Abstract
Flaxseed is used to fortify bread. In order to reduce cyanogenic glycosides compounds of flaxseed, ground flaxseed was incubated at 30 °C and heated in a kitchen microwave oven. The cyanogenic compounds of flaxseed were reduced to 13.4 %. Treated ground flaxseed was coated with Arabic gum solution containing ascorbic acid and hydrogenated fat and was stored at 25 °C for 80 days in order to prevent oxidation of flaxseed oil. Results showed that oxidation in coated samples was lower than that in control samples and that there was a significant difference between them (p < 0.01). Coated and uncoated ground flaxseed was added to wheat flour in 5, 15 and 25 % levels in order to produce fortified Taftoon bread. Rheological, physical and organoleptic tests were carried out in order to evaluate dough and bread properties. Results showed that with increasing coated and uncoated ground flaxseed percentages, a decrease in water absorption and an increase in stability, dough development and relaxation time of dough occurred. The lowest water absorption was observed by adding 25 % coated ground flaxseed with hydrogenated fat. The highest dough development and dough stability time were observed by adding 25 % coated ground flaxseed with Arabic gum. Results indicated that coated and uncoated ground flaxseed has a good effect on decreasing the staling rate compared to the control bread. Results of organoleptic test showed that bread with 5 and 15 % coated and uncoated ground flaxseed had better acceptability.
Keywords: Flaxseed, Oxidation, Taftoon bread, Rheology of dough
Introduction
Flaxseed (Linum usitatissimum) is an annual plant of the linaceae family, whose fruit contains a seed known as flaxseed or linseed (Singh et al. 2011). Flaxseed has considerable nutritional value. Frequent consumption of flaxseed provides benefits for human health due to its lignans and alpha linolenic acid (ALA) (Hall et al. 2006; Touré and Xueming 2010). Also, flaxseed has potential beneficial effects on immune function, inflammatory diseases and probably prevention of platelet aggregation and cancer, especially breast and colon cancer (Singh et al. 2011). The most important compound of flaxseed is oil, whose content varies between 38 and 45 % depending on location, cultivar, and environmental conditions (Hall et al. 2006). Flaxseed oil is low in saturated fat (9 % of total fatty acids), moderate in monosaturated fat (18 %), and rich in polyunsaturated fat (73 %) (Cunnane et al. 1993). Flaxseed is a rich source of ALA, as 45 to 52 % of total flaxseed oil is ALA; and this exceptionally high content of ALA in flaxseed makes it unique among oilseeds crops (Hall et al. 2006). Moreover, flaxseed is a rich source of soluble and insoluble dietary fiber. Soluble fiber fractions are the mucilage gums, and the major insoluble fiber fraction consists of cellulose and lignan. Secoisolariciresinol diglucoside (SDG) is the most important lignan present in flaxseed (Singh et al. 2011; Strandås et al. 2008). Flaxseed is also a rich source of protein between 10.5 and 31 % (Hall et al. 2006; Singh et al. 2011).
Milling or grinding of flaxseed enhances more nutrient availability to the consumer; hence, seeds must be milled or ground to access their full nutritional value (Kuijsten et al. 2005). However, there are two major concerns when using milled flaxseed as a food ingredient. Flaxseed is a rich source of ALA with three double bonds. Since autoxidation reaction rate increases by increasing the number of double bonds present in a fatty acid, ALA is most affected by oxidation. So, damaged seeds are apt to develop rancidity, and development of objectionable odors and flavors result in rancidity (Schorno et al. 2010).
Another consideration in the application of various forms of flaxseeds in human foods is related to the presence of antinutritional cyanogenic glycosides compounds. Although flaxseed is the richest source of ALA and lignan, its daily intake and its consumption is limited due to the high content of antinutritional cyanogenic glycosides compounds (100–300 mg hydrogen cyanide/kg seed) (Hall et al. 2006; Niedźwiedź-Siegień and Gierasimiuk 2001). In the absence of qualitative toxicological data, the provisional tolerable daily intake has been set at 12 μg cyanide/kg body weight. With regard to the levels of cyanogenic glycosides, the intake of flaxseed should be limited to 10–20 g whole flaxseed per day, and the National Food Administration in Sweden advises against usage of crushed or milled flaxseeds since the bioavailability of cyanides increases with disintegration (Strandås et al. 2008).
Various methods could be used to reduce or remove the cyanogenic glycosides compounds in the flaxseed. Wanasundara et al. (1993) investigated the use of solvents extraction as a method to reduce cyanogenic glycosides content of flaxseed meal. They found that extraction of flaxseed with alkanol-ammonia-water/hexane resulted in enhanced removal of cyanogenic compounds from 41 to 16 mg/100 g (total cyanogenic glycosides as HCN equivalents) compared to using hexane alone. Also, they found that cyanogenic glycosides could be further removed by increasing solvent volumes, duration of the extraction period and number of extraction stages. Feng et al. (2003) reported that autoclaving and microwave heating reduced hydrogen cyanide of flaxseed by 29.7 and 83.2 %, respectively. Imran et al. (2013) found that cyanogenic compounds, measured as hydrocyanic acid (HCN), in full-fat flaxseed were reduced from 60.8 to 86.6 % using extrusion processing. Optimum extrusion conditions of barrel exit temperature, screw speed, and feed rate were found to be 143.6 °C, 133.5 rpm, and 57.8 kg/h for maximum (89.1 %) reduction of HCN.
Enrichment of flour and bread can improve bread nutritional value. Flaxseed is one of nutrients that can be used to fortify bread since it is a rich source of ALA, lignans, fiber and protein. Also, flaxseed has beneficial health effects and functional properties. Alpaslan and Hayta (2006) reported that with an increase in substitution ratio of ground flaxseed in bread, flavor decreased and crust color become darker. Koca and Anil (2007) studied the rheological properties of wheat flour blended with flaxseed flour. Their result showed that water absorption and stability of dough was increased, otherwise, extensibility of dough was decreased. Menteş et al. (2008) evaluated that effect of ground flaxseed on bread quality, staling, and unsaturated fatty acid composition. They reported that the use of ground flaxseed at a 10 % level markedly increased loaf volume, specific loaf volume and retarded bread staling. Also, they reported that during bread staling, the changes in peroxide values and fatty acid distribution were not significant. Khattab et al. (2012) observed that bread containing flaxseed had better texture and was rich in protein, fat, ash and fiber.
