Abstract
Objectives
Epidemiological studies have shown a relationship between long-term use of proton pump inhibitors and bone metabolism. However, this relationship has not yet become established. The aim of the present study was to analyze the mechanical properties and bone mineral density (BMD) of rats that were subjected to long-term omeprazole use.
Methods
Fifty Wistar rats weighing between 200 and 240 g were divided equally into five groups: OMP300 (omeprazole intake at a dose of 300 μmoL/kg/day); OMP200 (200 μmoL/kg/day); OMP40 (40 μmoL/kg/day); OMP10 (10 μmoL/kg/day); and Cont (control group; intake of dilution vehicle). The solutions were administered for 90 consecutive days. After the rats had been sacrificed, their BMD, the mechanical properties of the dissected femurs and their serum Ca++ levels were analyzed.
Results
The BMD of the OMP300 group was lower than that of the controls (p = 0.006). There was no difference on comparing the OMP200, OMP40 and OMP10 groups with the controls. The maximum strength and rigidity of the femur did not differ in the experimental groups in comparison with the controls. The OMP300 group had a statistically lower serum Ca++ concentration than that of the controls (p = 0.049), but the other groups did not show any difference in relation to the controls.
Conclusion
Daily intake of 300 μmoL/kg/day of omeprazole decreased the BMD of the femur, but without changes to the rigidity and strength of the femur in adult rats.
Keywords: Bone, Bone density, Omeprazole, Rats
Resumo
Objetivos
Estudos epidemiológicos mostram uma relação entre o uso em longo prazo de inibidores de bomba de prótons e o metabolismo ósseo, porém essa relação ainda não está estabelecida. O objetivo deste estudo foi analisar as propriedade mecânicas e a densidade mineral óssea (DMO) de ratos submetidos ao uso de omeprazol em longo prazo.
Métodos
Cinquenta ratos Wistar, entre 200 e 240 g, foram divididos igualmente em cinco grupos: OMP300 (ingestão de omeprazol na dose de 300 μmoL/Kg/dia), OMP200 (200 μmoL/Kg/dia), OMP40 (40 μmoL/Kg/dia), OMP10 (10 μmoL/Kg/dia) e Cont (grupo controle; ingestão do veículo de diluição). A administração das soluções ocorreu durante 90 dias seguidos. Após a eutanásia, foram analisadas a DMO, as propriedades mecânicas dos fêmures dissecados e a dosagem de Ca++ sérico.
Resultados
A DMO do grupo OMP300 foi menor do que a do Cont (p = 0,006). Não houve diferença na comparação entre os grupos OMP200, OMP40 e OMP10 em relação ao Cont. A força máxima e rigidez do fêmur não foram diferentes nos grupos experimentais quando comparados ao Cont. O grupo OMP300 teve concentrações séricas de Ca++ estatisticamente menores do que o grupo Cont (p = 0,049) sem diferença entre os demais grupos em relação ao Cont.
Conclusão
A ingestão diária de 300 μmoL/Kg/dia de omeprazol diminuiu a DMO do fêmur, porém sem alterações na rigidez e na força do fêmur de ratos adultos.
Palavras-chave: Osso, Densidade óssea, Omeprazol, Ratos
Introduction
Proton pump inhibitors (PPIs) are the main drugs used for treating diseases such as duodenal ulcers and reflux esophagitis.1, 2 Because these drugs present few adverse effects when administered correctly, they have come to be used not only for acute symptoms in clinical practice, but also for long-term purposes, even though such indications are highly debatable.3, 4, 5
PPIs act mainly toward suppressing gastric acid secretion by the parietal cells of the stomach, since they inhibit the enzyme H + K + ATPase, and this acid suppression may last for up to 48 h.6
Epidemiological studies have indicated that there is a relationship between prolonged use of PPIs and bone metabolism,7, 8, 9 although this relationship is still not totally established. Yang et al.4 reported that administration of omeprazole (20 mg/day), which is one of the most important PPIs, is capable of significantly diminishing bone mineral density (BMD). It is believed that the mechanism responsible for this consists of elevation of gastric pH, which would interfere with calcium absorption.4, 10, 11 This occurs because some salts, such as calcium, are insoluble in basic pH and would therefore be absorbed less readily.8 However, a study by Hyun et al.3 suggested that using omeprazole would tend to diminish bone reabsorption and impede the progression toward osteoporosis. Therefore, the relationship between using PPIs and bone demineralization and the risk of fractures associated with prolonged use of omeprazole remains unclear.9
Given that there is evidence that prolonged use of PPIs may change the behavior of bone cells, our objective here was to analyze the bone mineral density and mechanical properties of rat femurs that were subjected to long-term use of omeprazole.
