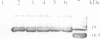Abstract
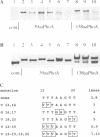
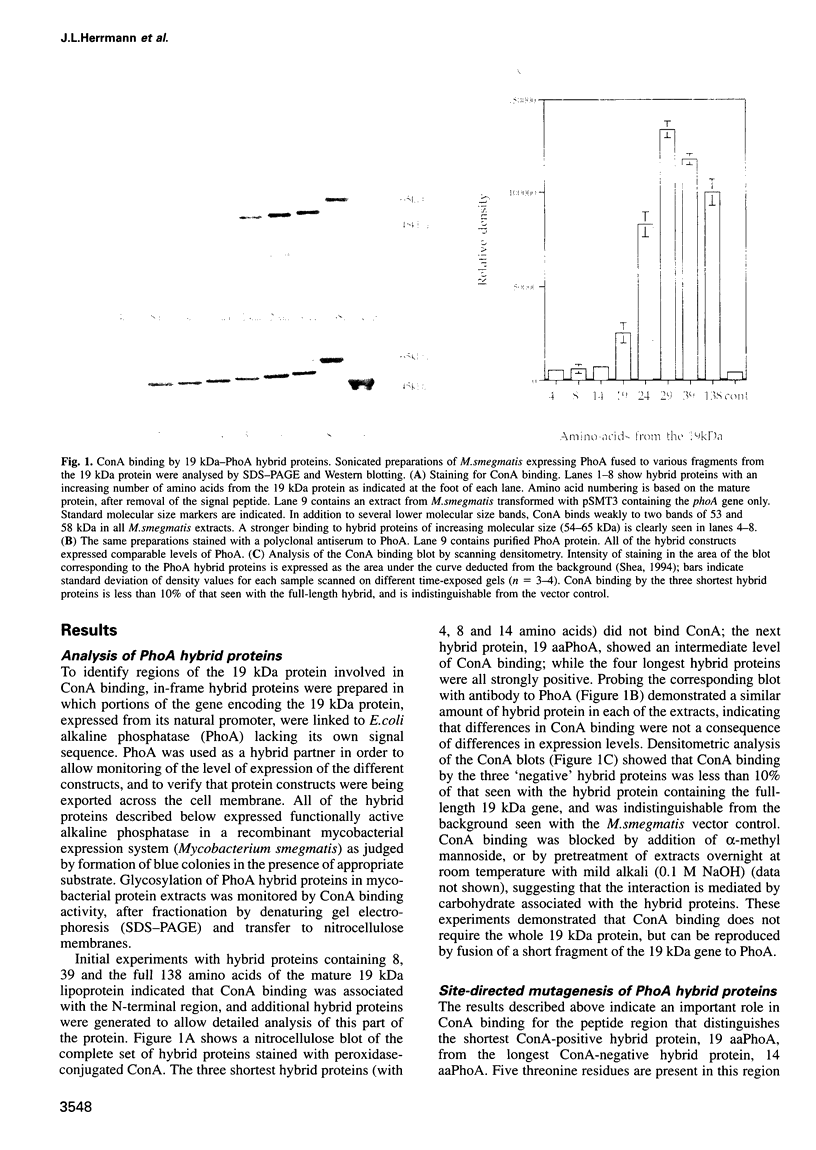
Protein glycosylation has an important influence on a broad range of molecular interactions in eukaryotes, but is comparatively rare in bacteria. Several antigens from Mycobacterium tuberculosis, the causative agent of human tuberculosis, have been identified as glycoproteins on the basis of lectin binding, or by detailed structural analysis. By production of a set of alkaline phosphatase (PhoA) hybrid proteins in a mycobacterial expression system, the peptide region required for glycosylation of the 19 kDa lipoprotein antigen from M.tuberculosis was defined. Mutagenesis of two threonine clusters within this region abolished lectin binding by PhoA hybrids and by the 19 kDa protein itself. Substitution of the threonine residues also resulted in generation of a series of smaller forms of the protein as a result of proteolysis. In a working model to account for these observations, we propose that the role of glycosylation is to regulate cleavage of a proteolytically sensitive linker region close to the acylated N-terminus of the protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Ljungqvist L., Olsen M. Evidence that protein antigen b of Mycobacterium tuberculosis is involved in phosphate metabolism. J Gen Microbiol. 1990 Mar;136(3):477–480. doi: 10.1099/00221287-136-3-477. [DOI] [PubMed] [Google Scholar]
- Andersen A. B., Yuan Z. L., Hasløv K., Vergmann B., Bennedsen J. Interspecies reactivity of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Mar;23(3):446–451. doi: 10.1128/jcm.23.3.446-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Andersen A. B., Sørensen A. L., Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995 Apr 1;154(7):3359–3372. [PubMed] [Google Scholar]
- Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994 Jun;62(6):2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbridge K. R., Booth R. J., Watson J. D., Lathigra R. B. Nucleotide sequence of the 19 kDa antigen gene from Mycobacterium tuberculosis. Nucleic Acids Res. 1989 Feb 11;17(3):1249–1249. doi: 10.1093/nar/17.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock K., Schuster-Kolbe J., Altman E., Allmaier G., Stahl B., Christian R., Sleytr U. B., Messner P. Primary structure of the O-glycosidically linked glycan chain of the crystalline surface layer glycoprotein of Thermoanaerobacter thermohydrosulfuricus L111-69. Galactosyl tyrosine as a novel linkage unit. J Biol Chem. 1994 Mar 11;269(10):7137–7144. [PubMed] [Google Scholar]
- Booth R. J., Williams D. L., Moudgil K. D., Noonan L. C., Grandison P. M., McKee J. J., Prestidge R. L., Watson J. D. Homologs of Mycobacterium leprae 18-kilodalton and Mycobacterium tuberculosis 19-kilodalton antigens in other mycobacteria. Infect Immun. 1993 Apr;61(4):1509–1515. doi: 10.1128/iai.61.4.1509-1515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castric P. pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology. 1995 May;141(Pt 5):1247–1254. doi: 10.1099/13500872-141-5-1247. [DOI] [PubMed] [Google Scholar]
- Collins M. E., Patki A., Wall S., Nolan A., Goodger J., Woodward M. J., Dale J. W. Cloning and characterization of the gene for the '19 kDa' antigen of Mycobacterium bovis. J Gen Microbiol. 1990 Jul;136(7):1429–1436. doi: 10.1099/00221287-136-7-1429. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Church V. L., Gale J. M., Tobey R. A. DNA contents of replication without DNA density labeling. Anal Biochem. 1990 May 15;187(1):1–9. doi: 10.1016/0003-2697(90)90409-3. [DOI] [PubMed] [Google Scholar]
- Dobos K. M., Swiderek K., Khoo K. H., Brennan P. J., Belisle J. T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995 Aug;63(8):2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson P. R., Herzberg M. C. Evidence for the covalent linkage of carbohydrate polymers to a glycoprotein from Streptococcus sanguis. J Biol Chem. 1993 Nov 15;268(32):23780–23783. [PubMed] [Google Scholar]