In this study, firstly we made an effort to reduce cyanogenic glycosides compounds in ground flaxseed by roasting them in a microwave oven, and then we reduced oxidation in ground flaxseed during storage at 25 °C by coating them. Finally the rheological properties of dough and physical properties of bread were assessed and optimum level of replacing flaxseed in bread formula was obtained in order to produce fortified Taftoon bread (Iranian traditional bread, that is flat, single layered and leavened with a round shape, 40 to 50 cm diameter and 3 to 5 mm thickness and flour extraction rate of 78 to 90 %.) containing flaxseed.
Material and method
Chemical composition
Wheat flour and flaxseed (brown variety) were purchased from local company. Arabic gum and ascorbic acid were purchased from Merck chemicals Co (Sepahan Teb, Isfahan). Hydrogenated fat was obtained from local factory (Nahal Rooz-Brojerd). Chemical properties of the wheat flour and flaxseed containing moisture (method 44-15); ash (method 08-01) and fiber (method 32-10) were determined by AACC methods (Committee 2000). Nitrogen content was measured by micro-Kjeldahl method and was converted to protein value by using a factor of 5.7. The total lipid content was determined by Soxhlet extraction using petroleum ether and reflux periods of 6 h.
Determination and removal of cyanogenic glycosides in flaxseed
Flaxseed was ground by a laboratory hammer grinder (Retschke 5657 Haan, West Germany) and sieved. Ground flaxseed was incubated for 18 h at 30 °C, and then microwave processing was performed with 600 W output, under the operating frequency of 2,450 MHz (Samsung, Korea). One hundred grams of flaxseed was laid on a 20 cm diameter glass tray and cooked under maximum output for 2.5 min.
As theoretically 1 μmol of the cyanogenic glycosides yields 1 μmol of HCN (hydrogen cyanide) (Kobaisy et al. 1996), it was considered that the amount of cyanogen glycosides in flaxseed can be determined by releasing HCN from the glycosides using enzymes (β-glucosidase) of freshly ground flaxseed. The HCN content in flaxseed in this study was determined by alkaline titration (Feng et al. 2003).
Preparation of different samples of ground flaxseed
Treated ground flaxseeds were coated with Arabic gum solution (10 %) and hydrogenated fat. In order to coat ground flaxseed with Arabic gum, ten percent w/v solution of Arabic gum was prepared by dissolving the powder in distilled water under gentle constant stirring and was stirred for at least 2 h at a constant temperature (25 °C), then it was allowed to hydrate for about 24 h at refrigerated temperature (4–8 °C). Ascorbic acid was added to solution 1 % w/v and stirred. Treated ground flaxseed was suspended in the solution with a proportion of 1 to 5 (ground flaxseed/ solution, w/w), and it was stirred for about 2 h. The solution was drained, and then dispersed on a Teflon plate. Finally, it was dried in oven at 30 °C for 8 h. In order to coat ground flaxseed with hydrogenated fat, the hydrogenated fat was gently heated until it became liquid. Then treated ground flaxseed was suspended in it and it was stirred for 30 min. After that it was drained and refrigerated. Treated ground flaxseed (GF), coated ground flaxseed with Arabic gum (CGFGA) and coated ground flaxseed with hydrogenated fat (CGFHF) were stored at 25 °C for 80 days. Oxidative stability during storage was characterized using peroxide value (PV) according to the Official Method, Cd 8b-90 (Society and Firestone 1989) and thiobarbituric acid (TBA) number using the method of Pokorny and Dieffenbacher (1989) on the oil extracted from the ground flaxseed using hexane for 5 h followed by solvent removal under vacuum at 40 °C.
Preparation of blended wheat flour/flaxseed
In order to assess the rheological characteristic of dough and baking bread, the wheat flour was replaced with ground flaxseed, coated ground flaxseed with Arabic gum and coated ground flaxseed with hydrogenated fat at 5, 15 and 25 % levels base of flour and it was blended.
Rheological characterization
In order to evaluate the effect of replacement of wheat flour with 5, 15 and 25 % of ground flaxseed, coated ground flaxseed with Arabic gum and coated ground flaxseed with hydrogenated fat on rheological characteristics of dough, farinograph test, expansion of dough and stress relaxation test were performed.
Farinograph characteristic
Water absorption, dough development time, dough stability, degree of softening and farinogeraph quality number were determined by farinograph (Brabender, Model No. 827504, and Duisburg, Germany) according to AACC method 54-21.
Expansion of dough
Expansion of dough during fermentation was obtained by measuring the difference in the height of dough during fermentation. One hundred grams of different dough samples were placed in a cylindrical can and then placed in a fermenter (30 °C and saturated relative humidity) for 1 h. Difference in the height of dough before and after fermentation was measured by ultrasonic. The height difference was multiplied by the area of the can, and increase in volume was obtained.
Stress relaxation
Stress relaxation characteristic of cylindrical sample was determined by compressing the sample up to a strain of 0.2 cm/cm at a compression rate of 100 mm/ min by a texture analyzer (TA INSTRUN, Model No 1140, England). When the compression was achieved at the desired level, the cross head surface was stopped, and the dough was allowed to relax. The force at different relaxation times was continuously monitored and the relaxation curve was plotted. The relaxation curve has been modeled by Eq. (1) and later normalized (Eq. 2) by Peleg and Normand (1983). The normalized model was applied by Bhattacharya and Narasimha (1997) on blackgram dough to calculate the stress decay. Also, Bhattacharya (2010) assessed the suitability of model on moth bean flour dough.
| 1 |
| 2 |
Where, F0 is the initial force, Ft is the decaying force after time t and k1 and k2 are constant parameters. According to this equation, the reciprocal of the constant kl indicates the initial decay rate, and the reciprocal of k2 is representative of a hypothetical asymptotic level of the normalized relaxation parameter [F(0) - F(t)]/F(0).k2 of Eq. (2) depicts the degree of solidity of dough, which can be between the value of k2~l and ∞, in which 1 is for a material that is liquid and ∞ for an ideal elastic solid where the stress does not relax at all (Peleg and Normand 1983). The constant of Eq. (2) was obtained by MATLAB software fitting the best curve based on the Eq. (2) on the plotted stress relaxation curve.