Materials and methods
Type of study
This was an experimental study using an animal model.
Animals
The procedures used in this study followed the standards described by the Brazilian College for Animal Experimentation (COBEA) in 1991 and the International Guiding Principles for Biomedical Research Involving Animals12 and were approved by the Ethics Committee for Animal Research of the University of Vale do Sapucaí.
Fifty male adult rats of Wistar lineage, weighing 200–240 g, were used in this study. The animals were kept under normal environmental and temperature conditions (21 ± 2 °C; humidity of 55–60%; and light/dark cycles of 12 h). They received water ad libitum and feed suitable for rats. Fasting of 6 h was maintained during the daytime period, before the daily protocol was started.
The rats were divided equally into five groups: (1) OMP300 – omeprazole intake at a dose of 300 μmol/kg; (2) OMP200 – 200 μmol/kg; (3) OMP40 – 40 μmol/kg; (4) OMP10 – 10 μmol/kg; and (5) control (Cont) – intake only of the dilution vehicle.
Solutions
All the experiments were performed using grade I purified water from the Milli-Q system and using analytical grade reagents. The drug solutions were prepared as described by Larsson et al.13 The omeprazole granules (8.5%; Pharma Nostra, Lavras, Minas Gerais, Brazil) were ground up with the aid of a mortar and were dispersed in a vehicle containing 0.25% hydroxyethyl cellulose 4400 (Galena Química, Lavras, Minas Gerais, Brazil) in a 0.1 M solution of sodium bicarbonate (pH ≈ 7.4). The suspensions were prepared at concentrations of 10, 40, 200 and 300 μmol/kg.
Experimental protocol
Over a 90-day period, the animals of the experimental groups received their respective doses of omeprazole and the animals of the control group received the dilution vehicle. The doses were administered orally by means of gavage. Fig. 1 shows the experimental design over the course of the 90 days of the experiment.
Fig. 1.
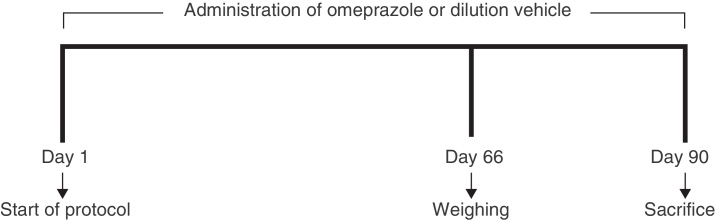
Experimental design during the exposure period.
At the end of the 90 days of receiving the compounds, the animals were again weighed. They were then anesthetized by means of intramuscular injection of xylazine hydrochloride (95 mg/kg) and ketamine hydrochloride (12 mg/kg) and sacrificed by means of exsanguination, using cardiac puncture.14, 15 The right and left femurs of each animal were extracted from their body segments and the muscle and connective tissues were totally removed. The animals and the specimens were weighed simultaneously. After weighing and measurement, the structures were kept in chilled saline solution until the day of the densitometric analysis and mechanical tests (not more than 48 h after the dissection).
Densitometric analysis
The right femurs were subjected to analysis in a dual-emission X-ray densitometer for small animals (Lunar® DPX Alpha). The images were all acquired with the femurs in the same position, immersed in water at a depth of 2 cm. The following imaging options were selected: appendicular; type I bone; high-resolution mode; 76 kVp; 150 μA; collimation at the fine adjustment; and areas of standardized size comprising 40 mm in width and 20 mm in length. In accordance with the manufacturer's recommendations, the apparatus was calibrated using a phantom that the manufacturer supplied.
The analyses on the imaging examinations were performed with the aid of the same computer software as had been used for image acquisition. Using a selection tool, the bones were fully demarcated and information on their mass and area, and consequently their mineral density, was gathered.
Mechanical analysis
The animals’ left femurs were analyzed mechanically by means of a three-point mechanical flexion test (Fig. 2). To perform these mechanical tests, a universal test machine was used (EMIC®, model DL 10000), with a load cell of capacity 500 N (certified by EMIC®), both of which belonged to the Bioengineering Laboratory of the Ribeirão Preto School of Medicine, University of São Paulo, along with accessories specially developed for three-point flexion tests on the bones of small animals.16, 17, 18
Fig. 2.
