Abstract
Basil seed (Ocimum basilicum L.) has practical amounts of gum with good functional properties. In this work, extraction of gum from Basil seed was studied. Effect of pH, temperature and water/seed ratio on the kinetic and thermodynamic parameters; entropy, enthalpy and free energy of extraction were investigated. The maximum gum yield was 17.95 % at 50 °C for pH=7 and water/seed ratio 30:1. In this study, the experimental data were fitted to a mathematical model of mass transfer and equations constants were obtained. The kinetic of Basil seed gum extraction was found to be a first order mass transfer model. Statistical results indicated that the model used in this study will be able to predict the gum extraction from Basil seed adequately. It also found that ΔH and ΔS were positive and ΔG was negative indicating that the extraction process was spontaneous, irreversible and endothermic. The ΔH, ΔS and ΔG values were 0.26–7.87 kJ/mol, 8.12–33.2 J/mol K and 1.62–4.42 kJ/mol, respectively.
Keywords: Entropy, Enthalpy, Hydrocolloid, Kinetic, Mass transfer, Polysaccharides
Introduction
Gums are known as complex polysaccharides from various sources, e.g. endosperm of plant seeds (Guar gum), plant exudates (Tragacanth), tree or shrub exudates (Arabic, Karaya, and tragacanth gums), sea weed extracts (Agar), bacteria (Xanthan gum), and animal sources (chitin and chondroitin sulphate) (Williams and Phillips 2000).
According to the increasing demand for hydrocolloids, with specific functionality in the recent years, finding new hydrocolloid sources with appropriate properties is an active area of study. A large number of plants can produce the complex polysaccharides commercially known as plant-based gums. The plant gum exudates and seed gums are the complex polysaccharides/carbohydrate polymers commonly used as a dietary fiber, thickening agent, foaming agent, film, emulsifier, stabilizer and drug delivery agent. The hydrocolloids from seeds can be used extremely in food formulations because of their appropriate price, easy availability and proper functionality (Mirhosseini and Amid 2012; Razavi et al. 2012; Salehi and Kashaninejad 2015). Basil seed (Ocimum basilicum L.) is mucilaginous endemic plant which is grown in different regions of Asia, Europe, and Middle East, especially in various regions of Iran. This seed has reasonable amounts of gum with good functional properties which is comparable with commercial food hydrocolloids (Razmkhah et al. 2010). Some potential of the Basil seed gum as a new source of hydrocolloid have been recently investigated by Razmkhah et al. (2010).
Extraction process is one of the most widely used unit operations in food industry (Pinelo et al. 2006). Aqueous extraction is one of the most common techniques applied for the extraction of the seed mucilaginous material. The extraction yield of gum from seeds is influenced mainly by the conditions under which the process of liquid–solid extraction is carried out, using a solvent to separate a soluble fraction from a permeable solid. Kinetic study of solid liquid extraction depends on the nature of the seed, temperature of the process, solvent pH, reaction time and the ratio of the solid to the solvent (water/seed) (Amid and Mirhosseini 2012; Sulaiman et al. 2013).
The purpose of present study was to investigate the gum extraction process from Basil seed. This study also focuses on determination of the gum yield, effects of pH and temperature, reaction time and water/seed ratio. These optimum parameters were applied to a kinetic model based on mass transfer coefficient. Thermodynamic parameters which include entropy, enthalpy and Gibbs free energy of extraction of gum were also determined.
Materials and methods
Extraction of gum
Basil seeds were purchased from a local market in Gorgan, Iran. The cleaned Basil seeds were soaked in distilled water (for 0.5 to 25 min). In the extraction processes, there are multiple independent variables affecting the gum yield. The effect of water/seed ratio (20:1 and 30:1 at two levels), pH (5–9 at three levels) and temperature (20–80 °C at three levels) on the Basil seed gum extraction was studied. Separation of the hydrocolloid from the swollen seeds was achieved by passing the seeds through an extractor equipped with a rotating plate that scraped the gum layer on the seed surface (Salehi and Kashaninejad 2014). The extracted solution was then filtered and dried in an air forced oven at 50 °C (convection oven, Memmert Universal, Schwabach, Germany). The gum yield (gum content) of the Basil seed was calculated as below (Salehi et al. 2014; Salehi and Kashaninejad 2014):
| 1 |
Kinetic model
A relevant kinetic data is required to analyze and design a gum extraction process especially in industrial scale. In these experiments, we used mass transfer kinetic model to represent our experiment data. For this model we assumed that the main mechanism which controls the rate of extraction of gum is mass transfer of gum from the seed to the water. Mass transfer rate can be written as (Liauw et al. 2008):
| 2 |
where dWA/dt, mass transfer rate of the Basil seed gum (g/s); CA and CAi, concentration of Basil seed gum in distilled water at time t (g/m3) and at equilibrium (g/m3), respectively; k, mass transfer coefficient, ms−1; A, surface area for mass transfer process, m2.