Preparation of bread
The bread was prepared by direct method. Control flour (wheat flour without adding flaxseed) and flour supplemented with GF, CGFGA and CGFHF at 5, 15 and 25 % level mixed with 1 % salt, 1 % yeast and water were determined by farinograph for 5 min. Different prepared dough samples were fermented for 1 h at 30 °C. After dividing, intermediate fermentation was performed for 10 min and then the dough was formed to round shape and baked at 270 °C for 4 min. Bread (round shape with 40 cm diameter) was cooled and stored at 25 °C in a plastic bag until testing time.
Texture of bread and staling test
The hardness of bread was measured using puncture test by texture analyzer equipped with a 50 N load cell (TA INSTRUN, Model No 1140, England). The force required to punch bread slice was measured with a TA probe (14 mm. diameter) at a speed of 100 mm/min, and maximum shear stress was calculated using Eq. (3):
| 3 |
Where, S is shear stress, F is force (N), D is diameter of probe and T is the thickness of bread. Texture measurements were performed at three points of each sample, and mean values were reported. In order to assess staling of bread texture, measurements were performed three times, 1, 48 and 72 h after baking.
Sensory analysis
The sensory acceptance test (crust color, aroma, flavor, texture and overall acceptance) for control and composite flour breads were evaluated using a 9-point hedonic scale (1 “disliked extremely”, 9 “liked extremely”), according to Stone and Sidel (2004). The sensory analysis was counted with 20 untrained panelists.
Statistical analyses
The data obtained by three replications were subjected to analysis of variance (ANOVA) and least significance difference (LSD) test to determine the difference between means at a significant level of p < 0.01.
Result and discussion
Chemical composition of flaxseed
Moisture, ash, crude fiber, oil and protein content of flaxseed were determined to be 4.78, 3.02, 22.51, 40.1 and 19.59 % respectively.
Determination and removal of cyanogenic glycosides compounds
The reported HCN contents in flaxseed vary greatly depending on cultivar variation and differences in detection methods (Hall et al. 2006). Although flaxseed has cyanogenic glycosides and decomposed enzyme since the cyanogenic glycosides and catabolizing enzymes are obviously kept apart within the intact seed, cyanide is only produced after mechanical tissue interruption is damaged by herbivore or fungal attack (Niedźwiedź-Siegień and Gierasimiuk 2001). In order to minimize cyanogenic glycosides compound of flaxseed, it was considered that the cyanogenic glycosides should first be enzymatically decomposed to HCN and then evaporated. The primary amount of hydrogen cyanide in flaxseed was 162.04 mg/1,000 g seed, which was decreased to 25.02 mg/1,000 after incubating and microwave roasting. This reduction could be the result of the heat deactivation of glycosidase, the evaporation of HCN after formation from hydrolysis, or both. Heat induces great formation of HCN, so in incubating section and microwave roasting, cyaonogenic glycosides were hydrolyzed to HCN, and then evaporated. A similar result was reported by Feng et al. (2003).
Oxidative stability
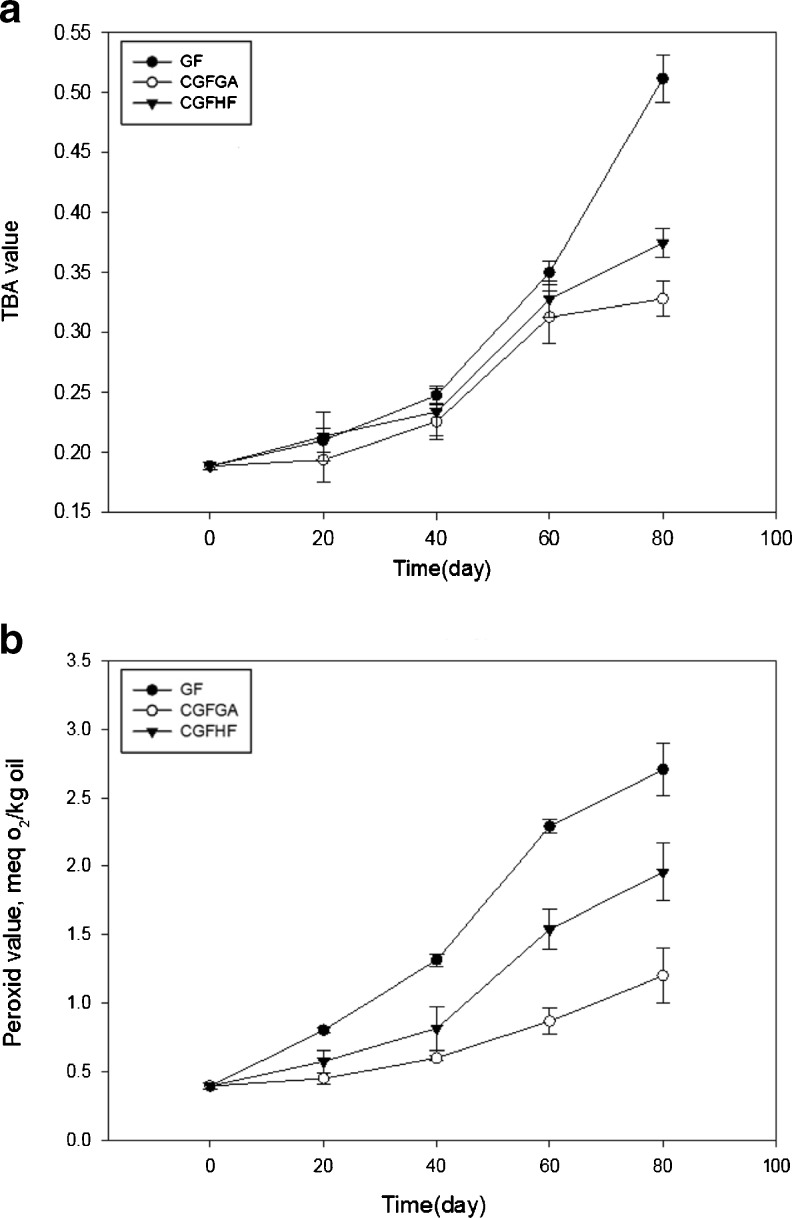
Peroxide value can be used as a measure of primary oxidation products in different foods (Menteş et al. 2008). Peroxides can degrade further into secondary oxidative products such as aldehydes and ketones to give additional loss to product sensory quality (Liu et al. 2010). Reaction between aldehydes and thiobarbituric acid is the base of measuring TBA value (Pokorny and Dieffenbacher 1989). Change to peroxide and TBA value of different samples (coated and uncoated ground flaxseed) over time were measured, and results are presented in Fig. 1. Ground flaxseed had the highest peroxide and TBA value over the storage period, and CGFGA had the lowest value. Peroxide values began to rise at day 20 in the GF (p < 0.01), whereas the CGFGA and CGFHF remained unchanged over the 40 days storage period (p < 0.01) (Fig. 1b). These results indicate that the production of significant levels of primary oxidative products (peroxides) occurred only in the GF. TBA values began to rise after 40 days for the GF (p < 0.01), whereas the CGFGA and CGFHF remained unchanged over the 60 days storage period (p < 0.01) (Fig. 1a). These results can be due to the role of coating material since Arabic gum and hydrogenated fat can form a film around ground flaxseed and keep it safe from oxygen attack. Liu et al. (2010) reported that encapsulation of flaxseed oil with gelatin-Arabic gum matrix decrease oxidation in flaxseed oil.