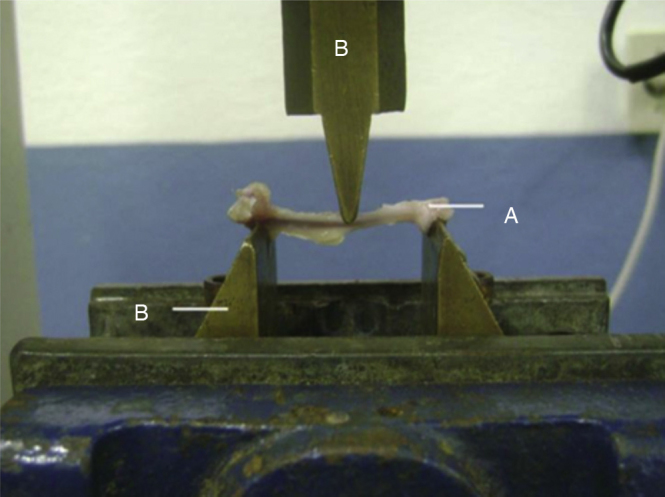
Three-point mechanical flexion test: (A) bone structure; (B) apparatus for applying force. Ribeirão Preto School of Medicine, USP.
The mechanical properties analyzed in the three-point flexion tests were the maximum strength (Fmax) and rigidity (Rig). The velocity at which the force was applied in the three-point flexion tests was 1 mm/min. The span between the test points was 30 mm and an accommodation time of 30 s was used.
Serum Ca++ assay
The blood that had been collected through cardiac puncture was used to perform a serum calcium assay, by means of a diagnostic kit supplied by the company Doles (Goiânia, Brazil).
After centrifugation (at 1000 g, for 5 min) of the blood collected by means of cardiac puncture, the serum was collected and the serum calcium assays were performed by means of a diagnostic kit supplied by the company Doles (Goiânia, Brazil). The method was based on formation of a stained complex between calcium and cresolphthalein in an alkaline medium. For the analyses, a Femto 650® spectrophotometer was used (Femto Ind. e Com. de Instrumentos Ltda., São Paulo, Brazil), adjusted to a wavelength of 570 nm.
Statistical analysis
The statistical analysis on the data was performed using the SPSS software (SPSS, Chicago, USA), version 15.0. To test the normality of the sample, the Kolmogorov–Smirnov test was used. Because the data distribution was found to be normal, comparisons of parameters between the control group and the other experimental groups were made using Student's t-test. To compare the animals’ weights during the experiment and the weights of the femurs, the one-way ANOVA test was used. The significance level of 5% (p ≤ 0.05) was used and the results were expressed as means ± standard deviations.
Results
During the experiment, one animal belonging to the OMP300 group was lost.
The animals’ mean weight did not differ between the groups on day 1 (p = 0.52), day 66 (p = 0.74) or day 90 (p = 0.45) of the experiment (Table 1).
Table 1.
Initial, mean and final weights of the animals, maximum force (Fmax) and rigidity (Rig).
| Group | Initial weight (g) | Mean weight (g) | Final weight (g) | Fmax (N) | Rig (N/mm) |
|---|---|---|---|---|---|
| OMP300 | 196.88 ± 14.90 | 357.55 ± 39.61 | 379.44 ± 43.94 | 92.35 ± 11.61 | 154.92 ± 33.52 |
| OMP200 | 213.80 ± 36.06 | 365.90 ± 44.71 | 391.70 ± 49.79 | 97.19 ± 12.09 | 152.34 ± 26.73 |
| OMP40 | 223.40 ± 39.90 | 355.10 ± 57.83 | 382.10 ± 59.283 | 91.22 ± 14.93 | 130.30 ± 18.50 |
| OMP10 | 221.90 ± 40.39 | 378.80 ± 35.04 | 413.60 ± 33.59 | 97.01 ± 15.93 | 149.52 ± 34.91 |
| CONT | 215.70 ± 39.20 | 362.30 ± 22.63 | 383.50 ± 35.83 | 99.76 ± 6.10 | 155.23 ± 37.24 |
OMP300 (omeprazole, 300 μmol/kg), OMP200 (omeprazole 200 μmol/kg), OMP40 (omeprazole 40 μmol/kg), OMP10 (omeprazole 10 μmol/kg), Cont (control). Results are presented as mean ± standard deviation.
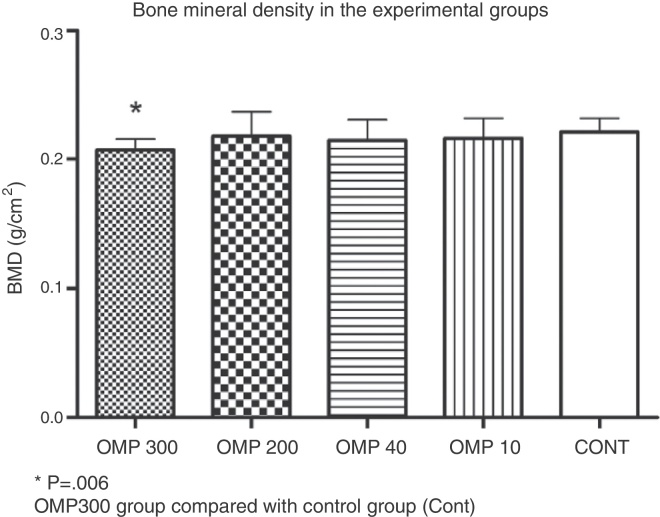
The bone density in the OMP300 group (0.20 ± 0.008 g/cm2) was lower than that of the Cont group (0.22 ± 0.107 g/cm2; p = 0.006). The other groups did not show any differences in comparison with the control group: OMP200 (0.21 ± 0.019 g/cm2; p = 0.644), OMP40 (0.21 ± 0.015 g/cm2; p = 0.305) and OMP10 (0.21 ± 0.016 g/cm2; p = 0.410) (Fig. 3).
