Abstract
Synthetic colors have been widely used in various industries including food, textile, cosmetic and pharmaceuticals. However toxicity problems caused by synthetic pigments have triggered intense research in natural colors and dyes. Among the natural Sources, pigment producing microorganisms hold a promising potential to meet present day challenges. Furthermore natural colors not only improve the marketability of the product but also add extra features like anti oxidant, anti cancer properties etc. In this review, we present various sources of microbial pigments and to explore their biological and clinical properties like antimicrobial, antioxidant, anticancer and anti inflammatory. The study also emphasizes upon key parameters to improve the bioactivity and production of microbial pigments for their commercial use in pharmacological and medical fields.
Keywords: Natural colors, Microbial pigments, Food, Fermentation, Bioactivity, Chemical structure
Introduction
Colors are the most pleasing and first parameter to be noticed about any article by the receptor. Artificial or synthetic colors mostly used by the food processing and cosmetic industries are reliable and economical as compared to the natural colorants which are expensive, less stable, and possess lower intensity (Joshi et al. 2003). Organizations like the World Health Organization (WHO), the U.S. Food and Drug Administration (FDA), and the European Food Standards Authority (EFSA) have recommended the safe dosage of artificial colors in food, drug and cosmetic items (Clydesdale 1993; Wissgot and Bortlik 1996; Wodicka 1996). However, many synthetic colorants have been banned or being banned due to their hyper-allergenicity, carcinogenicity and other toxicological problems. These adverse effects of synthetic colors have made the scientific community skewed towards natural colors (Reyes et al. 1996). Many research efforts have been made to replace synthetic pigments with natural pigments because nature is a rich source of colored pigment producing organisms including plants, animals and microorganisms. Recent Research has prominently projected the value of natural colors over that of artificial/synthetic colors. In 2011, global sales of natural colors amounted to an estimated $600 millions, up by almost 29 % from 2007, depicting annual growth in excess of 7 %.
Microorganisms are known as a potential source for bio-pigment production due to their advantages over plants in terms of availability; stability; cost efficiency; labor; yield and easy downstream processing (Joshi et al. 2003). Varieties of bio pigments have been produced such as carotenoids, melanins, flavins, quinines, monascins, violancein using microorganisms (Duffose 2006). Cultivation of microorganisms can be attained through solid state and submerged fermentation on natural raw material / industrial organic waste. Many of the microbial pigments not only act as coloring agents in various food processing and cosmetics industry but also possess anticancer, antioxidant, anti-inflammatory, anti microbial activities (Venil and Lakshmanaperumalsamy 2009). Furthermore there is huge demand for coloring agents in industries like textile, plastic, paint, paper and printing.
Only limited research studies are available on exploration of microorganisms for color/pigment production especially in Indian scenario which really points towards exploring microbial pigments in more detail. The present review will lead us to explore the potential of microorganisms to produce pigments and further discusses about various strategies for their production and applications thereof in various fields.
Microbial sources of natural color
Microorganisms are the most versatile tools in biotechnology to produce variety of molecules including enzymes, antibiotics, organic acids and pigments. Recent studies have shown that microorganisms are a promising source for natural colors. The presence of pigments has been reported among the entire microbial world including Bacteria, Fungi, Yeast, algae & protozoa (Table 1). These microorganisms can be isolated/cultured/purified from various environmental sources such as water bodies, soil, plants, insects and animals (Fig. 1).
Table 1.
List of pigment producing microorganisms and their proposed bioactivities
| Sr no. | Pigment | Color | Microorganism | Activities | Status | Reference |
|---|---|---|---|---|---|---|
| 1 | Astaxanthin | Pink-red | Haematococcus pluvialis Agrobacterium aurantiacum* | Antioxidant, photoprotectant, Anti-cancer, Anti-inflammatory | RP | Reyes et al. 1996 |
| 2 | Canthaxanthin | Orange | Bradyrhizobium Spp.* | Antioxidant, Anti-cancer | RP | Lorquin et al. 1997; Mathews-Roth 1982; Chew et al. 1998; Duffose 2006 |
| 3 | Cycloprodigiosin | Red | Pseudoalteromonas denitrificans | Anti-plasmodial, Anti-cancer | DS | Kim et al. 1999; Yamamoto et al. 1999 |
| 4 | Granadaene | Orange–red | Streptococcus agalactiae | Antioxidant, detoxify ROS | DS | George and Nizet 2009; Rosa-Fraile 2006 |
| 5 | Heptyl prodigiosin | Red | α-Proteobacteria | Anti-plasmodial | DS | Lazaro et al.2002 |
| 6 | Indigoidine | Blue | Corynebacterium insidiosum | Anti-microbial, Phaeobacter sp | DS | Starr 1958; Cude et al. 2012 |
| 7 | Prodigiosin | Red | Serratia marcescens, Pseudoalteromonas rubra | Anti-cancer, DNA Cleavage, Immunosuppressant | IP | Feher et al. 2008; Deorukhkar et al. 2007; Melvin et al. 2000; Tsuji et al. 1990 |