Fig. 1.
Changes to a TBA and b Peroxide value of ground flaxseed (GF), coated ground flaxseed with gum arabic (CGFGA) and coated ground flaxseed with hydrogenated fat (CGFHF) during storage at 25 °C for 80 days
Farinograph characteristics
The farinographic properties of dough with different levels of GF, CGFGA and CGFHF are presented in Table 1. Results showed that water absorption of dough incorporated with ground flaxseed was different from that in control dough. It was observed that replacing wheat flour with GF, CGFGA and CGFHF cause a decrease in water absorption. The lowest amount of water absorption was observed in replacing wheat flour with CGFHF. Also results showed that increasing flaxseed substitution ratio caused a decrease in water absorption value (Table 1). The decrease in water absorption was attributed to the decrease in gluten content. Furthermore, flaxseed is rich in oil, which can coat gluten and starch and reduce water absorption. Chin et al. (2010) noted that fat and shortening do not dissolve in water and reduce water absorption during dough making because it coats the gluten protein and starch granules, and prevents them from absorbing water to form the structure during the mixing process. Results showed that coating ground flaxseed causes more decrease in water absorption, which can be due to the coating material specially the hydrogenated fat.
Table 1.
Rheological characteristic of dough containing different percent of ground flaxseed (GF), coated ground flaxseed with gum arabic (CGFGA) and coated ground flaxseed with hydrogenated fat (CGFHF)
| Substitution level % | Farinograph characteristic | Expansion of dough during fermentation (cm3) | |||
|---|---|---|---|---|---|
| Water absorption % | Dough development time (min) | Stability time (min) | Degree of softening (Fu) | ||
| 0 | 61.5 | 3.00 bcd ± 0.2 | 2.30 bcde ± 0.14 | 128.5 a ± 4.95 | 1274.53 a ± 34.06 |
| 5 GF | 60.5 | 3.21abcd ± 0.12 | 2.2 cde ± 0.14 | 101.0 b ± 4.24 | 1128.62 d ± 31.74 |
| 15 GF | 60 | 3.23 abcd ± 0.15 | 2.55 abcd ± 0.07 | 71.0 c ± 9.89 | 1036.36 c ± 25.25 |
| 25 GF | 59.5 | 3.52 abc ± 0.08 | 2.70 abc ± 0.14 | 64.0 cd ± 7.07 | 939.80 f ± 25.74 |
| 5 CGFGA | 61 | 3.45 d ± 0.15 | 2.85 ab ± 0.63 | 110.5 ab ± 0.07 | 1141.50 dc ± 16.19 |
| 15 CGFGA | 59 | 3.50 abc ± 0.3 | 2.75 abc ± 0.7 | 75.5 c ± 6.36 | 1068.54 e ± 33.47 |
| 25 CGFGA | 57 | 3.90 ab ± 0.4 | 3.00 a ± 0. 14 | 62.5 cd ± 6.39 | 697.70 f ± 26.01 |
| 5 CGFHF | 58 | 2.8 cd ± 0.1 | 1.85 e ± 0.07 | 100.5 b ± 10.6 | 1188.68 bc ± 29.27 |
| 15 CGFHF | 55 | 3/00 bcd ± 0.2 | 2.05 de ± 0.35 | 73.5 c ± 6.36 | 1210.16 b ± 27.74 |
| 25 CGFHF | 52 | 3.85 a ± 0.15 | 2.35 bcde ± 0.21 | 45.0 d ± 2.82 | 1180.12 bc ± 31.75 |
Mean values in the same column followed by different superscript letters are significantly different (P < 0.01)
The results represent the averages of 3 determinations ± standard deviation
There was no significant (P < 0.01) increase in dough development time with added flaxseed as compared to the control dough (Table 1). It was observed that replacing wheat flour with GF, CGFGA and CGFHF increased dough development time, and the highest increase was observed in replacing it with CGFGA at the level of 25 %. The longer dough development time could result from the dilution of gluten. Also, it can be attributed to the increased fiber content associated with ground flaxseed, and it was difficult to mix fiber and wheat flour homogeneously. The stability value of flaxseed dough was comparable with that of the control dough and had no significant difference (p < 0.01). Results showed that replacing wheat flour with GF and CGFGA increased dough stability time, and that the highest amount was observed in replacing it with CGFGA and in 25 % replacing. Also, results showed increasing substitution ratio of flaxseed increases dough stability time (Table 1). It can be due to the effect of flaxseed gum. Chen et al. (2006) reported that addition of flaxseed gum to the dough causes the gluten continuous phase of dough to appear denser and more uniform than the control dough without flaxseed gum. Stability time of CGFHF dough is less than that in other samples, it can be due to the effect of hydrogenated fat that can coat gluten and interfere into the formation of gluten network. As the results showed, water absorption of CGFGA is less than GF, indicating that water absorption of arabic gum is less. So in this case, water is more available for glutenin and geliadin, and gluten network can form better, so stability time increases although there is no significant difference between dough containing GF with dough containing CGFGA (p < 0.01).