Since the extraction was conducted in a batch process and the volume was constant throughout the experiment, Eq. (2) can be written as (Sulaiman et al. 2013):
| 3 |
| 4 |
Where, K, is volumetric mass transfer coefficient. To solve Eq. (4) by integration, following condition was used where the mass of Basil seed gum is zero in liquid (WA) at the beginning of the extraction process. Considering this condition, integration of Eq. (4) resulted as:
| 5 |
Rearranging Eq. (5) in terms of yield per mass of Basil seed gum, the kinetic model used in this study was:
| 6 |
Where, YA and YAi is yield of Basil seed gum in liquid at time, t (h) and equilibrium; and K, volumetric mass transfer coefficient. To determine the value of K (h−1) and YAi a log linear regression method was used to calculate numerically. Curve Expert program version 1.34 was used to fit the data in order to obtain the mass transfer value and the yield of gum.
Mass transfer within the seed
The study of mass transfer within the seed was conducted to determine that extraction of gum using water was controlled by the internal diffusion. Thiele modulus was used to investigate the mass transfer within the seed. To determine the value of Thiele modulus, effective diffusivity (Deff), m2 s−1 was calculated. Fick’s second law was used to determine effective diffusivity by assuming Deff is constant with the Y, yield at time, t and initial yield of the gum. Pinelo et al. (2006) calculated effective diffusivity using following equation:
| 7 |
Where, r is the radius of seeds diameter (m).
By using Eq. (7), a plot lnY versus time of experiment was plotted. The value of the slope was used to determine the effective diffusivity. This equation has been especially utilized by a large number of previous works to determine diffusivity values from experimental drying data (Palumbo et al. 1977), from leaching experiments (Schwartzberg and Chao 1982) or extraction process (Pinelo et al. 2006; Sulaiman et al. 2013).
To determine the effect of mass transfer within the seed on the extraction of the gum, Thiele Modulus, ϕ was calculated based on Giri and Sharma (2000) equation:
| 8 |
The value dp represents the seed diameter, K is the extraction rate, s−1, ρp is the density of the seed. The system is assumed to have no internal mass transfer limitation if the Thiele Modulus is <2, and the system suffers from the internal mass transfer limitation if it is above 10 (Giri and Sharma 2000).
Thermodynamic parameters
Thermodynamic parameters (ΔH, ΔS and ΔG) for the gum extraction of Basil seed using distilled water were estimated using following equations (Liauw et al. 2008):
| 9 |
| 10 |
Where F is equilibrium constant, YT is the yield percent of gum at temperature T, YU is the percent unextracted gum in Basil seed, R, is a gas constant, ΔH, (kJ/mol) is enthalpy, ΔS, (kJ/mol K) is entropy and ΔG, (kJ/mol) is a free energy of extraction. The plot of lnF against 1/T is used to find the value of ΔH and ΔG from the slope, and ΔS from intercept (Salehi and Kashaninejad 2015).
Statistical analysis
All measurements were conducted by triplicate and a completely randomized design was used for statistical analysis. The data was statistically analyzed by factorial analysis of variance (ANOVA) at the 0.05 level of significance. The statistical software used to evaluate the experimental design results was Minitab (Version 16, Minitab Inc, Launcher).
Results and discussion
In general, the extraction yield was influenced by the interaction effect of aqueous extraction variables. The maximum value of gum obtained from extraction of Basil seed were 17.95 % at 50 °C, pH=7 and water to seed 30 to 1. This value was greater than the extraction yield reported for Flaxseed gum 7.9 % (Cui et al. 1994) and Yanang gum 4.54 % (Singthong et al. 2009).
The ANOVA and p-value significant levels were used to check the factors effect significance on the gum extraction yield (Table 1). Table 1 depicts the sum of squares (SS) used to estimate the factors’ effect and the F-ratios defined as the ratio of the respective mean square effect and the mean-square-error, respectively. From the p value, each factor main effect seems to be statistically significant at p < 0.05. A regression equation based on the studied coefficients is represented as follows:
| 11 |
Table 1.
