parts of Amaranth sp. plants seem to be very promising. Leaves extracts can be used to alleviate fever, intestinal bleeding, diarrhoea, excessive menstruation and colic (Maiyo et al. 2010). Additionally, compounds present in amaranth and widely recognized for their antimicrobial activity, such as tannins, phlobatanins, flavonoids and terpenoids (Maiyo et al. 2010) urged us to subject amaranth sprouts to an in vitro test against pathogenic microorganisms. Under this study we evaluated the biological effect of the obtained extracts of sprouts and seeds on selected bacteria (Gram + and Gram-) and yeast. Our interest was particularly focused on observing how selenium supplementation affected growth of Staphylococcus aureus, Eschericshia coli and Candida albicans. Regretfully, none activity against the evaluated microorganisms was detected in all the extracts of edible amaranth seeds and sprouts at concentrations up to 10 mg/ml. Similar observation was reported by Kumar et al. (1997) for other types of amaranth seeds. So far no reports on influence of amaranth sprouts on bacteria or fungi growth have been published, therefore our results were compared with activity of leaves, bearing the closest similarity to sprouts. Maiyo et al. (2010) indicated that extracts of amaranth leaves (A. hybridus; A. caudatus) were active against E. coli but this inhibitory effect was observed at minimum inhibitory concentration (MIC) ranging between 200 and 755 mg/ml, which was significantly higher than used under the current study. Amaranthus caudatus was also active against S. aureus at MIC of 155 mg/ml. Undetected antimicrobial effect may be attributed to low contents of active compounds in the tested extracts. On the other hand, high concentrations of extracts were evaluated by Maiyo et al. (2010) who found no effect on C. albicans growth, which coincides with our findings.
Conclusion
The evaluated amaranth sprouts attractiveness as a functional food is attributable both to their chemical compounds profile and attractive colour.
Particularly amaranth sprouts seem to be an important source of alfa-linolenic acid and other unsaturated fatty acids. Having low S/U ratio amaranth sprouts are recommended for people with disturbed lipid profiles.
They can also serve as a moderate selenium accumulator, though the presented studies clearly show that more detailed experimentation in order to determine whether positive/negative interactions occur are required. Regretfully, the presented research failed to prove selenium supplementation to have any beneficial effect on the biological activity of antioxidant, antibacterial or antifungal character, or on the chemical composition of the harvested amaranth sprouts. It is also crucial to understand likely functional consequences of metabolic manipulations, as ignoring probable synergistic interactions in foods may result in missed opportunities for targeting health outcomes and causing potential harm to live organism (Finley et al. 2005). Therefore, further biological evaluation of amaranth sprouts on adequate cell lines and live organisms is highly required.
Acknowledgments
This work was supported in parts by grants from the Polish Ministry of Science and Higher Education, project K/DSC/000805, K/DSC/001960 and K/ZDS/001294. This fatty analysis project was executable through the support given by National Science Centre, Poland DEC-2011/01/B/NZ7/00038. Excellent technical assistance of E. Gajdzik is highly appreciated.
Conflict of interest
The Authors have no conflict of interest to declare.
Footnotes
The preliminary results from this paper were presented on the Sixth International Symposium of the European Amaranth Association in Slovakia 2012.
References
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Berti C, Riso P, Brusamolino A, Porrini M. Effect on appetite control of minor cereal and pseudocereal products. Br J Nutr. 2005;94:850–858. doi: 10.1079/BJN20051563. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Kaplan DI, Adriano DC. Effects of selenium on germination and radicle elongation of selected agronomic species. Environ Exp Bot. 1989;2:493–498. doi: 10.1016/0098-8472(89)90028-2. [DOI] [Google Scholar]