| 8 | Pyocyanin | Blue, green | Pseudomonas Spp.* | Cytotoxicity, Neutrophil apoptosis, Ciliary dysmotility, Pro-inflammatory | IP | Baron and Rowe 1981 |
| 9 | Rubrolone | Streptomyces echinoruber** | DS | Iacobucci and Sweeney 1981; Schüep et al. 1978 | ||
| 10 | Scytonemin | Cyanobacteria | Anti-inflammatory, Anti-proliferative | – | Stevenson et al. 2002 | |
| 11 | Staphyloxanthin | Golden | Staphylococcus aureus | Antioxidant, detoxify ROS | – | Liu et al. 2005a, 2005b; Clauditz et al. 2006 |
| 12 | Tryptanthrin | Cytophaga/Flexibacteria AM13,1Strain | – | – | Wagner-D¨obler et al. 1996 | |
| 13 | Undecylprodigiosin | Red | Streptomyces spp | Anti-bacterial, anti-oxidative, UV-protective, Anti-cancer | – | Gerber 1975; Stankovic et al. 2012; Liu et al. 2005a, 2005b |
| 14 | Violacein | Purple | Janthinobacterium lividum, Pseudoalteromonas tunicate, Pseudoalteromonas spp. Chromobacterium violaceum | Antioxidant, detoxify ROS | – | Duran et al. 2012; Matz et al. 2004; Konzen et al. 2006 |
| 15 | Xanthomonadin | Yellow | Xanthomonas oryzae | protection against photodamage | – | Rajagopal et al. 1997 |
| 16 | Zeaxanthin | Yellow | Staphylococcus aureus, Flavobacterium spp.**, Paracoccus Zeaxanthinifaciens, Sphingobacterium Multivorum | – | DS | Hammond and White 1970 |
| Fungi | ||||||
| 17 | Ankaflavin | Yellow | Monascus spp.* | Anti-tumor, Anti-inflammatory | IP | Hsu et al. 2011 |
| 18 | Anthraquinone | Red | Penicillium oxalicum* | Anti-fungal, virucidal | IP | Andersen et al. 1991; Agarwal et al. 2000; Venil and Lakshmanaperumalsamy 2009 |
| 19 | Canthaxanthin | Orange, Pink | Monascus roseus | Antioxidant, Anti-cancer | – | Mathews-Roth 1982; Chew et al. 1998; Cooney et al. 1966; Dufossé 2009 |
| 20 | Lycopene | Red | Fusarium Sporotrichioides*, Blakeslea trispora* | Antioxidant, Anti-cancer | RP/DS | Di Mascio et al. 1989; Giovannucci et al. 2002 |
| 21 | Monascorubramin | Red | Monascus spp.* | Anti-micrbial, Anti-cancer | IP | Blanc et al. 1994 |
| 22 | Naphtoquinone | Deep blood red | Cordyceps unilateralis* | Anticancer, Anti-bacterial, Trypanocidal | RP | Prathumpai et al. 2006; Nematollahi et al. 2012; Ventura et al. 2009 |
| 23 | Riboflavin | Yellow | Ashbya gossypi* | Anti-cancer, anti-oxidant, protection against cardiovascular diseases, in vision | IP | Unagul et al. 2005; Hong et al. 2008; Powers 2003 |
| 24 | Rubropunctatin | Orange | Monascus spp.* | Anti-cancer | IP | Blanc et al. 1994; Zheng et al. 2010 |
| 25 | β-carotene | Yellow-orange | Blakeslea trispora*, Fusarium sporotrichioides, Mucor, circinelloides, Neurospora crassa, Phycomyces, Blakesleeanus | Anti-cancer, Antioxidant, suppression of cholesterol synthesis | IP | Costa et al. 2005; Dufossé 2009; Lopes et al. 2009; Cerdá-Olmedo 2001; Terao 1989 |
| Algae | ||||||
| 26 | Astaxanthin | Red | Haematococcus pluvialis | Antioxidant, photoprotectant, Anti-cancer, Anti-inflammatory | - | Terao 1989; Guerin et al. 2003 |
| 27 | β-carotene | Orange | Dunaliella salina | Anti-cancer, Antioxidant, suppression of cholesterol synthesis | – | Kobayashi et al. 1993; Jacobson and Wasileski 1994; Fuhrman et al. 1997 |
| Yeast | ||||||
| 28 | Astaxanthin | Red, Pink-red | Phaffia rhodozyma*, Xanthophyllomyces, Dendrorhous* | Antioxidant, photoprotectant, Anti-cancer, Anti-inflammatory | DS | Ramirez et al. 2000; Florencio et al. 1998; Flores-Cotera and Sanchez 2001 |
| 29 | Melanin | Black | Saccharomyces, Neoformans | – | – | Vinarov et al. 2003 |
| 30 | Torularhodin | Orange-red | Rhodotorula spp. | Antioxidant, Anti-microbial | – | Sakaki et al. 2000; Ungureanu and Ferdes 2012 |
| Archea | ||||||
| 31 | Canthaxanthin | Orange | HaloferaxAlexandrines | Antioxidant, Anti-cancer | – | Lorquin et al. 1997; Mathews-Roth 1982; Chew et al. 1998; Duffose 2006 |
| Protozoan | ||||||
| 32 | Hemozoin | Brown– black | Plasmodium spp. | – | – | George and Nizet 2009; Kumar 2007 |
*industrial status adopted from Dufossé(2006)
DS Development stage; IP Industrial production; RP Research project
Fig. 1.

Representation of various colors producing microorganisms on a Petri plate
Fermentation strategy
Advancements in fermentation techniques have lead to the easy production and isolation of color pigments. Microbial pigments can be produced either by solid substrate fermentation or by submerged fermentation. In solid substrate fermentation (SSF), the cultivation of microbial biomass occurs on the surface of a solid substrate (Araújo et al. 2010; Grossart et al. 2009). This SSF technique has many potential advantages including savings in wastewater and yield of the metabolites. On the other hand, in submerged fermentation, microorganisms are cultivated in liquid medium aerobically with proper agitation to get homogenous growth of cells and media components (Cho et al. 2002). Furthermore researchers investigated the influence of various process parameters such as carbon source, temperature, pH, aeration rate on pigment production as well (Vasanthabharathi et al. 2011). However, due to the high cost of using synthetic medium, there is a need to develop new low cost process and extraction procedure for the production of pigments. Efforts are on to utilize the waste organic material for large scale production of microbial pigments. Some studies have focused on production of carotenoids from waste material such as whey, apple pomade and crushed pasta (Lampila et al. 1985). Such kind of waste utilization procedures not only lower down the production cost but also act as potent waste management tool as well.