Expansion of dough
During mixing dough, gas is occluded and concentrated in the liquid phase of dough forming small gas bubbles. During fermentation, as a result of the yeast activity, glucose converts into carbon dioxide and ethanol. The produced carbon dioxide first saturates the dough aqueous liquor phase and then diffuses to pre-existing gas bubbles and so an initial expansion of dough can be observed (Pareyt et al. 2011). The results of the expansion of dough during fermentation are shown in Table 1. Results showed that with the replacement of wheat flour with GF and CGFGA, expansion decreased and had a significant difference (p < 0.01) with control dough. Also, it was observed that increasing the replacement level of wheat flour with GF and CGFGA causes more decrease in the expansion of dough. There was a significant difference (p < 0.01) between decrease in expansion of dough in 5, 15 and 25 % level of replacing wheat flour with GF and CGFAG. This result can be because of the ground flaxseed; the large size of particle (less than 1 mm) can decrease the expansion of gas bubbles. Also, because of the replacement of flaxseed, gluten content dilutes and the ability of gluten network to stabilize gas decreases. Borges et al. (2011) reported that the replacement of wheat flour with flaxseed causes lower bread dough ability to retain the fermentation gases. Replacing wheat flour with CGFHF causes a better increase in dough volume and has a significant difference from other samples during fermentation. It can be due to hydrogenated fat. As described above, hydrogenated fat that cannot be dissolved in water and whose solid fraction can coat gluten matrix during mixing and therefore in the proving stage, strengthens the gluten matrix and allows for more gas retention during proofing. As a result, the proofed loaf is less sensitive to mechanical shocks during transfer to the oven, and the increased gas retention is translates into more oven spring (Chin et al. 2010).
Stress relaxation
When dough is subjected to mechanical force, it shows a viscoelastic behavior. It means that by applying a mechanical force to the dough, the dimension of dough partially changes, and when the force is removed, the deformation is not fully reversed (Belton 2003).
The results of stress relaxation test are presented in Table 2. The reciprocal of the constant k1 indicates the initial decay rate. Thus, dough with a high k1 value indicates a low decay rate and shows elastic behavior (Bhattacharya et al. 2006). The results showed that the replacement of wheat bread with flaxseed causes a significant increase (p < 0.01) in F during relaxation and k1. Typically, by increasing the substitution ratio, these parameters increased. Higher F indicates solid behavior in dough, and higher k1 and k2 indicate that the elasticity of dough increased (Bhattacharya et al. 2006). These results can be due to flaxseed gum in flaxseed hull that can form gel, and can increase surface tension that causes an increase in the elasticity of dough (Qian et al. 2012). Furthermore, replacing wheat flour with flaxseed reduces water absorption and causes F and elasticity of dough to increase. Bhattacharya and Narasimha (1997) reported that low moisture dough (such as in the case of dough with 31.9 % moisture) resists more at higher moisture (39.7 %) dough yielding a high value of initial stress. An increase in moisture content decreased k1, and k2 (Bhattacharya and Narasimha 1997). Also, Bhattacharya et al. (2006) reported that an increase in moisture content decreased k1 values. Also, results showed that replacing wheat flour with CGFHF causes more F and k1 and have decreased lowering the F during relaxation. In addition, this replacing had a significant difference from the control dough and dough supplemented with GF and CGFGA. It is due to hydrogenated fat that is added to dough since hydrogenated fat decreases moisture in dough, and coated gluten that can reduce viscoelastic properties of dough. Fu et al. (1997) suggested that added fat actually delayed the onset of viscous flow, while simultaneously attenuating the short-time elastic properties of the gluten fraction of the dough.
Table 2.