Analysis of variance for gum extraction process of Basil seed
| Source | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| pH | 2 | 31.14 | 15.57 | 778.72 | 0.000 |
| T | 2 | 7.14 | 3.57 | 178.51 | 0.000 |
| R | 1 | 0.68 | 0.68 | 34.19 | 0.000 |
| pH × T | 4 | 3.30 | 0.82 | 41.26 | 0.000 |
| pH × R | 2 | 0.51 | 0.25 | 12.74 | 0.000 |
| T × R | 2 | 0.23 | 0.12 | 5.81 | 0.011 |
| pH × T × R | 4 | 0.35 | 0.08 | 4.47 | 0.011 |
| Error | 18 | 0.36 | 0.02 | ||
| Total | 35 | 43.73 |
T temperature, R water/seed ratio, DF degree of freedom, SS sum of squares, MS mean squares, F fhactor F, p probability
Although, the gum extraction yield was related to the single main effects of all aqueous extraction variables, but it was positively proportional to the interaction effects of pH with water/seed ratio and temperature (Amid and Mirhosseini 2012).
Effect of pH
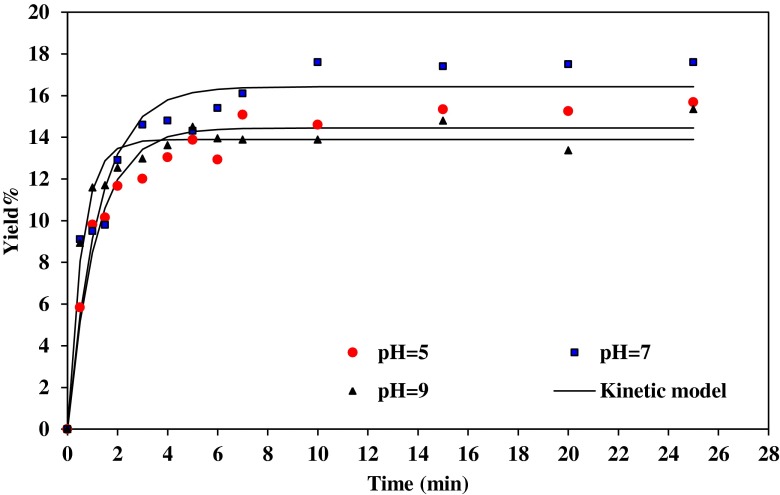
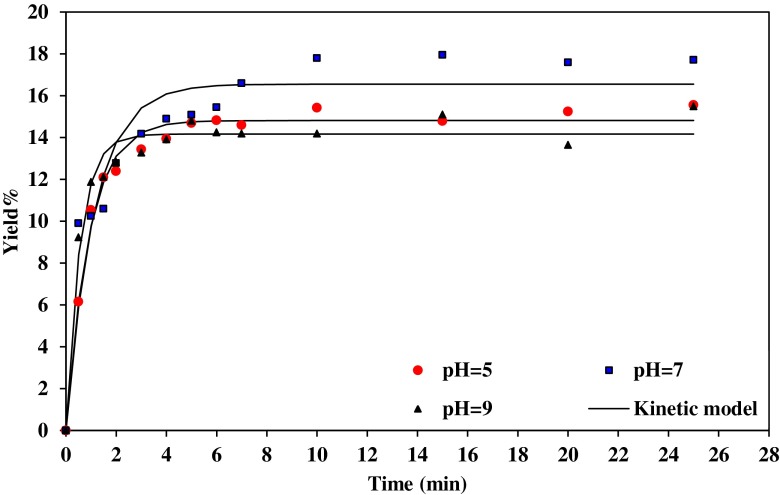
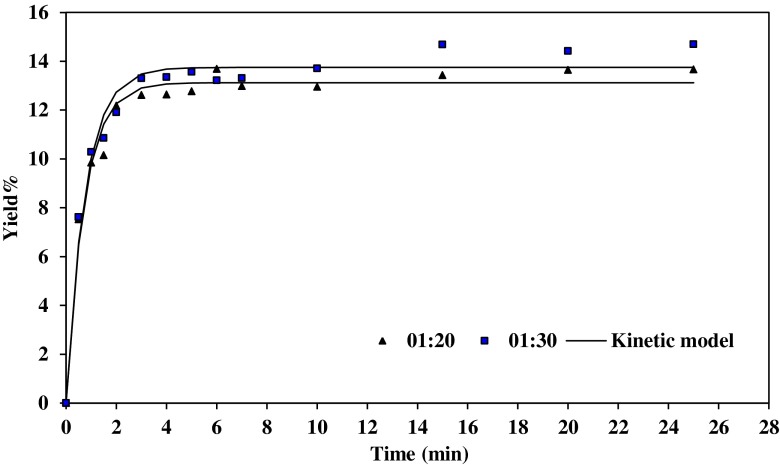
Figures 1 and 2, illustrates the gum extraction yield of Basil seed at water/seed ratio 20:1 and 30:1, respectively, at different pH and 50 °C. The extraction rate was rapid at the beginning of the process and slows gradually because when the seed was exposed to the fresh water, the gum on the surface of the seed was solubilized and gum gets extracted quickly. This causes fast increase in extraction rate. At the initial extraction rate, the gum concentration was low in the solution and mass transfer effect causes the gum to diffuse quickly from the seed to the water. When the maximum amount of extractable gum was reached, the gum yield remains the same even after extending the extraction time.