Bio-pharmacological activities
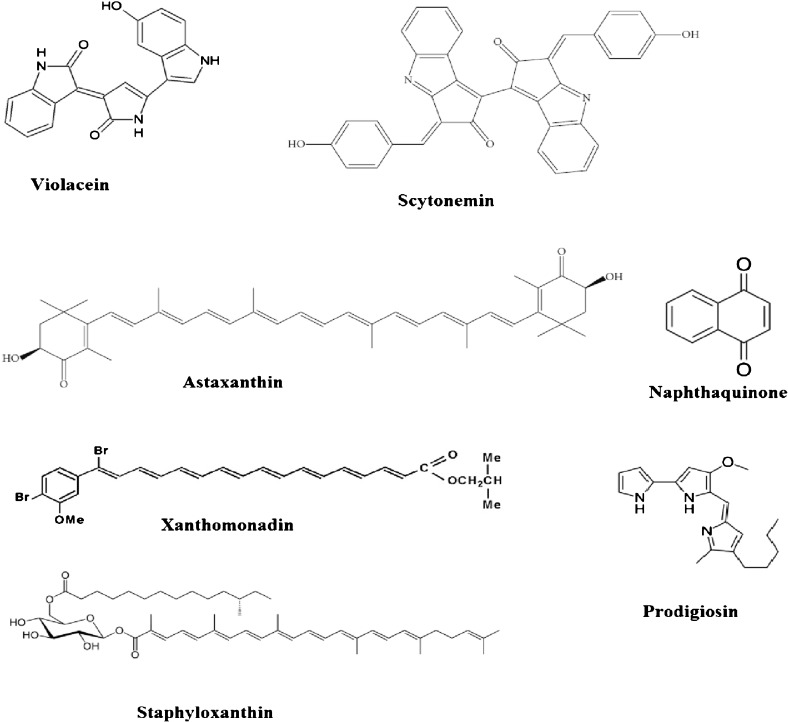
Microorganisms are known to produce a variety of biologically and pharmacologically active compounds. An increasing number of studies have been carried out to find antioxidant, anticancer, antimicrobial activities using microbial pigments. It can be an alternative for synthetic compounds in food and pharmaceutical technology so as to develop new drugs in order to treat various pathological disorders. Medicinal significance of some of the important microbial pigments is discussed below along with their chemical structures (Fig. 2).
Fig. 2.
Chemical structures of some pharmacologically active microbial pigments
Antioxidant
The chronic diseases such as cancer, diabetes, cardiovascular and autoimmune disorders are known to associate with free radicals. Microbial pigments like Carotenoid, naphthaquinone and Violacein have been shown to have a potent antioxidant activity due to their biological functions (Duran et al. 2012; Lampila et al. 1985; Patel et al. 2007). Bacterial pigment xanthomonadin showed antioxidant activity by inhibiting photodynamic lipid peroxidation in liposome and offered protection against photodamage (Rajagopal et al. 1997). Studies revealed that yellow pigment called staphyloxanthin, from Staphylococcus aureus prevents carbon tetrachloride induced oxidative stress in swiss albino mice (Kurjogi et al. 2010). Patel et al. (2007) were successful in producing an antioxidant pigment naphthaquinone from Comamonas testosteroniand and they proposed its protective role against superoxide free radicals. Violacein, an another versatile microbial pigment has shown protection against oxidative damage in gastric ulceration by stimulating mucosal defence mechanism (Antonisamy and Ignacimuthu 2010; De Azevedo et al. 2000; Duran et al. 2003)
Antimicrobial
The development of drug resistance in human pathogenic microorganisms prompted researchers to look for better antimicrobial agents. In current scenario, the treatment of infectious diseases has become difficult due to the emergence of multidrug resistance pathogens (Keith et al. 2000). Such evolutionary changes in pathogenic microorganisms necessitate for the development of a newer generation of antimicrobial agent. Therefore the question of investigations into the natural antimicrobial agents is a valid one to tackle such problems (Tuli et al. 2013). Nakamura et al. (2003) reported that violacein not only caused growth inhibition but also the death of bacteria. Furthermore violacein is known to possess anti fungal (Shirata et al. 1997), antiviral (Andrighetti-Frohner et al. 2003) and antiprotozoal activity (Costa et al. 2005; Lopes et al. 2009; Nakamura et al. 2003). Endophytic fungal pigment was found to be superior to the commercial antibiotic Streptomycin against human pathogenic bacteria, Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhi and Vibrio cholera (Visalakchi and Muthumary 2010) Prodigiosin, a red color pigment from serratia marcescens was also shown as an antibacterial agent against gram + ve and gram -ve bacteria (Mekhael and Yousif 2009). However with the emergence of antimicrobial resistant bacterial strains, there is a need to search for new and novel antibiotics and the pigments are required to be investigated further based upon their promising bioactivities. Most of the studies mentioned above point towards their bacteriostatic role showing antibiotic like activity. The need is to improve ways to produce, purify and characterize such antimicrobial agents (pigments).
Anticancer
The role of microbial pigments to induce apoptosis and cell cycle inhibition has been reported by many studies (Montaner et al. 2000; Pandey et al. 2007). Apoptosis is mainly characterized by a series of distinct changes in cell morphology such as blebbing, loss of cell attachment, cytoplasmic contraction, DNA fragmentation and many other biochemical changes including activation of caspases through extrinsic and/ or intrinsic mitochondrial pathways. Prodigiosin, from Serratia marcescens showed a potent apoptotic effect against human cervix carcinoma cells in a dose dependent manner with a mean IC50 of 700 nM (Kavitha et al. 2010). Anti proliferative effect of prodigiosin has also been investigated in the standard 60 cell line panels of human tumor cells derived from lung, colon, renal, ovarian, brain cancers, melanoma and leukemia (Venil and Lakshmanaperumalsamy 2009). Furthermore prodigiosin analogues and its synthetic indole derivatives have shown in vitro anticancer activity (Han et al. 2001). Ferreira et al. (2004) investigated the cytotoxic effect of violacein on HL60 leukemia cells through tumor necrosis factor (TNF) signaling cascade, which leads to translocation of nuclear factor ҡB (NFҡB), and activation of p38 mitogen-activated protein kinase (p38 MAPK) and caspase-8 (Sakaki et al. 2000). In addition involvement in apoptotic pathway, microbial pigments, are also known to arrest cell cycle at certain check points. Scytonemin, a yellow green pigment isolated from aquatic cyanobacteria showed anti-proliferative effect by inhibiting the activity of cell cycle regulatory protein kinase (Stevenson et al. 2002).