- Cartes P, Gianfreda L, Mora ML. Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant Soil. 2005;276(1–2):359–367. doi: 10.1007/s11104-005-5691-9. [DOI] [Google Scholar]
- Chinrasri O, Chantiratikul P, Thosakham W, Atiwetin P, Chumpawadee S, Saenthaweesuk S, Chantiratikul A. Effect of selenium-enriched bean sprout and other selenium sources on productivity and selenium concentration in eggs of laying hens. Asian-Aust J Anim Sci. 2009;12:1661–1666. doi: 10.5713/ajas.2009.90220. [DOI] [Google Scholar]
- Chung TY, Nwokolo EN, Sim JS. Compositional and digestibility changes in sprouted barley and canola seeds. Plant Foods Hum Nutr. 1989;39:267–278. doi: 10.1007/BF01091937. [DOI] [PubMed] [Google Scholar]
- Dávalos A, Gómez-Cordovés C, Bartolomé B. Commercial dietary antioxidant supplements assayed for their antioxidant activity by different methodologies. J Agric Food Chem. 2003;51:2512–2519. doi: 10.1021/jf021030j. [DOI] [PubMed] [Google Scholar]
- Djanaguiraman M, Devi DD, Shanker AK, Sheeba JA, Bangarusamy U. Selenium – an antioxidative protectant in soybean during senescence. Plant Soil. 2005;272:77–86. doi: 10.1007/s11104-004-4039-1. [DOI] [Google Scholar]
- Dumont E, Vanhaecke F, Cornelis R. Selenium speciation from food source to metabolites: a critical review. Anal Bioanal Chem. 2006;385(7):1304–1323. doi: 10.1007/s00216-006-0529-8. [DOI] [PubMed] [Google Scholar]
- Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB. Thorough study of reactivity of various compound classes toward the Folin − Ciocalteu reagent. J Agric Food Chem. 2010;58(14):8139–8144. doi: 10.1021/jf1005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando T, Bean G. A comparison of the fatty acids and sterols of seeds of weedy and vegetable species of Amaranthus spp. J Am Oil Chem Soc. 1985;6:89–91. doi: 10.1007/BF02541498. [DOI] [Google Scholar]
- Finley JW, Sigrid-Keck A, Robbins RJ, Hintze KJ. Selenium enrichment of broccoli: Interactions between selenium and secondary plant compounds. J Nutr. 2005;135:1236–1238. doi: 10.1093/jn/135.5.1236. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gorinstein S, Vargas O, Jaramillo N, Salas I, Ayala A, Arancibia-Avila P, Toledo F, Katrich E, Trakhtenberg S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur Food Res Technol. 2007;22:321–328. doi: 10.1007/s00217-006-0417-7. [DOI] [Google Scholar]
- Halvorsen BL, Holte K, Myhrstad MC, Barikmo I, Hvattum E, Remberg SF, Wold AB, Haffner K, Baugerød H, Andersen LF, Moskaug Ø, Jacobs DR, Jr, Blomhoff R. A systematic screening of total antioxidants in dietary plants. J Nutr. 2002;13:461–471. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- He HP, Corke H. Oil and squalene in amaranthus grain and leaf. J Agric Food Chem. 2003;51:7913–7920. doi: 10.1021/jf030489q. [DOI] [PubMed] [Google Scholar]
- Jahaniaval F, Kakuda Y, Marcone MF. Fatty acid and triacylglycerol compositions of seed oils of five Amaranthus accessions and their comparison to other oils. J Am Oil Chem Soc. 2000;77:847–852. doi: 10.1007/s11746-000-0135-0. [DOI] [Google Scholar]
- Kápolina E, Shah M, Caruso JA, Fedor P. Selenium speciation studies in Se-enriched chives (Allium schoenoprasum) by HPLC–ICP-MS. Food Chem. 2007;101:1398–1406. doi: 10.1016/j.foodchem.2006.03.048. [DOI] [Google Scholar]
- Kumar S, Bagchi GD, Darokar MP. Antibacterial activity observed in the seeds of some Coprophilous plants. Pharm Biol. 1997;35:179–184. doi: 10.1076/phbi.35.3.179.13293. [DOI] [Google Scholar]
- Lintschinger J, Fuchs N, Moser J, Kuehnelt D, Goessler W. Selenium-enriched sprouts. A raw material for fortified cereal-based diets. J Agric Food Chem. 2000;48:5362–5368. doi: 10.1021/jf000509d. [DOI] [PubMed] [Google Scholar]
- Liu L, Howe P, Zhou YF, Hocart C, Zhang R. Fatty acid profiles of leaves of nine edible wild plants: an Australian study. J Food Lipids. 2002;9(1):65–71. doi: 10.1111/j.1745-4522.2002.tb00209.x. [DOI] [Google Scholar]
- Maiyo ZC, Ngure RM, Matasyoh JC, Chepkorir R. Phytochemical constituents and antimicrobial activity of leaf extracts of three Amaranthus plant species. Afr J Biotechnol. 2010;9:3178–3182. [Google Scholar]
- Márton M, Mándoki Z, Csapó-Kiss Z, Csapó J. The role of sprouts in human nutrition. A review. Acta Univ Sapientiae Alimentaria. 2010;3:81–117. [Google Scholar]