Fig. 1.
Effect of pH on gum extraction process at 50°c and water/seed ratio 20:1, experimental and the predicted values
Fig. 2.
Effect of pH on gum extraction process at 50 °C and water/seed ratio 30:1, experimental and the predicted values
The graph illustrates that more carbohydrates were extracted in neutral water. The highest value of gum was obtained in the pH=7. The gum yield was decreased averagely from 16.2 to 14.5 %, with decrease in pH from 7 to 5 (water/seed ratio 30:1).
Wu et al. (2007) also reported that the optimum conditions for the extraction procedure of crude polysaccharides from boat-fruited sterculia seeds were pH equal 7.
Tables 2 and 3 shows the calculated values of the mass transfer coefficients (K) and the equilibrium yield of gum (YAi), at various temperatures, pH and water/seed ratio using Eq. 6. Parameters of kinetic model, YAi and K were estimated by non linear square fit from equation to experimental data using Curve Expert 1.34.
Table 2.
Calculated parameters for gum extraction process of Basil seed at water/seed ratio 20:1
| pH | Temperature (°C) | K (h−1) | YAi (wt%) | R |
|---|---|---|---|---|
| 5 | 20 | 51.99 | 13.57 | 0.993 |
| 5 | 50 | 52.95 | 14.45 | 0.977 |
| 5 | 80 | 81.99 | 13.12 | 0.988 |
| 7 | 20 | 34.19 | 14.46 | 0.989 |
| 7 | 50 | 48.75 | 16.43 | 0.956 |
| 7 | 80 | 79.06 | 14.74 | 0.942 |
| 9 | 20 | 72.44 | 13.71 | 0.958 |
| 9 | 50 | 104.41 | 13.89 | 0.981 |
| 9 | 80 | 67.49 | 13.71 | 0.986 |
Table 3.
Calculated parameters for gum extraction process of Basil seed at water/seed ratio 30:1
| pH | Temperature (°C) | K (h−1) | YAi (wt%) | R |
|---|---|---|---|---|
| 5 | 20 | 48.11 | 14.00 | 0.971 |
| 5 | 50 | 64.92 | 14.82 | 0.993 |
| 5 | 80 | 78.19 | 13.75 | 0.986 |
| 7 | 20 | 45.13 | 16.30 | 0.985 |
| 7 | 50 | 53.49 | 16.56 | 0.951 |
| 7 | 80 | 84.39 | 14.61 | 0.971 |
| 9 | 20 | 74.74 | 14.06 | 0.956 |
| 9 | 50 | 108.17 | 14.17 | 0.983 |
| 9 | 80 | 72.57 | 13.96 | 0.986 |
The mass transfer coefficient values were 34.19–104.41 (h−1) and 48.11–108.17 (h−1), at water/seed ratio 20:1 and 30:1, respectively.