Immuno regulation
Earlier evidences suggest that microbial pigment has potent immuno modulatory effects. Immuno suppressive activity of prodigiosine, metacycloprodigiosin and prodigiosine analogues has been reported through inhibition of polyclonal proliferation of T cells (Han et al. 2001; Kavitha et al. 2010). Recent studies have shown that violacein affects the T cell and IgE mediated inflammatory and anaphylactic response in sheep RBC-induced hypersensitivity and ovalbumin–induced active paw anaphylaxis (Antonisamy and Ignacimuthu 2010). Another important class of pigments comprises of carotenoids produced by bacteria, fungus and algae which are known to enhance immune response. Lo et al. (2013) reported the mechanistic approach to identify the role of carotenoid lutin to induce matrix metalloproteinase-9 expression and phagocytosis through intracellular ROS generation and ERK1/2, p38 MAPK, and RARβ activation in murine macrophages.
Anti inflammatory
It has long been understood that the inflammatory activities are related to cancer progression. Cancer cells are known to express variety of cytokines, chemokines and their receptors which play an important role to mediate inflammatory responses (Arias et al. 2007; Farrow et al. 2004; Nelson et al. 2004; Wang et al. 2009). Many anti-cancer compounds can be used to treat inflammatory diseases as well (Rayburn et al. 2009). Previous studies reported that expression of cytokines, such as IL-6, IL-8, G-CSF, IFN-γ, and MIP-1β were up-regulated in carcinoma (Rayburn et al. 2009). Therefore it is essential to inhibit such inflammatory mediators so as to develop a potent strategy to tackle and prevent cancer. Stevenson et al. (2002) evaluated the anti inflammatory as well as anti proliferative effect of scytonemin pigment extracted from cynobacteria. Recent studies investigated the molecular mechanisms of scytonemin, responsible for anti inflammatory effect in lipopolysaccharide (LPS)-stimulated macrophages. Kang et al. (2011) found significantly inhibition of nitric oxide (NO), in addition to downregulation of inducible nitric oxide (iNOS); TNF-α and IL-1β mRNA by scytonemin. Further more study demonstrated the attenuation of LPS-induced NF- B/Rel activation in macrophage cells (Kang et al. 2011).
Industrial role of microbial pigments
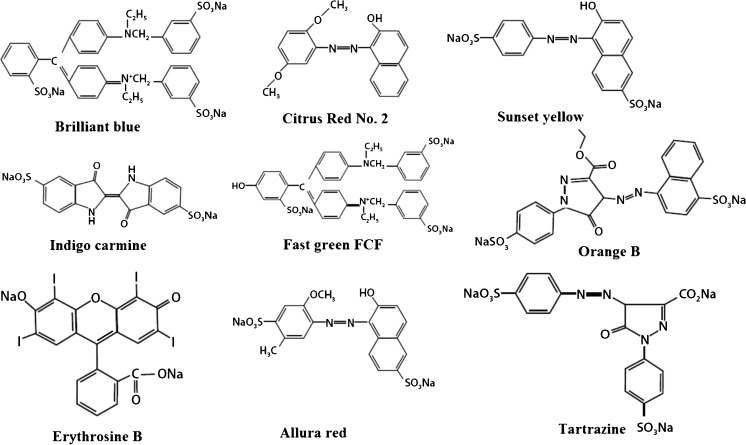
Many Companies like food, cosmetics and pharmaceuticals are widely using synthetic pigments because they are cheaper, more stable, and brighter than natural colors. Chemical structures of some synthetic coloring agents such as Brilliant Blue, Indigo Carmine, Citrus Red No. 2, Fast Green FCF, Orange B, Erythrosine B, Allura Red and Tartrazine have been shown in Fig. 3. Studies are suggestive of the fact that inadequate intake of such artificial colors in food supply may lead to harmful effects including carcinogenicity, genotoxicity and neurotoxicity (Hayashi and Matsui 2000; Ishidate and Sofuni 1984; Matula and Downie 1984; McGregor and Brown 1988; Price and Suk 1978; Patterson and Butler 1982; Sasaki and Kawaguchi 2002).. More positive adaptability and acceptance of customers’ towards natural colors is encouraging industries to use them into their products. The natural colorings, like Monascus pigments, astaxanthin from Xanthophyllomyces dendrorhous, Arpink Red from Penicillium oxalicum, riboflavin from Ashbya gossypii, β-carotene (a Precursor to vitamin A) from Blakeslea trispora are already being used in many food items (Duffose 2006). However, efforts are being made to reduce the production cost of such fermentation based microbial pigments. In order to assess the production cost, economic comparison has been drawn between natural and synthetic pigments (Table 2).
Fig. 3.
Chemical structures of various synthetic coloring agents used by industries
Table 2.
Economics for pigment production
| Color | Synthetic Pigment | Natural Pigment | ||||
|---|---|---|---|---|---|---|
| Insect/Plant | Bacterial/Fungal | |||||
| Name* | Price/100 g* | Name | Price/100 g# | Name | Price/100 g# | |
| Violet | Erioglaucine | 140 | NA | NA | Violacein | 5 X 107 |
| Red | Toluidine | 800 | Cochinel (Insect) | 50–80 | Prodigiosin | 5 × 107 |
| Allura Red AC | 80–90 | Annato extract (Plant) | 80 | |||
| Orange /Yellow | Orange G | 150 | Saffaron (Plant) | 1400 | Carotenoids | 1000 |
| Tetrazine | 2100–2200 | |||||
*Sigma, # Venil et al. (2013)
Conclusions and future perspectives
Public perception towards natural colors has been increased due to health safety and eco-friendly nature. Microbial pigments being an important source for natural colors possess wide range of medicinal properties. More rigorous efforts are required to have a cheap organic substrate for the growth of color producing microorganisms. Also one need to look into the influence of various process parameters on the rate of production of microbial pigments. Studies should be more directed towards delineating the mechanism of action behind pharmacological activity of microbial pigments which would be very helpful in designing a novel strategy for the management of dreadful diseases like cancer. Future investigations need to be more focused on the chemical structure of microbial pigments and their structure-function relationship.