- Mosovska S, Birosova L. Antimycotic and antifungal activities of amaranth and buckwheat extracts. Asian J Plant Sci. 2012;11:160–162. doi: 10.3923/ajps.2012.160.162. [DOI] [Google Scholar]
- Nowak J, Kaklewski K, Ligocki M. Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol Biochem. 2004;36(10):1553–1558. doi: 10.1016/j.soilbio.2004.07.002. [DOI] [Google Scholar]
- Nsimba RY, Kikuzaki H, Yotaro K. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008;106:760–766. doi: 10.1016/j.foodchem.2007.06.004. [DOI] [Google Scholar]
- Orhan I, Özçelik B, Kartal M, Aslan S, Ener B, Özgüven M. Quantification of daidzein, genistein and fatty acids in soybeans and soy sprouts, and some bioactivity studies. Acta Biol Cracov. 2007;49(2):61–68. [Google Scholar]
- Paśko P, Sajewicz M, Gorinstein S, Zachwieja Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008;20:661–672. doi: 10.1556/AChrom.20.2008.4.11. [DOI] [Google Scholar]
- Paśko P, Bartoń H, Zagrodzki P, Gorinstein S, Fołta M, Zachwieja Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009;115:994–998. doi: 10.1016/j.foodchem.2009.01.037. [DOI] [Google Scholar]
- Paśko P, Barton H, Zagrodzki P, Gorinstein S. Effect of amaranth seeds (Amaranthus cruentus) in deit on some biochemical parameters and essential trace elements in blood of high fructose – fed rats. Nat Prod Res. 2011;25:844–849. doi: 10.1080/14786419.2010.513976. [DOI] [PubMed] [Google Scholar]
- Paśko P, Bartoń H, Zagrodzki P, Chłopicka J, Iżewska A, Gawlik M, Gawlik M, Gorinstein S. Effect of amaranth seeds in diet on oxidative status in plasma and selected tissues of high fructose – fed rats. Food Chem. 2011;126:85–90. doi: 10.1016/j.foodchem.2010.10.081. [DOI] [Google Scholar]
- Pedrero Z, Madrid Y, Camára C. Selenium species bioaccessibility in enriched radish (Raphanus sativus): A potential dietary source of selenium. J Agric Food Chem. 2006;54:2412–2417. doi: 10.1021/jf052500n. [DOI] [PubMed] [Google Scholar]
- Pyrzynska K. Selenium speciation in enriched vegetables. Food Chem. 2009;114(4):1183–1191. doi: 10.1016/j.foodchem.2008.11.026. [DOI] [Google Scholar]
- Ryan E, Galvin K, O’Connor TP, Maguire AR, O’Brien NM. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum Nutr. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- Seppänen M, Turakainen M, Hartikainen H. Selenium effects on oxidative stress in potato. Plant Sci. 2003;165(2):311–319. doi: 10.1016/S0168-9452(03)00085-2. [DOI] [Google Scholar]
- Sirichakwal PP, Puwastien P, Polngam J, Kongkachuichai R. Selenium content of Thai foods. J Food Comp Anal. 2005;18:47–59. doi: 10.1016/j.jfca.2003.10.010. [DOI] [Google Scholar]
- Stibilj V, Kreft I, Smrkolj P, Osvald J. Enhanced selenium content in buckwheat (Fagopyrum esculentum Moench) and pumpkin (Cucurbita pepo L.) seeds by foliar fertilisation. Eur Food Res Technol. 2004;219:142–144. doi: 10.1007/s00217-004-0927-0. [DOI] [Google Scholar]
- Szlósarczyk M, Piech W, Paśko P, Opoka W, Krzek J. Voltammetric determination of zinc, copper and selenium in selected raw plant material. Anal Lett. 2011;44:1–10. doi: 10.1080/00032719.2010.551695. [DOI] [Google Scholar]
- Terry N, Zayed AM, de Souza MP, Tarun AS. Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:401–432. doi: 10.1146/annurev.arplant.51.1.401. [DOI] [PubMed] [Google Scholar]
- Xue T, Hartikainen H. Association of antioxidative enzymes with the synergistic effect of selenium and UV irradiation in enhancing plant growth. Agric Food Sci Finl. 2000;9(2):177–186. [Google Scholar]
- Xue T, Hartikainen H, Piironen V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil. 2001;237:55–61. doi: 10.1023/A:1013369804867. [DOI] [Google Scholar]
- Zagrodzki P, Joniec A, Gawlik M, Gawlik M, Krośniak M, Fołta M, Bartoń H, Paśko P, Chłopicka J, Zachwieja Z. High fructose model of oxidative stress and metabolic disturbances in rats. Part I. Antioxidant status of rats’ tissues. Bull Vet Inst Pulawy. 2007;51:407–412. [Google Scholar]
- Zayed A, Lytle CM, Terry N. Accumulation and volatilization of different chemical species of selenium by plants. Planta. 1998;206(2):284–292. doi: 10.1007/s004250050402. [DOI] [Google Scholar]