Effect of temperature
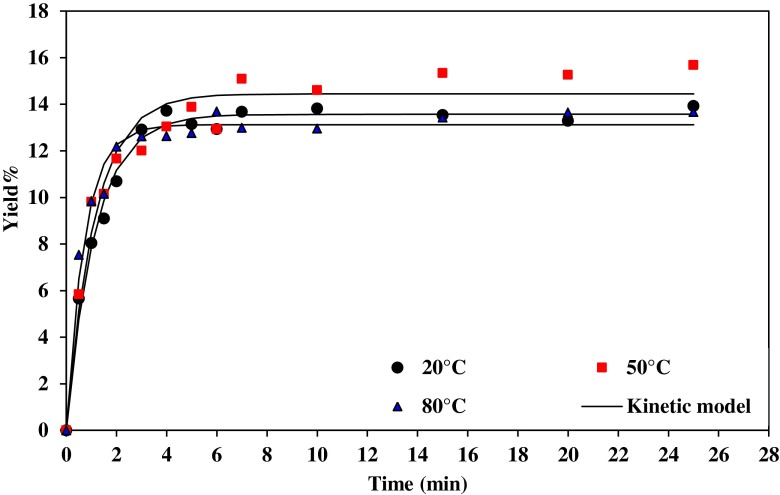
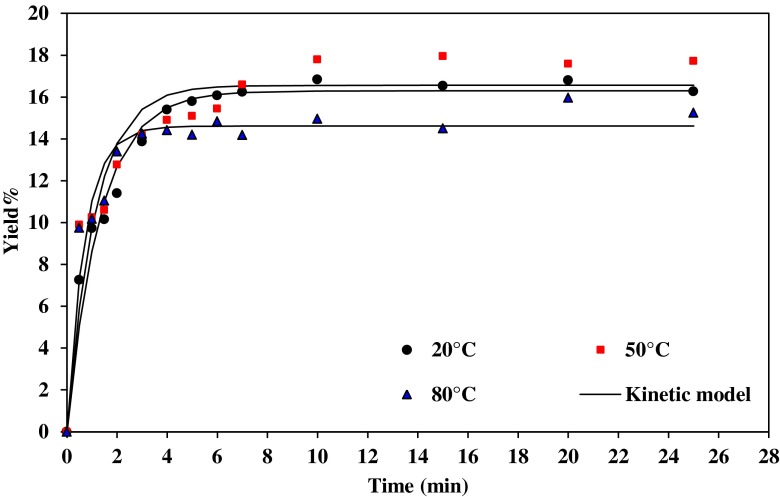
Figures 3 and 4, illustrates the gum extraction yield of Basil seed at water/seed ratio 20:1 and 30:1, respectively, at different temperature and pH=5. The increase in temperature increases the gum yield during extraction process. Increasing the temperature causes the reaction time to be reduced as extraction occurs faster. The gum yield was increased from 15.10 to 15.58 %, with increase in temperature from 20 to 50 °C but it decreased from 15.58 to 14.4 %, with increase in temperature from 50 to 80 °C (water/seed ratio 20:1). Temperature as an independent variable increases the ability of the solvent to solubilize the compounds and, decreasing the viscosity of the liquid solvent which is allowing better penetration of the solvent into the solid matrix (Eikani et al. 2012). Mazza and Biliaderis (1989) reported that temperature was the major factor affecting the extraction yields of flaxseed gum irrespective of the changes in pH or water to seed ratio. In addition, Wu et al. (2007) found that the extraction temperature and time were the most important factors affecting the response variables (extraction yield, purityand relative viscosity of crude polysaccharides). This result is in agreement with reports of other authors in extracting polysaccharides (Vinogradov et al. 2003; Yin and Dang 2008). The extraction at the elevated temperature resulted in faster and easier mass transfer of water-soluble polysaccharide from the cell wall into the extract (Amid and Mirhosseini 2012).
Fig. 3.
Effect of temperature on gum extraction process at pH=5 and water/seed ratio 20:1
Fig. 4.
Effect of temperature on gum extraction process at pH=5 and water/seed ratio 30:1
Although the extraction yield of polysaccharides was also high at 100 °C, increasing temperature will bring about the increase in cost for the extraction process from the industrialization point of view (Ye and Jiang 2011).
At higher temperatures, the viscosity of mucilage decreases and makes the slurry less sticky and the mucilage can be easily released. As a result, the mass transfer coefficient of the extraction process increases with temperature thus effecting the diffusion and viscosity. It is found that the mass transfer coefficients increase with the increase in the temperature of extraction (Tables 2 and 3). The K (h−1) value varies from 74.74 to 108.17 for temperature from 20 to 50 °C (pH=9 and water/seed ratio 30:1). The increase of the polysaccharides diffusion coefficient and the enhanced solubility of the polysaccharides in the extracting solvent at higher temperatures caused the increase of the polysaccharides mass going out from the mushroom particles into the solution (Ye and Jiang 2011).
The extraction coefficient increased with increasing the extraction temperature due to the increase of the polysaccharides solubility (Braga et al. 2006).
Effect of water/seed ratio
Figure 5 shows the effect of water/seed ratio and extraction time on extraction of Basil seed gum at 80 °C and pH=5. Water to seed ratio will significantly affect extract yield. If ratio of water to seed is too small, gums in Basil seed cannot be completely extracted up. If ratio of water to seed is too big, this will cause high process cost. The gum yield was increased averagely from 14.75 to 15.03 %, with increase in water/seed ratio from 20:1 to 30:1 under same conditions.
Fig. 5.
Effect of water/seed ratio on gum extraction process at 80°c and pH=5
Sepulveda et al. (2007) reported that when the volume ratio of water to seeds was increased, a greater mucilage yield obtained from Opuntia spp. seeds. Bendahou et al. (2007) reported that the extraction yield of polysaccharides significantly increases as the ratio of water to seed was increased, which could be due to an increased driving force for the mass transfer of the polysaccharides. Singthong et al. (2009) also reported a higher extraction yield for Yanang leaves gum at a low ratio of solid to water.