References
- Agarwal SK, Singh SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol. 2000;72(1):43–46. doi: 10.1016/S0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Andersen DO, Weber ND, Wood SG, Hughes BG, Murray BK, North JA. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antivir Res. 1991;16(2):185–196. doi: 10.1016/0166-3542(91)90024-L. [DOI] [PubMed] [Google Scholar]
- Andrighetti-Frohner CR, Antonio RV, Creczynski-Pasa TB, Barandi CRM, Simo˜es CMO. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Mem Inst Oswaldo Cruz. 2003;98:834–848. doi: 10.1590/S0074-02762003000600023. [DOI] [PubMed] [Google Scholar]
- Antonisamy P, Ignacimuthu S. Immunomodulatory, analgesic and antipyretic effects of violacein isolated from chromobacterium violaceum. Phytomedicine. 2010;17:300–304. doi: 10.1016/j.phymed.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Araújo HWCD, Fukushima K, Takaki GMC. Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a Low cost substrate. Molecules. 2010;15:6931–6940. doi: 10.3390/molecules15106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias JI, Aller MA, Arias J. Cancer cell; using inflammation to invade the host. Mol Cancer. 2007;6:29–39. doi: 10.1186/1476-4598-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron SS, Rowe JJ. Antibiotic action of pyocyanin. Antimicrob Agents Chemother. 1981;20:814–820. doi: 10.1128/AAC.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc PJ, Loret MO, Santerre AL, Pareilleux A, Prome D, Prome JC, Laussac JP, Goma G. Pigments of Monascus. J Food Sci. 1994;59:862–865. doi: 10.1111/j.1365-2621.1994.tb08145.x. [DOI] [Google Scholar]
- Cerdá-Olmedo E. Phycomyces and the biology of light and color. FEMS Microbiol Rev. 2001;25:503–512. doi: 10.1111/j.1574-6976.2001.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Chew BP, Park JS, Wong MW, Wong TS. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1998;19(3A):1849–1853. [PubMed] [Google Scholar]
- Cho YJ, Hwang HJ, Kim SW, Song CH, Yun JW. Effect of carbon source and aeration rate on broth rheology and fungal morphology during red pigment production by Paecilomyces sinclairii in a batch bioreactor. J Biotechnol. 2002;95:13–23. doi: 10.1016/S0168-1656(01)00445-X. [DOI] [PubMed] [Google Scholar]
- Clauditz A, Resch A, Wieland KP, Peschel A, Götz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74(8):4950–4953. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clydesdale FM. Color as a factor in food choice. Crit Rev Food Sci Nutr. 1993;33(1):83–101. doi: 10.1080/10408399309527614. [DOI] [PubMed] [Google Scholar]
- Cooney JJ, Marks HW, Smith AM. Isolation and identification of canthaxanthin from Micrococcus roseus. J Bacteriol. 1966;92:342–345. doi: 10.1128/jb.92.2.342-345.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa FTM, Justo GZ, Dura’n N, Nogueira PA, Lopes SCP: The use of violacein in its free and encapsulated form in polymeric systems against malaria. Brazilian Patent PIBr 2005, 056399–0
- Cude WN, Mooney J, Tavanaei AA, Hadden MK, Frank AM, Gulvik CA, May AL, Buchan A. Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp. strain Y4I. Appl Environ Microbiol. 2012;78(14):4771–4780. doi: 10.1128/AEM.00297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Azevedo MBM, Melo PS, Almeida ABA, Souza-Brito ARM, Haun M, Dura’n N. Antiulcerogenic activity of violacein/beta-cyclodextrin inclusion complexes and violacein. Proc Int Sym Controlled Release Bioact Mater. 2000;27:508–509. [Google Scholar]
- Deorukhkar AA, Chander R, Ghosh SB, Sainis KB. Identification of a red-pigmented bacterium producing a potent anti-tumor N-alkylated prodigiosin as Serratia marcescens. Res Microbiol. 2007;158(5):399–404. doi: 10.1016/j.resmic.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274(2):532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Duffose L. Microbial production of food grade pigments, food grade pigments. Food Technol Biotechnol. 2006;44(3):313–321. [Google Scholar]
- Dufossé L (2009) Microbial and microalgal carotenoids as colourants and supplements. In Carotenoids Birkhäuser Basel 83–98
- Dura’n N, Justo GZ, Melo PS, De Azevedo MBM, Souza-Brito ARM, Almeida ABA, Haun M. Evaluation of the antiulcerogenic activity of violacein and its modulation by the inclusion complexation with beta-cyclodextrin. Can J Physiol Pharmacol. 2003;81:387–396. doi: 10.1139/y03-033. [DOI] [PubMed] [Google Scholar]
- Duran M, Ponezi AN, Faljoni-Alario A, Teixeira MF, Justo GJ, Duran N. Potential applications of violacein: a microbial pigment. Med Chem Res. 2012;21(7):1524–1532. doi: 10.1007/s00044-011-9654-9. [DOI] [Google Scholar]
- Farrow B, Sugiyama Y, Chen A, Uffort E, Nealon W, Mark BE. Inflammatory mechanisms contributing to pancreatic cancer development. Ann Surg. 2004;239(6):763–769. doi: 10.1097/01.sla.0000128681.76786.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher D, Barlow RS, Lorenzo PS, Hemscheidt T. A 2-substituted prodiginine, 2-(p-hydroxybenzyl)prodigiosin, from Pseudoalteromonas rubra. J Nat Prod. 2008;71(11):1970–1972. doi: 10.1021/np800493p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CV, Bos CL, Versteeq HH, Justo GZ, Duran N, Peppelenbosch MP. Molecular mechanism of violacein-mediated human leukemia cell death. Blood. 2004;104(5):1459–1464. doi: 10.1182/blood-2004-02-0594. [DOI] [PubMed] [Google Scholar]