- Espitia C., Mancilla R. Identification, isolation and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin Exp Immunol. 1989 Sep;77(3):378–383. [PMC free article] [PubMed] [Google Scholar]
- Fifis T., Costopoulos C., Radford A. J., Bacic A., Wood P. R. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect Immun. 1991 Mar;59(3):800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe T. R., Barathi J., Barnini S., Zhang Y., Abou-Zeid C., Tang D., Mukherjee R., Young D. B. Transformation of mycobacterial species using hygromycin resistance as selectable marker. Microbiology. 1994 Jan;140(Pt 1):133–138. doi: 10.1099/13500872-140-1-133. [DOI] [PubMed] [Google Scholar]
- Garbe T., Harris D., Vordermeier M., Lathigra R., Ivanyi J., Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993 Jan;61(1):260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig G. J., Kamerling J. P., Vliegenthart J. F., Morag E., Lamed R., Bayer E. A. Novel oligosaccharide constituents of the cellulase complex of Bacteroides cellulosolvens. Eur J Biochem. 1992 Apr 15;205(2):799–808. doi: 10.1111/j.1432-1033.1992.tb16844.x. [DOI] [PubMed] [Google Scholar]
- Gerwig G. J., de Waard P., Kamerling J. P., Vliegenthart J. F., Morgenstern E., Lamed R., Bayer E. A. Novel O-linked carbohydrate chains in the cellulase complex (cellulosome) of Clostridium thermocellum. 3-O-Methyl-N-acetylglucosamine as a constituent of a glycoprotein. J Biol Chem. 1989 Jan 15;264(2):1027–1035. [PubMed] [Google Scholar]
- Gooley A. A., Classon B. J., Marschalek R., Williams K. L. Glycosylation sites identified by detection of glycosylated amino acids released from Edman degradation: the identification of Xaa-Pro-Xaa-Xaa as a motif for Thr-O-glycosylation. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1194–1201. doi: 10.1016/0006-291x(91)91019-9. [DOI] [PubMed] [Google Scholar]
- Haas R., Kahrs A. F., Facius D., Allmeier H., Schmitt R., Meyer T. F. TnMax--a versatile mini-transposon for the analysis of cloned genes and shuttle mutagenesis. Gene. 1993 Aug 16;130(1):23–31. doi: 10.1016/0378-1119(93)90342-z. [DOI] [PubMed] [Google Scholar]
- Hansen J. E., Lund O., Engelbrecht J., Bohr H., Nielsen J. O., Hansen J. E. Prediction of O-glycosylation of mammalian proteins: specificity patterns of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase. Biochem J. 1995 Jun 15;308(Pt 3):801–813. doi: 10.1042/bj3080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Lee B. W., Dillon B. J., Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson H. F. Anchorage and release of Gram-positive bacterial cell-surface polypeptides. Trends Microbiol. 1995 Sep;3(9):333–335. doi: 10.1016/s0966-842x(00)88969-6. [DOI] [PubMed] [Google Scholar]
- Kluepfel D., Vats-Mehta S., Aumont F., Shareck F., Morosoli R. Purification and characterization of a new xylanase (xylanase B) produced by Streptomyces lividans 66. Biochem J. 1990 Apr 1;267(1):45–50. doi: 10.1042/bj2670045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsford M. L., Gilkes N. R., Singh B., Moser B., Miller R. C., Jr, Warren R. A., Kilburn D. G. Glycosylation of bacterial cellulases prevents proteolytic cleavage between functional domains. FEBS Lett. 1987 Dec 10;225(1-2):163–167. doi: 10.1016/0014-5793(87)81150-x. [DOI] [PubMed] [Google Scholar]
- Laqueyrerie A., Militzer P., Romain F., Eiglmeier K., Cole S., Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995 Oct;63(10):4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2687–2691. doi: 10.1073/pnas.73.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Christian R., Kolbe J., Schulz G., Sleytr U. B. Analysis of a novel linkage unit of O-linked carbohydrates from the crystalline surface layer glycoprotein of Clostridium thermohydrosulfuricum S102-70. J Bacteriol. 1992 Apr;174(7):2236–2240. doi: 10.1128/jb.174.7.2236-2240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J., Rouse D. A., Morris S. L. Nucleotide sequence analysis and serologic characterization of the Mycobacterium intracellulare homologue of the Mycobacterium tuberculosis 19 kDa antigen. Mol Microbiol. 