The mass transfer coefficient (K) increases around 6.14 % when water/seed ratio increased from 20:1 to 30:1 (Tables 2 and 3). The availability of high liquid content which led to an increase in the driving force of mucilage out of the seeds into the extract. Therefore, the presence of insufficient amount of water or high amount of seeds may reduce the mass transfer coefficient.
Response surface methodology (RSM) was applied to optimize the extraction of crude polysaccharides from boat-fruited sterculia seeds by Wu et al. 2007. They reported that extraction temperature, pH, extraction time and water to seed ratio were found to have a significant influence on the yield and purity of the extracted crude polysaccharides.
Mass transfer within the seed
Effect of mass transfer within the seed was studied to determine if the diffusion to the surface controls and water diffuses well into the seeds. The following data was used in Eqs. (7 and 8) in order to calculate the Thiele Modulus, density of Basil seed: 1.038 g/cm3, effective diffusivity data (Table 4) and average value of seed diameter, dp =1.37 mm.
Table 4.
Effective diffusivity and thiele modulus on the internal mass transfer
| pH | Temperature (°C) | 20:1 | 30:1 | ||
|---|---|---|---|---|---|
| Deff (m2 s−1) | ϕ | Deff (m2 s−1) | ϕ | ||
| 5 | 20 | 5.85 × 10−10 | 19.21 | 5.78 × 10−10 | 18.59 |
| 5 | 50 | 5.88 × 10−10 | 19.34 | 6.07 × 10−10 | 21.07 |
| 5 | 80 | 5.90 × 10−10 | 24.02 | 5.94 × 10−10 | 23.38 |
| 7 | 20 | 7.16 × 10−10 | 14.08 | 6.14 × 10−10 | 17.47 |
| 7 | 50 | 7.24 × 10−10 | 16.72 | 6.14 × 10−10 | 19.02 |
| 7 | 80 | 7.16 × 10−10 | 21.41 | 9.01 × 10−10 | 19.72 |
| 9 | 20 | 5.92 × 10−10 | 22.54 | 7.13 × 10−10 | 20.86 |
| 9 | 50 | 6.03 × 10−10 | 26.81 | 6.07 × 10−10 | 27.20 |
| 9 | 80 | 7.01 × 10−10 | 19.99 | 5.94 × 10−10 | 22.52 |
Effective diffusivity values can be strongly affected by conditions under which the extraction process is carried out. Temperature, for instance, was reported to significantly increase diffusivity (Loncin and Merson 1979). The results shows that the effective diffusivity values were 5.78 × 10−10 m2s−1–9.01 × 10−10 m2s−1.
Table 4 also represents the Thiele Modulus calculate for the Basil seed gum solution at 20:1 and 30:1 water/seed ratio at temperature 20–80 °C and pH of 5, 7 and 9. Thiele Modulus ranges between 14.08 and 27.20. The value of Thiele Modulus indicates water diffuses with reacting. The reaction increases the internal mass transfer diffusion limitation (Giri and Sharma 2000). However, the system was affected by the mass transfer within the seed. This was due to the value of the Thiele modulus, which was >10.
Thermodynamic parameters
Table 5 show the values of equilibrium constant and thermodynamic parameters for the gum extraction process of Basil seed. The values of the enthalpy were in the range of 0.26–7.87 kJ/mol. The value obtained was in the range (4–13.5 kJ/mol) obtained by other researches for extraction of melon, rubber seed and olive cake oil (Ibemesi and Attah 1990; Meziane and Kadi 2008). Positive enthalpy change indicates the endothermic nature of the extraction process and requires energy during the process.
Table 5.
Thermodynamic parameters for gum extraction process of Basil seed
| 1:20 | 1:30 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | T (K) | F | ΔH, kJ/mol | ΔS, kJ/mol K | ΔG, kJ/mol | F | ΔH, kJ/mol | ΔS, kJ/mol K | ΔG, kJ/mol |
| 5 | 293 | 1.94 | 7.87 | 3.23 × 10−2 | −1.62 | 2.35 | 4.46 | 2.22 × 10−2 | −2.08 |
| 5 | 323 | 2.60 | −2.57 | 2.78 | −2.74 | ||||
| 5 | 353 | 1.88 | −1.86 | 2.16 | −2.25 | ||||
| 7 | 293 | 3.79 | 6.03 | 3.16 × 10−2 | −3.24 | 4.08 | 6.29 | 3.32 × 10−2 | −3.42 |
| 7 | 323 | 4.75 | −4.18 | 5.18 | −4.42 | ||||
| 7 | 353 | 3.08 | −3.30 | 2.69 | −2.91 | ||||
| 9 | 293 | 2.21 | 0.52 | 8.35 × 10−3 | −1.93 | 2.39 | 0.26 | 8.12 × 10−3 | −2.12 |
| 9 | 323 | 2.26 | −2.19 | 2.41 | −2.36 | ||||
| 9 | 353 | 2.19 | −2.30 | 2.28 | −2.42 | ||||
Entropy of the solution increases due to the gum molecules extraction. The positive value of entropy change describes the process is irreversible (Amin et al. 2010). The values of the entropy were in the range of 8.12–33.2 J/mol K.