Coefficients of Eq. 2 of dough containing different samples of ground flaxseed (GF), coated ground flaxseed with gum arabic (CGFGA) and coated ground flaxseed with hydrogenated fat (CGFHF) with different percent
| Substitution level % | F0 | K1(s) | K2 |
|---|---|---|---|
| 0 | 5.4 g ± 0.75 | 1.67 h ± 0.02 | 1.023 ef ± 0.005 |
| 5 GF | 6.69 fg ± 0.91 | 2.43 e ± 0.40 | 1.045 ef ± 0.019 |
| 15 GF | 9.30 d ± 0.55 | 2.21 f ± 0.02 | 1.096 bc ± 0.011 |
| 25GF | 11.25 ab ± 0.08 | 2.19 f ± 0.01 | 1.072 cd ± 0.005 |
| 5 CGFGA | 7.48 ef ± 0.83 | 2.07 g ± 0.01 | 1.003 f ± 0.077 |
| 15 CGFGA | 8.59 de ± 0.5 | 2.24 e ± 0.12 | 1.022 ef ± 0.005 |
| 25 CGFGA | 9.46 cd ± 0.67 | 2.69 d ± 0.01 | 1.074 bcd ± 0.006 |
| 5 CGFHF | 10.89 bc ± 0.85 | 4.17 c ± 0.09 | 1.044 de ± 0.006 |
| 15 CGFHF | 11.69 ab ± 0.82 | 5.32 b ± 0.09 | 1.106 b ± 0.038 |
| 25 CGFHF | 12.65 a ± 0.67 | 7.84 a ± 0.01 | 1.163 a ± 0.005 |
Mean values in the same column followed by different superscript letters are significantly different (P < 0.01)
The results represent the averages of 3 determinations ± standard deviation
Texture analyzer and staling test
The results showed that the flaxseed substitution caused a softer texture and delayed the bread staling (Table 3). The difference between control bread and breads made with 15 and 25 % substitution level of GF, CGFGA and CGFHF flour was statistically significant (p < 0.01). Softer texture of bread containing flaxseed can be due to the shortening in the flaxseed since shortening can soften the crumb of bread. Autio and Laurikainen (1997) reported this role of fat in bread. Also, results indicated that bread containing CFFGA and CGFHF had a softer texture than control bread and bread containing GF. It is the result of presence of Arabic gum and hydrogenated fat although no significant difference was observed between bread containing GF and CGFGA at similar levels of replacement. Hydrogenated fat can make the crumb of bread softer as described above, and it is the reason that a significant difference was observed between bread containing GF and CGFHF. Also, results indicated that the bread staling was delayed by substituting ground flaxseed flour and by increasing substitution ratio; staling was lower, which can be due to flaxseed gum and shortening in the flaxseed. Chen et al. (2006) reported that addition of flaxseed gum causes a gluten continuous phase of dough which appears denser and more uniform than dough without flaxseed gum. Starch granules are securely embedded in modified gluten network and therefore delay staling of bread. Shortening reduces moisture migration from center of the loaf to the drier outer crust region which causes it to lose its crispiness (Chin et al. 2010). Also, Rogers et al. (1988) suggested that the slower firming rate can be due to the interaction between shortening lipids and endogenous flour lipids. A similar result was reported by Menteş et al. (2008).
Table 3.
texture characteristics of bread produce d with different dough containing different percent of ground flaxseed (GF), coated ground flaxseed with gum arabic (CGFGA) and coated ground flaxseed with hydrogenated fat (CGFHF)
| Substitution level % | Maximum shear stress (N/Cm2) | |||
|---|---|---|---|---|
| 0 | 48 | 72 | ||
| 0 | 8.60 a ± 0.41 | 9.42 a ± 0.07 | 12.12 a ± 0.11 | Y = 1.76X + 6.52 |
| 5 GF | 8.13 a ± 0.44 | 8.83 ab ± 0.88 | 9.82 b ± 0.71 | Y = 0.84X + 7.23 |
| 15 GF | 6.82 bc ± 0.72 | 7.47 bcde ± 1.02 | 8.60 bcde ± 0.14 | Y = 0.89X + 5.85 |
| 25 GF | 6.53 bcd ± 0.27 | 7.64 abcde ± 0.89 | 8.56 bcde ± 0.46 | Y = 1.01X + 5.54 |
| 5 CGFGA | 8.34 a ± 0.28 | 8.54 abc ± 0.15 | 9.11 bc ± 0.39 | Y = 0.38X + 7.89 |
| 15 CGFGA | 7.08 b ± 0.58 | 7.67 abcde ± 1.88 | 8.72 bcd ± 1.48 | Y = 0.82X + 6.19 |
| 25 CGFGA | 5.75de ± 0.39 | 6.11 e ± 1.07 | 7.78 cdf ± 0.40 | Y = 1.01X + 4.52 |
| 5 CGFHF | 8.08 a ± 0.45 | 8.45 abcd ± 0.34 | 8.89 cde ± 0.11 | Y = 0.40X + 7.66 |
| 15 CGFHF | 5.98 cde ± 0.31 | 6.98 cde ± 0.87 | 7.41 ef ± 0.19 | Y = 0.71X + 5.36 |
| 25 CGFHF | 5.55 e ± 0.47 | 6.64 de ± 0.41 | 7.03 f ± 0.31 | Y = 0.73X + 4.93 |
Mean values in the same column followed by different superscript letters are significantly different (P < 0.01)
The results represent the averages of 3 determinations ± standard deviation
Also, slower firming rate was observed in bread supplemented with CGFGA and CGFHF that can be due to the presence of hydrogenated fat and Arabic gum used as a coated material. As described above, hydrogenated fat (shortening) can decrease the firming rate. Furthermore, hydrocolloids are able to modify gluten and starch properties, decrease retrogradation processes of starch and so decrease the firming rate (Bárcenas 2009).
Sensory evaluation
Table 4 presents the results obtained in the sensory acceptance test (color, aroma, flavor, texture and overall acceptance). Results showed that bread supplemented with ground flaxseed flour had better aroma, flavor and overall acceptance. By increasing the supplement ratio, these parameters received a better score. Bread containing GF, CGFGA and CGFHF at 5 % replacing obtained a better score by the panelists and the score decreased by increasing replacing level. Aroma of bread containing CGFGA and CGFHF received a better score than the control bread and bread containing GF. It can be due to the effect of coating ground flaxseed, which reduces oxidation and rancidity aroma in ground flaxseed. Best result was observed at 5 % replacing and bread containing CGFHF. Overall acceptance was better by replacing GF, CGFGA and CGFHF although it decreases with an increase in replacing ratio, and the best result was observed in replacing CGFHF.
Table 4.