1992 Jun;6(11):1431–1439. doi: 10.1111/j.1365-2958.1992.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Navarre W. W., Daefler S., Schneewind O. Cell wall sorting of lipoproteins in Staphylococcus aureus. J Bacteriol. 1996 Jan;178(2):441–446. doi: 10.1128/jb.178.2.441-446.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell B. C., Hagen F. K., Tabak L. A. The influence of flanking sequence on the O-glycosylation of threonine in vitro. J Biol Chem. 1992 Dec 15;267(35):25010–25018. [PubMed] [Google Scholar]
- O'Connell B., Tabak L. A., Ramasubbu N. The influence of flanking sequences on O-glycosylation. Biochem Biophys Res Commun. 1991 Oct 31;180(2):1024–1030. doi: 10.1016/s0006-291x(05)81168-4. [DOI] [PubMed] [Google Scholar]
- Ong E., Kilburn D. G., Miller R. C., Jr, Warren R. A. Streptomyces lividans glycosylates the linker region of a beta-1,4-glycanase from Cellulomonas fimi. J Bacteriol. 1994 Feb;176(4):999–1008. doi: 10.1128/jb.176.4.999-1008.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995 Oct;3(10):401–404. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- Parge H. E., Forest K. T., Hickey M. J., Christensen D. A., Getzoff E. D., Tainer J. A. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature. 1995 Nov 2;378(6552):32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Tarentino A. L., Hauer C. R. Novel, specific O-glycosylation of secreted Flavobacterium meningosepticum proteins. Asp-Ser and Asp-Thr-Thr consensus sites. J Biol Chem. 1995 Jun 2;270(22):13192–13196. doi: 10.1074/jbc.270.22.13192. [DOI] [PubMed] [Google Scholar]
- Reinhold B. B., Hauer C. R., Plummer T. H., Reinhold V. N. Detailed structural analysis of a novel, specific O-linked glycan from the prokaryote Flavobacterium meningosepticum. J Biol Chem. 1995 Jun 2;270(22):13197–13203. doi: 10.1074/jbc.270.22.13197. [DOI] [PubMed] [Google Scholar]
- Schneewind O., Fowler A., Faull K. F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995 Apr 7;268(5207):103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- Schneewind O., Mihaylova-Petkov D., Model P. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 1993 Dec;12(12):4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shea T. B. Technical report. An inexpensive densitometric analysis system using a Macintosh computer and a desktop scanner. Biotechniques. 1994 Jun;16(6):1126–1128. [PubMed] [Google Scholar]
- Stimson E., Virji M., Makepeace K., Dell A., Morris H. R., Payne G., Saunders J. R., Jennings M. P., Barker S., Panico M. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995 Sep;17(6):1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- Stover C. K., Bansal G. P., Hanson M. S., Burlein J. E., Palaszynski S. R., Young J. F., Koenig S., Young D. B., Sadziene A., Barbour A. G. Protective immunity elicited by recombinant bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J Exp Med. 1993 Jul 1;178(1):197–209. doi: 10.1084/jem.178.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe I. C., Russell R. R. Lipoproteins of gram-positive bacteria. J Bacteriol. 1995 Mar;177(5):1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm J., Perilli M. G., Duez C., Trias J., Orefici G., Fattorini L., Amicosante G., Oratore A., Joris B., Frère J. M. Transcription and expression analysis, using lacZ and phoA gene fusions, of Mycobacterium fortuitum beta-lactamase genes cloned from a natural isolate and a high-level beta-lactamase producer. Mol Microbiol. 1994 May;12(3):491–504. doi: 10.1111/j.1365-2958.1994.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Wilson I. B., Gavel Y., von Heijne G. Amino acid distributions around O-linked glycosylation sites. Biochem J. 1991 Apr 15;275(Pt 2):529–534. doi: 10.1042/bj2750529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991 Jan;142(1):55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]