- Florencio JA, Soccol CR, Furlanetto LF, Bonfim TMB, Krieger N, Baron M, Fontana JD. A factorial approach for a sugarcane juice-based low cost culture medium: increasing the astaxanthin production by the red yeast Phaffia rhodozyma. Bioprocess Eng. 1998;19:161–164. [Google Scholar]
- Flores-Cotera LB, Sanchez S. Copper but not iron limitation increases astaxanthin production by Phaffia rhodozyma in a chemically defined medium. Biotechnol Lett. 2001;23:793–797. doi: 10.1023/A:1010358517806. [DOI] [Google Scholar]
- Fuhrman B, Elis A, Aviram M. Hypocholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem Biophys Res Commun. 1997;233(3):658–662. doi: 10.1006/bbrc.1997.6520. [DOI] [PubMed] [Google Scholar]
- George YL, Nizet V. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 2009;17(9):406–413. doi: 10.1016/j.tim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber NN. A new prodiginine (prodigiosin like) pigment from Streptomyces. Antimalarial Act Several Prodiginines J Antibiot. 1975;28:194–199. doi: 10.7164/antibiotics.28.194. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94(5):391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- Grossart HP, Thorwest M, Plitzko I, Brinkhoff T, Simon M, Zeeck A. Production of a blue pigment (glaukothalin) by marine rheinheimera spp. Int J Microbiol. 2009;2009:701735. doi: 10.1155/2009/701735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21(5):210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Hammond RK, White DC. Inhibition of carotenoid hydroxylation in Staphylococcus aureus by mixed-function oxidase inhibitors. J Bacteriol. 1970;103:607–610. doi: 10.1128/jb.103.3.607-610.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SB, Park SH, Jeon YJ, Kim YK, Kim HM, Yang KH. Prodigiosin blocks T cell activation by inhibiting interleukin - 2Rα expression and delays progression of autoimmune diabetes and collagen induced arthritis. J Pharm Exp Ther. 2001;299:415–425. [PubMed] [Google Scholar]
- Hayashi M, Matsui M. Genotoxicity evaluation datasheet of food additives by the MHW (1980–1998) Environ Mutagen Res. 2000;22:27–44. [Google Scholar]
- Hong MY, Seeram NP, Zhang Y, Heber D. Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J Nutr Biochem. 2008;19(7):448–458. doi: 10.1016/j.jnutbio.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Hsu YW, Liang YH, Kuo YH, Pan TM. Anti-tumor and anti-inflammatory properties of ankaflavin and monaphilone A from Monascus purpureus NTU 568. J Agri Food Chem. 2011;59(4):1124–1130. doi: 10.1021/jf103652n. [DOI] [PubMed] [Google Scholar]
- Iacobucci GA, Sweeney LG. Process for enhancing the sunlight stability of rubrolone. US patent. 1981;4:285,985. [Google Scholar]
- Ishidate MJ, Sofuni T. Primary mutagenicity screening of food additives currentlyused in Japan. Food Chem Toxicol. 1984;22(8):623–636. doi: 10.1016/0278-6915(84)90271-0. [DOI] [PubMed] [Google Scholar]
- Jacobson G, Wasileski J (1994) Production of food colorants by fermentation. In Bioprocess Production of Flavor, Fragrance, and Color Ingredients. Ed. A. Gabelman, John Wiley and Sons Inc 205–237.
- Joshi V, Attri D, Bala A, Bhushan S. Microbial Pigments. Indian J Biotechnol. 2003;2:362–369. [Google Scholar]
- Kang JS, Cho SA, Han SB, Lee K, Kim HM. Scytonemin inhibits lipopolysaccharide-induced production of nitric oxide, tumor necrosis factor-α and interleukin-1β by blocking NF-{kappa}B/Rel signaling in macrophages. J Immunol. 2011;186:112.13. doi: 10.4049/jimmunol.1100007. [DOI] [Google Scholar]
- Kavitha R, Aiswarya S, Ratnawali MG. Anticancer activity of red pigment from Serratia marcescens in human cervix carcinoma. Int J ChemTech Res. 2010;2(1):784–787. [Google Scholar]
- Keith S, Kaye MD, Donald Kaye MD. Multidrug-resistant pathogens: mechanisms of resistance and epidemiology. Curr Infect Disease Rep. 2000;2(5):391–398. doi: 10.1007/s11908-000-0065-1. [DOI] [PubMed] [Google Scholar]
- Kim HS, Hayashi M, Shibata Y. Cycloprodigiosin hydrochloride obtained from Pseudoalteromonas denitrificans is a potent antimalarial agent. Biol Pharm Bull. 1999;22(5):532–534. doi: 10.1248/bpb.22.532. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kakizono T, Nagai S. Enhanced carotenoid biosynthesis by oxidative stress in acetate induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol. 1993;59:867–873. doi: 10.1128/aem.59.3.867-873.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konzen M, De Marco D, Cordova CA, Vieira TO, Antônio RV, Creczynski-Pasa TB. Antioxidant properties of violacein: possible relation on its biological function. Bioorg Med Chem. 2006;14(24):8307–8313. doi: 10.1016/j.bmc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kumar S. Antimalarial drugs inhibiting hemozoin (beta-hematin) formation: a mechanistic update. Life Sci. 2007;80:813–828. doi: 10.1016/j.lfs.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Kurjogi MM, Sanakal RD, Kaliwal BB. Antibiotic susceptibility and antioxidant activity of Staphylococcus aureus pigment staphyloxanthin on carbon tetrachloride (ccl4) induced stress in swiss albino mice. Int J Biot Appl. 2010;2(2):33–40. doi: 10.9735/0975-2943.2.2.33-40. [DOI] [Google Scholar]
- Lampila LE, Wallen SE, Bullerman LB. A review of factors affecting biosynthesis of carotenoids by the order Mucorales. Mycopathologia. 1985;90:65–80. doi: 10.1007/BF00436853. [DOI] [PubMed] [Google Scholar]
- Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202(2):209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Cui CB, Duan L, Gu QQ, Zhu WM. Potentin Vitro anticancer activity of metacycloprodigiosin and undecylprodigiosin from a sponge-derived actinomycete Sac-charopolyspora sp. nov. Archives Pharm Res. 2005;28(12):1341–1344. doi: 10.1007/BF02977899. [DOI] [PubMed] [Google Scholar]
- Lo HM, Chen CL, Yang CM, Wu PH, Tsou CJ, Chiang KW, Wu WB. The carotenoid lutein enhances matrix metalloproteinase-9 production and phagocytosis through intracellular ROS generation and ERK1/2, p38 MAPK, and RARβ activation in murine macrophages. J Leukoc Biol. 2013;93(5):723–35. doi: 10.1189/jlb.0512238. [DOI] [PubMed] [Google Scholar]
- Lopes SCP, Blanco YC, Justo GZ, Nogueira PA, Rodrigues FLS, Goelnitz U, Wunderlich G, Facchini G, Brocchi M, Dura’n N, Costa FTM. Violacein extracted from Chromobacterium violaceum inhibits Plasmodium growth in vitro and in vivo. Antimicrob Agents Chemother. 2009;53:2149–2152. doi: 10.1128/AAC.00693-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorquin J, Molouba F, Dreyfus BL. Identification of the carotenoid pigment canthaxanthin from photosynthetic Bradyrhizobium strains. Appl Environ Microbiol. 1997;63:1151–1154. doi: 10.1128/aem.63.3.1151-1154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews-Roth MM. Antitumor activity of β-carotene, canthaxanthin and phytoene. Oncology. 1982;39(1):33–37. doi: 10.1159/000225601. [DOI] [PubMed] [Google Scholar]
- Matula TI, Downie RH. Genetic toxicity of erythrosine in yeast. Mutat Res. 1984;138(2–3):153–156. doi: 10.1016/0165-1218(84)90038-7. [DOI] [PubMed] [Google Scholar]
- Matz C, Deines P, Boenigk J, Arndt H, Eberl L, Kjelleberg S, Jurgens K. Impact of violacein producing bacteria on survival and feeding of bacteriovorans nanoflagellates. Appl Environ Microbiol. 2004;70:1593–1599. doi: 10.1128/AEM.70.3.1593-1599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor DB, Brown A. Responses of the L5178Y tk+/tk- mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen. 1988;12(1):85–154. doi: 10.1002/em.2860120111. [DOI] [PubMed] [Google Scholar]
- Mekhael R, Yousif SY. The role of red pigment produced by Serratia marcescens as antibacterial and plasmid curing agent. J Duhok Univ. 2009;12(1):268–274. [Google Scholar]
- Melvin MS, Tomlinson JT, Saluta GR, Kucera GL, Lindquist N, Manderville RA. Double-strand DNA cleavage by copper prodigiosin. J Am Chem Soc. 2000;122(26):6333–6334. doi: 10.1021/ja0000798. [DOI] [Google Scholar]
- Montaner B, Navarro S, Pique M, Vilaseca M, Martinell M, Giralt E, Gil J, Perez-Thomas R. Prodigiosin from the supernatant of serratia marcescens induce apoptosis in haematopoietic cancer cell lines. Br J Pharmacol. 2000;131(3):585–593. doi: 10.1038/sj.bjp.0703614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Asada C, Sawada T. Production of antibacterial violet pigment by psychrotropic bacterium RT102 strain. Biotechnol Bioprocess Eng. 2003;8:37–40. doi: 10.1007/BF02932896. [DOI] [Google Scholar]
- Nelson WG, De AMM, De TLW, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172:S6–11. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- Nematollahi A, Aminimoghadamfarouj N, Wiart C. Reviews on 1, 4-naphthoquinones from Diospyros L. J Asian Nat Prod Res. 2012;14(1):80–88. doi: 10.1080/10286020.2011.633515. [DOI] [PubMed] [Google Scholar]
- Pandey R, Chander R, Sainis KB. Prodigiosins; A novel family of immunosuppressants with anticancer activity. Ind J Biochem Biophy. 2007;44:295–302. [PubMed] [Google Scholar]
- Patel KC, Patel MA, Chauhan K, Anto H, Trivedi U. Production of an antioxidant naphthaquinone pigmant by comamonas testosteroni during growth on naphthalene. J Scientific Indus Res. 2007;66:605–610. [Google Scholar]
- Patterson RM, Butler JS. Tartrazine-induced chromosomal aberrations in mammalian cells. Food Chem Toxicol. 1982;20(4):461–465. doi: 10.1016/S0278-6915(82)80113-0. [DOI] [PubMed] [Google Scholar]
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77(6):1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- Prathumpai W, Phimmakong K, Srikitikulchai P, Wongsa P. Kinetic study of naphthoquinone and key metabolite production of C. unilateralis BCC1869. Thai J Biotechnol. 2006;7(2):39–43. [Google Scholar]
- Price PJ, Suk WA. In vitro and in vivo indications of the carcinogenicity and toxicity of food dyes. Int J Cancer. 1978;21:361–367. doi: 10.1002/ijc.2910210318. [DOI] [PubMed] [Google Scholar]
- Rajagopal L, Sundari CS, Balasubramanian D, Sonti RV. The bacterial pigment Xanthomonadin offers protection against photodamage. FEBS Lett. 1997;415:125–128. doi: 10.1016/S0014-5793(97)01109-5. [DOI] [PubMed] [Google Scholar]
- Ramirez I, Nunez ML, Valdivia R. Increased astaxanthin production by a Phaffia rhodozyma mutant grown on date juice from Yucca fillifera. J Ind Microbiol Biotechnol. 2000;24:187–190. doi: 10.1038/sj.jim.2900792. [DOI] [Google Scholar]
- Rayburn ER, Ezell SJ, Zhang R. Anti-inflammatory agents for cancer therapy. Mol Cell Pharmacol. 2009;1(1):29–43. doi: 10.4255/mcpharmacol.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes FG, Valim MF, Vercesi AE. Effect of organic synthetic food colours on mitochondrial respiration. Food Addit Contam. 1996;13(1):5–11. doi: 10.1080/02652039609374376. [DOI] [PubMed] [Google Scholar]