Sensory characteristic of bread produce d with different dough containing different percent of ground flaxseed (GF), coated ground flaxseed with gum arabic (CGFGA) and coated ground flaxseed with hydrogenated fat (CGFHF)
| Substitution level % | Aroma | Flavor | Color | Texture | Overall acceptance |
|---|---|---|---|---|---|
| 0 | 5.0 b ± 1.7 | 6.2 cd ± 1.5 | 7.4 abc ± 0.7 | 6.1 i ±1.5 | 6.5 ab ± 0.7 |
| 5 GF | 6.5 ab ± 1.4 | 7.0 abcd ± 1.4 | 7.8 ab ± 1.1 | 7.7c ± 1.1 | 7.0 ab ± 1.1 |
| 15 GF | 6.8 ab ± 1.6 | 7.1 abcd ± 1.2 | 6.3 bcd ± 2.2 | 7.7 c ± 0.8 | 6.9 ab ± 1.0 |
| 25 GF | 5.9 b ± 1.1 | 6.3 bcd ± 1.1 | 5.3 d ± 2.2 | 7.4 f ± 0.98 | 6.0 b ± 1.4 |
| 5 CGFGA | 6.5 ab ± 1.4 | 7.2 abc ± 1.3 | 8.0 a ± 0.9 | 8.0 a ± 0.67 | 7.4 a ± 1.7 |
| 15 CGFGA | 6.0 ab ± 1.7 | 5.9 d ± 1.6 | 6.8 abcd ± 1.6 | 7.0 g ± 0.9 | 6.5 ab ± 1.9 |
| 25 CGFGA | 7.5 a ± 1.8 | 6.0 cd ± 2.4 | 6.3 bcd ± 2.5 | 6.7 h ± 1.5 | 6.0 b ± 2.2 |
| 5 CGFHF | 6.9 ab ± 1.0 | 7.5 ab ± 0.9 | 8.2 a ± 0.6 | 7.8 b ± 0.7 | 7.7 a ± 0.95 |
| 15 CGFHF | 6.8 ab ± 1.4 | 7.5 ab ± 0.8 | 6.9 abcd ± 1.4 | 7.5 e ± 1.0 | 7.0 ab ± 1.2 |
| 25 CGFHF | 7.1 ab ± 1.37 | 7.5 ab ± 0.8 | 5.8 cd ± 1.4 | 7.6 d ± 1.1 | 6.5 ab ± 1.7 |
Mean values in the same column followed by different superscript letters are significantly different (P < 0.01)
The results represent the averages of 3 determinations ± standard deviation
Conclusion
The result showed that incubating ground flaxseed at 30 °C for 18 h and microwaving reduced 84.6 % cyanogenic glycosides content of flaxseed.
Coated ground flaxseed with Arabic gum and hydrogenated fat had more oxidative stability during storage at 25 °C for 80 days. Peroxide and TBA values of coated samples had a significant difference (p < 0.01) from the control sample, and sample coated with Arabic gum was more stable against oxidation.
Enriched Taftoon bread with different samples of ground flaxseed was produced. Rheological test of dough illustrated that replacing wheat flour with GF, CGFGA and CGFH results in a decrease in water absorption, expansion of dough during fermentation, and an increase in dough development time, dough stability time and elasticity of dough.
Results indicated that bread containing flaxseed has a softer texture and lower staling rate compared to the control bread. By replacing wheat flour with CGFGA and CGFHF, bread had a better and softer texture and lower staling rate than bread containing GF. Results of organoleptic test showed that bread containing GF, CGFGA and CGFHF had better acceptance by panelists; and some characteristics of bread such as aroma, flavor, texture and overall acceptance achieved a better score compared to the control bread by them. Although by increasing substitution level, the score of these parameters of bread decreased, bread containing coated sample in 5 and 15 % substitution was more acceptable by panelists, and bread containing CGFHF was the best among different samples.
Finally, we concluded that replacing wheat flour with 5 and 15 % coated ground flaxseed with Arabic gum or hydrogenated fat produces enriched Taftoon bread with acceptable rheological and organoleptic characteristics.
References
- Alpaslan M, Hayta M. The effects of flaxseed, soy and corn flours on the textural and sensory properties of a bakery product. J Food Qual. 2006;29:617–627. doi: 10.1111/j.1745-4557.2006.00099.x. [DOI] [Google Scholar]
- Autio K, Laurikainen T. Relationships between flour/dough microstructure and dough handling and baking properties. Trends Food Sci Technol. 1997;8:181–185. doi: 10.1016/S0924-2244(97)01039-X. [DOI] [Google Scholar]
- Bárcenas ME. Influence of different hydrocolloids on major wheat dough components (gluten and starch) J Food Eng. 2009;94:241–247. doi: 10.1016/j.jfoodeng.2009.03.012. [DOI] [Google Scholar]
- Belton PS. The molecular basis of dough rheology. In: Cauvain SP, editor. Bread making: improving quality. England: Woodhead Publishing; 2003. pp. 266–311. [Google Scholar]
- Bhattacharya S. Stress relaxation behaviour of moth bean flour dough: product characteristics and suitability of model. J Food Eng. 2010;97:539–546. doi: 10.1016/j.jfoodeng.2009.11.014. [DOI] [Google Scholar]
- Bhattacharya S, Narasimha H. Puncture and stress relaxation behavior of blackgram (phaseolus mungo) flour‐based papad dough. J Food Process Eng. 1997;20:301–316. doi: 10.1111/j.1745-4530.1997.tb00424.x. [DOI] [Google Scholar]
- Bhattacharya S, Narasimha H, Bhattacharya S. Rheology of corn dough with Arabic gum: stress relaxation and two-cycle compression testing and their relationship with sensory attributes. J Food Eng. 2006;74:89–95. doi: 10.1016/j.jfoodeng.2005.02.006. [DOI] [Google Scholar]
- Borges JTS, Pirozi MR, de Paula CD, Ramos DL, Chaves JBP. Physicochemical and sensory evaluation of french-type bread containing flaxseed flour. B Ctr Pesqui Proc Al. 2011;29:83–96. [Google Scholar]
- Chen H, Xu S, Wang Z, Tian J. Effect of flaxseed gum on the rheology of dough and its application to noodle processing. Trans Chin Soc Agric Eng. 2006;22:166–169. [Google Scholar]