The free energy values lie between 1.62 and 4.42 kJ/mol. This shows that the energy required to break the solute–solute and solvent–solvent interactions are less than the energy released in solute–solvent interactions.
Conclusions
Extraction of gum from native seeds and its use in the formulation of food products has recently become very attractive. Basil seed has practical amounts of gum with good functional properties which is comparable with commercial food hydrocolloids. In this study the effect of extraction temperature (at three levels, 20, 50 and 80 °C), pH (at three levels, 5, 7 and 9) and the ratio of water to seed (at two levels, 20 to 1 and 30 to 1) on mass transfer kinetics of Basil seed gum extraction were studied. The results showed that increasing the temperature from 20 to 50 °C, increased the gum extraction about 4.8 %. With increasing the ratio of water to seed, the rate of extraction of gum was also increased. The maximum value of gum obtained from extraction of Basil seed were 17.95 % at 50 °C, pH=7 and water to seed 30 to 1.
In this study, the experimental data were fitted to a mathematical model of mass transport and equations constants were obtained. The K (h−1) value varies from 34.19 to 108.17. Statistical results indicated that the model used in this study will be able to predict the gum extraction from Basil seed adequately and is probably useful for other gum-containing seeds. Thiele modulus value shows that the gum extraction was affected by the mass transfer within the particle.
The ΔH, ΔS and ΔG values were 0.26–7.87 kJ/mol, 8.12–33.2 J/mol K and 1.62–4.42 kJ/mol, respectively shows that the extraction process was spontaneous, irreversible and endothermic based on thermodynamic parameters.
References
- Amid BT, Mirhosseini H. Optimisation of aqueous extraction of gum from durian (Durio zibethinus) seed: a potential, low cost source of hydrocolloid. Food Chem. 2012;132(3):1258–1268. doi: 10.1016/j.foodchem.2011.11.099. [DOI] [PubMed] [Google Scholar]
- Amin S, Hawash G, Diwani E, El Rafei S. Kinetics and thermodynamics of oil extraction from jatropha curcas in aqueous acidic hexane solutions. J Am Sci. 2010;6(11):8. [Google Scholar]
- Bendahou A, Dufresne A, Kaddami H, Habibi Y. Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohydr Polym. 2007;68:601–608. doi: 10.1016/j.carbpol.2006.10.016. [DOI] [Google Scholar]
- Braga MEM, Moreschi SRM, Meireles MAA. Effects of supercritical fluid extraction on Curcuma longa L. and Zingiber officinale R. starches. Carbohydr Polym. 2006;63:340–346. doi: 10.1016/j.carbpol.2005.08.055. [DOI] [Google Scholar]
- Cui W, Mazza G, Oomah BD, Biliaderis CG. Optimization of an aqueous extraction process for flaxseed gum by response surface methodology. LWT-Food Sci Technol. 1994;27:363–369. doi: 10.1006/fstl.1994.1074. [DOI] [Google Scholar]
- Eikani MH, Golmohammad F, Homami SS. Extraction of pomegranate (Punica granatum L.) seed oil using superheated hexane. Food Bioprod Process. 2012;90(1):32–36. doi: 10.1016/j.fbp.2011.01.002. [DOI] [Google Scholar]
- Giri CC, Sharma DK. Mass-transfer studies of solvent extraction of coals in N-methyl-2-pyrrolidone. Fuel. 2000;79(5):577–585. doi: 10.1016/S0016-2361(99)00152-0. [DOI] [Google Scholar]
- Ibemesi J, Attah J. Temperature effects on the extraction of rubber and melon seed oils. J Am Oil Chem Soc. 1990;67(7):443–445. doi: 10.1007/BF02638958. [DOI] [Google Scholar]
- Liauw MY, Natan FA, Widiyanti P, Ikasari D, Indraswati N, Soetaredjo FE. Extraction of neem oil (Azadirachta indica A. Juss) using n-hexane and ethanol: studies of oil quality, kinetic and thermodynamic. ARPN J Eng Appl Sci. 2008;3(3):6. [Google Scholar]