- Rosa-Fraile M. Granadaene: proposed structure of the group B Streptococcus polyenic pigment. Appl Environ Microbiol. 2006;72:6367–6370. doi: 10.1128/AEM.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki H, Nakanishi T, Satonaka KY, Miki W, Fujita T, Komemushi S. Properties of a high-torularhodin mutant of Rhodotorula glutinis cultivated under oxidative stress. J Biosci Bioeng. 2000;89:203–205. doi: 10.1016/S1389-1723(00)88739-3. [DOI] [PubMed] [Google Scholar]
- Sasaki YF, Kawaguchi S. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res. 2002;519:103–119. doi: 10.1016/S1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Schüep W, Blount JF, Williams TH, Stempel A. Production of a novel red pigment, rubrolone, by Streptomyces echinoruber Sp. Nov II Chem Struct Elucidation J Antibiot. 1978;31(12):1226–1232. doi: 10.7164/antibiotics.31.1226. [DOI] [PubMed] [Google Scholar]
- Shirata A, Tsukamoto T, Yasui H, KatoH HS, Kojima A. Production of bluish-purple pigments by Janthinobacterium lividum isolated from the raw silk and dyeing with them. Nippon Sanshigaku Zasshi. 1997;66:377–385. [Google Scholar]
- Stankovic N, Radulovic V, Petkovic M, Vuckovic I, Jadranin M, Vasiljevic B, Nikodinovic-Runic J. Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties. Appl Microbial Biotechnol. 2012;96(5):1217–1231. doi: 10.1007/s00253-012-4237-3. [DOI] [PubMed] [Google Scholar]
- Starr MP. The blue pigment of Corynebacterium insidiosum. Arch Mikrobiol. 1958;30:325–334. doi: 10.1007/BF00411227. [DOI] [PubMed] [Google Scholar]
- Stevenson CS, Capper EA, Roshak AK. Scytonemin— a marine natural product inhibitor of kinases key in hyperproliferative inflammatory diseases. Inflamm Res. 2002;51(2):112–114. doi: 10.1007/BF02684014. [DOI] [PubMed] [Google Scholar]
- Terao J. Antioxidant activity of β-carotene-related carotenoids in solution. Lipids. 1989;24(7):659–661. doi: 10.1007/BF02535085. [DOI] [PubMed] [Google Scholar]
- Tsuji RF, Yamamoto M, Nakamura A, Kataoka T, Magae J, Nagai K, Yamasaki M. Selective immunosuppression of prodigiosin 25-C and FK506 in the murine immune system. J Antibiot. 1990;43(10):1293–1301. doi: 10.7164/antibiotics.43.1293. [DOI] [PubMed] [Google Scholar]
- Tuli HS, Sharma AK, Sandhu SS (2013) Pharmacological and Therapeutic potential of Cordyceps with special reference to Cordycepin. 3Biotech. DOI 10.1007/s13205-013-0121-9. [DOI] [PMC free article] [PubMed]
- Unagul P, Wongsa P, Kittakoop P, Intamas S, Srikitikulchai P, Tanticharoen M. Production of red pigments by the insect pathogenic fungus Cordyceps unilateralis. J Ind Microbiol Biotechnol. 2005;32:135–140. doi: 10.1007/s10295-005-0213-6. [DOI] [PubMed] [Google Scholar]
- Ungureanu C, Ferdes M. Evaluation of antioxidant and antimicrobial activities of torularhodin. Adv Sci Lett. 2012;18(1):50–53. doi: 10.1166/asl.2012.4403. [DOI] [Google Scholar]
- Vasanthabharathi V, Lakshminarayanan R, Jayalakshmi S. Melanin production from marine Streptomyces. Afr J Biotechnol. 2011;10(54):11224–11234. [Google Scholar]
- Venil CK, Lakshmanaperumalsamy P. An insightful overview on microbial pigment, prodigiosin. Elect J Biol. 2009;5(3):49–61. [Google Scholar]
- Venil CK, Zakaria ZA, Ahmad WA. Bacterial pigments and their applications. Process Biochem. 2013;48(7):1065–1079. doi: 10.1016/j.procbio.2013.06.006. [DOI] [Google Scholar]
- Ventura Pinto A, Lisboa de Castro S. The trypanocidal activity of naphthoquinones: a review. Molecules. 2009;14(11):4570–4590. doi: 10.3390/molecules14114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinarov A, Robucheva Z, Sidorenko T, Dirina E. Microbial biosynthesis and making of pigment melanin. Commun Agric Appl Biol Sci. 2003;68:325–326. [PubMed] [Google Scholar]
- Visalakchi S, Muthumary J. Antimicrobial activity of the new endophytic Monodictys castaneae SVJM139 pigment and its optimization. Afr J Microbiol Res. 2010;3(9):550–556. [Google Scholar]
- Wagner-D¨obler I, Beil W, Lang S, Meiners M, Laatsch H. Integrated approach to explore the potential of marine microorganisms for the production of bioactive metabolites. Adv Biochem Eng Biotechnol. 1996;74:207–238. doi: 10.1007/3-540-45736-4_10. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN, Richmond A. The role of chemokines in intestinal inflammation andcancer. Curr Opin Pharmacol. 2009;9:688–8. doi: 10.1016/j.coph.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissgot U, Bortlik K. Prospects for new food colorants. Trends Food Sci Technol. 1996;7:298–302. doi: 10.1016/0924-2244(96)20007-X. [DOI] [Google Scholar]
- Wodicka VO. Regulation of food: where have we been? Food Technol. 1996;50:106–109. [Google Scholar]
- Yamamoto C, Takemoto H, Kuno K, Yamamoto D, Tsubura A, Kamata K, Hirata H, Yamamoto A, Kano H, Seki T, Inoue K. Cycloprodigiosin hydrochloride, a new H+/Cl − symporter, induces apoptosis in human and rat hepatocellular cancer cell lines in vitro and inhibits the growth of hepatocellular carcinoma xenografts in nude mice. Hepatol. 1999;30(4):894–902. doi: 10.1002/hep.510300417. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Xin Y, Shi X, Guo Y. Anti-cancer effect of rubropunctatin against human gastric carcinoma cells BGC-823. Appl Microbiol Biotechnol. 2010;88(5):1169–1177. doi: 10.1007/s00253-010-2834-6. [DOI] [PubMed] [Google Scholar]