- Chin NL, Rahman RA, Hashim DM, Kowng SY. Palm oil shortening effects on baking performance of white bread. J Food Process Eng. 2010;33:413–433. doi: 10.1111/j.1745-4530.2008.00282.x. [DOI] [Google Scholar]
- Committee A.A.o.C.C.A.M (2000) Approved methods of the American Association of Cereal Chemists. AACC
- Cunnane S, Gaguli S, Menard C, Wolever T, Jenkins A. High a-linolenic acid flaxseed (Linurn usiiaiissimurn): some nutritional properties in humans. Br J Nutr. 1993;69:443–453. doi: 10.1079/BJN19930046. [DOI] [PubMed] [Google Scholar]
- Feng D, Shen Y, Chavez ER. Effectiveness of different processing methods in reducing hydrogen cyanide content of flaxseed. J Sci Food Agric. 2003;83:836–841. doi: 10.1002/jsfa.1412. [DOI] [Google Scholar]
- Fu J, Mulvaney S, Cohen C. Effect of added fat on the rheological properties of wheat flour doughs. Cereal Chem. 1997;74:304–311. doi: 10.1094/CCHEM.1997.74.3.304. [DOI] [Google Scholar]
- Hall C, Tulbek MC, Yingying X. Flaxseed. Adv Food Nutr Res. 2006;51:1–97. doi: 10.1016/S1043-4526(06)51001-0. [DOI] [PubMed] [Google Scholar]
- Imran M, Anjum FM, Butt MS, Siddiq M, Sheikh MA. Reduction of cyanogenic compounds in Flaxseed meal using thermal treatments. Int J Food Prop. 2013;16(8):1809–1818. doi: 10.1080/10942912.2011.608914. [DOI] [Google Scholar]
- Khattab R, Zeitoun M, Barbary OM. Evaluation of pita bread fortified with defatted flaxseed flour. Curr Nutr Food Sci. 2012;8:91–101. doi: 10.2174/157340112800840790. [DOI] [Google Scholar]
- Kobaisy M, Oomah B, Mazza G. Determination of cyanogenic glycosides in flaxseed by barbituric acid-pyridine, pyridine-pyrazolone, and high-performance liquid chromatography methods. J Agric Food Chem. 1996;44:3178–3181. doi: 10.1021/jf950838j. [DOI] [Google Scholar]
- Koca AF, Anil M. Effect of flaxseed and wheat flour blends on dough rheology and bread quality. J Sci Food Agric. 2007;87:1172–1175. doi: 10.1002/jsfa.2739. [DOI] [Google Scholar]
- Kuijsten A, Arts IC, van’t Veer P, Hollman PC. The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J Nutr. 2005;135:2812–2816. doi: 10.1093/jn/135.12.2812. [DOI] [PubMed] [Google Scholar]
- Liu S, Low N, Nickerson MT. Entrapment of flaxseed oil within gelatin-Arabic gum capsules. J Am Soc. 2010;87:809–815. [Google Scholar]
- Menteş Ö, Bakkalbaşşi E, Ercan R. Effect of the use of ground flaxseed on quality and chemical composition of bread. Food Sci Technol Int. 2008;14:299–306. doi: 10.1177/1082013208097192. [DOI] [Google Scholar]
- Niedźwiedź-Siegień I, Gierasimiuk A. Environmental factors affecting the cyanogenic potential of flax seedlings. Acta Physiol Plant. 2001;23:383–390. doi: 10.1007/s11738-001-0047-4. [DOI] [Google Scholar]
- Pareyt B, Finnie SM, Putseys JA, Delcour JA. Lipids in bread making: sources, interactions, and impact on bread quality. J Cereal Sci. 2011;54:266–279. doi: 10.1016/j.jcs.2011.08.011. [DOI] [Google Scholar]
- Peleg M, Normand M. Comparison of two methods for stress relaxation data presentation of solid foods. Rheol Acta. 1983;22:108–113. doi: 10.1007/BF01679835. [DOI] [Google Scholar]
- Pokorny J, Dieffenbacher A. Determination of 2-thiobarbituric acid value: direct method. Results of a collaborative study and the standardised method. J Pure Appl Chem. 1989;61:1165–1170. doi: 10.1351/pac198961061165. [DOI] [Google Scholar]
- Qian K, Cui S, Wu Y, Goff H. Flaxseed gum from flaxseed hulls: extraction, fractionation, and characterization. Food Hydrocoll. 2012;28:275–283. doi: 10.1016/j.foodhyd.2011.12.019. [DOI] [Google Scholar]
- Rogers D, Zeleznak K, Lai C, Hoseney R. Effect of native lipids, shortening, and bread moisture on bread firming. Cereal Chem. 1988;65:398–401. [Google Scholar]
- Schorno AL, Manthey FA, Hall CA. Effect of particle size and sample size on lipid stability of milled flaxseed (Linum usitatissimum L.) J Food Process Preserv. 2010;34:167–179. doi: 10.1111/j.1745-4549.2009.00463.x. [DOI] [Google Scholar]
- Singh K, Mridula D, Rehal J, Barnwal P. Flaxseed: a potential source of food, feed and fiber. Crit Food Sci Nutr. 2011;51:210–222. doi: 10.1080/10408390903537241. [DOI] [PubMed] [Google Scholar]
- Society A.O.C. Firestone D. Official methods and recommended practices of the American oil Chemists’ Society. Champaign: AOCS; 1989. [Google Scholar]
- Stone H, Sidel JL. Descriptive analysis. In: Stone H, Sidel JL, editors. Sensory evaluation practices. London: Academic; 2004. pp. 202–226. [Google Scholar]
- Strandås C, Kamal-Eldin A, Andersson R, Åman P. Phenolic glucosides in bread containing flaxseed. Food Chem. 2008;110:997–999. doi: 10.1016/j.foodchem.2008.02.088. [DOI] [PubMed] [Google Scholar]
- Touré A, Xueming X. Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bioactive compounds and health benefits. Compr Rev Food Sci Food Saf. 2010;9(3):261–269. doi: 10.1111/j.1541-4337.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- Wanasundara PKJPD, Amarowicz R, Kara MT, Shahidi F. Removal of cyanogenic glycosides of flaxseed meal. Food Chem. 1993;48(3):263–266. doi: 10.1016/0308-8146(93)90138-6. [DOI] [Google Scholar]