- Loncin M, Merson RL. Food engineering. Principles and selected applications. New York: Academic Press Inc; 1979. p. 494. [Google Scholar]
- Mazza G, Biliaderis CG. Functional properties of flaxseed mucilage. J Food Sci. 1989;54:1302–1305. doi: 10.1111/j.1365-2621.1989.tb05978.x. [DOI] [Google Scholar]
- Meziane S, Kadi H. Kinetics and thermodynamics of oil extraction from olive cake. J Am Oil Chem Soc. 2008;85(4):391–396. doi: 10.1007/s11746-008-1205-2. [DOI] [Google Scholar]
- Mirhosseini H, Amid BT. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res Int. 2012;46:387–398. doi: 10.1016/j.foodres.2011.11.017. [DOI] [Google Scholar]
- Palumbo SA, Komanowsky M, Metzger V, Smith JL. Kinetics of pepperoni drying. J Food Sci. 1977;42:1030–1033. doi: 10.1111/j.1365-2621.1977.tb12660.x. [DOI] [Google Scholar]
- Pinelo M, Sineiro J, Núñez MJ. Mass transfer during continuous solid– liquid extraction of antioxidants from grape byproducts. J Food Eng. 2006;77(1):57–63. doi: 10.1016/j.jfoodeng.2005.06.021. [DOI] [Google Scholar]
- Razavi SMA, Mohammadi Moghaddam T, Emadzadeh B, Salehi F. Dilute solution properties of wild sage (Salvia macrosiphon) seed gum. Food Hydrocoll. 2012;29:205–210. doi: 10.1016/j.foodhyd.2012.02.020. [DOI] [Google Scholar]
- Razmkhah S, Razav SMA, Behzad K, Mazaheri Tehrani M. The effect of pectin, sage seed gum and basil seed gum on physicochemical and sensory characteristics of non fat concentrated yoghurt. Iran Food Sci Technol Res J. 2010;6(1):27–36. [Google Scholar]
- Salehi F, Kashaninejad M. Effect of different drying methods on rheological and textural properties of Balangu seed gum. Dry Technol. 2014;32(6):720–727. doi: 10.1080/07373937.2013.858264. [DOI] [Google Scholar]
- Salehi F, Kashaninejad M (2015) Kinetics and thermodynamics of gum extraction from wild sage seed. Int J Food Eng. doi:10.1515/ijfe-2014-0079
- Salehi F, Kashaninejad M, Behshad V. Effect of sugars and salts on rheological properties of Balangu seed (Lallemantia royleana) gum. Int J Biol Macromol. 2014;67:16–21. doi: 10.1016/j.ijbiomac.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Schwartzberg HG, Chao RY. Solute diffusivities in leaching processes. Food Technol. 1982;36:73–86. [Google Scholar]
- Sepulveda E, Sanez C, Aliaga E, Aceituno C. Extraction and characterization of mucilage in Opuntia spp. J Arid Environ. 2007;68:534–545. doi: 10.1016/j.jaridenv.2006.08.001. [DOI] [Google Scholar]
- Singthong J, Ningsanond S, Cui SW. Extraction and physicochemical characterisation of polysaccharide gum from Yanang (Tiliacora triandra) leaves. Food Chem. 2009;114:1301–1307. doi: 10.1016/j.foodchem.2008.11.008. [DOI] [Google Scholar]
- Sulaiman S, Aziz ARA, Aroua MK. Optimization and modeling of extraction of solid coconut waste oil. J Food Eng. 2013;114:228–234. doi: 10.1016/j.jfoodeng.2012.08.025. [DOI] [Google Scholar]
- Vinogradov EV, Brade L, Brade H, Holst O. Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter baumannii strain 24. Carbohydr Res. 2003;338:2751–2756. doi: 10.1016/j.carres.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Williams PA, Phillips GO. Introduction to food hydrocolloids. In: Phillips GO, Williams PA, editors. Handbook of hydrocolloids. Boca Raton: CRC Press; 2000. pp. 1–19. [Google Scholar]
- Wu Y, Cui SW, Tang J, Gu X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007;105:1599–1605. doi: 10.1016/j.foodchem.2007.03.066. [DOI] [Google Scholar]
- Ye C, Jiang CJ. Optimization of extraction process of crude polysaccharides from Plantago asiatica L. by response surface methodology. Carbohydr Polym. 2011;84:495–502. doi: 10.1016/j.carbpol.2010.12.014. [DOI] [Google Scholar]
- Yin G, Dang Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr Polym. 2008;74:603–610. doi: 10.1016/j.carbpol.2008.04.025. [DOI] [Google Scholar]