Fig. 3.
Graph showing the bone mineral density results from each experimental group. Values are expressed as mean ± standard deviation. OMP300 (omeprazole, 300 μmol/kg), OMP200 (omeprazole 200 μmol/kg), OMP40 (omeprazole 40 μmol/kg), OMP10 (omeprazole 10 μmol/kg), Cont (control); BMD, bone mineral density.
There were no differences in the results relating to the maximum strength of the femurs, in comparing the Cont group with the other groups: OMP300 (p = 0.09); OMP200 (p = 0.55); OMP40 (p = 0.11) and OMP10 (p = 0.62). Likewise, there were no differences relating to the rigidity values, in comparing the Cont group with the other groups: OMP300 (p = 0.98); OMP200 (p = 0.84); OMP40 (p = 0.08) and OMP10 (p = 0.72). The strength and rigidity values are laid out in Table 1.
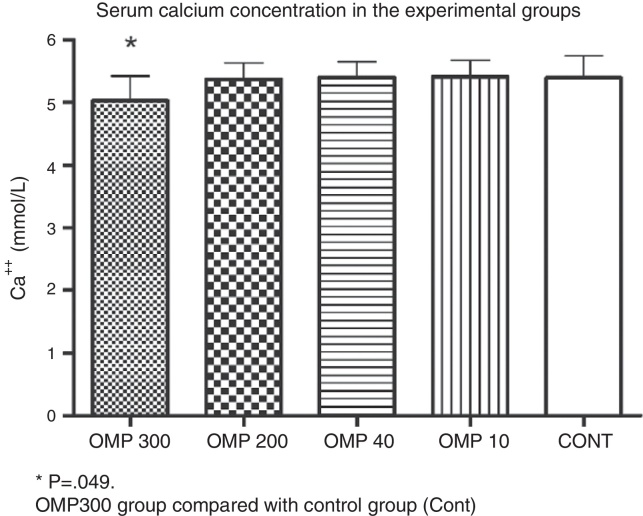
The serum concentration of Ca++ in the OMP300 group (5.03 ± 0.39 mmol/L) was lower than that of the Cont group (5.39 ± 0.35 mmol/L; p = 0.049). The other groups did not show any differences in comparison with the control group: OMP200 (5.37 ± 0.26 mmol/L; p = 0.860), OMP40 (5.40 ± 0.25 mmol/L; p = 0.970) and OMP10 (5.41 ± 0.26 mmol/L; p = 0.910) (Fig. 4).
Fig. 4.
Serum calcium concentration (Ca++). Values are expressed as mean ± standard deviation. OMP300 (omeprazole, 300 μmol/kg), OMP200 (omeprazole 200 μmol/kg), OMP40 (omeprazole 40 μmol/kg), OMP10 (omeprazole 10 μmol/kg), Cont (control).
Discussion
In the present study, the bone characteristics of rats that received four different daily doses of omeprazole (300, 200, 40 and 10 μmol/kg/day) for 90 consecutive days were evaluated. The results from this study demonstrate the influence of the dosage of this drug on bone mineral density, given that the group that received the highest dose (OMP300) presented lower bone mineralization in comparison with the control group (Cont). This result may have been related to the decrease in serum Ca++ concentration in this group. However, use of omeprazole was not seen to influence the mechanical properties of the bone tissue.
Osteoporotic fractures are considered to be a worldwide public health problem. The mortality rate during the first year after hip fractures is around 30%.19 It has been estimated that in the USA, 1.5 million people every year are affected by fractures relating to osteoporosis. This is an event that may reduce quality of life and increase the risk of death.5 However, the relationship between the risks of hip fracture and prolonged use of PPIs remains unclear.
In a study conducted in 1993, Mizunashi et al.20 evaluated the effect of PPI therapy on the serum levels of parathormone (PTH) in a small group of patients with gastric ulcers (seven men and 12 women, with a mean age of 67 ± 13 years). The study showed that after eight weeks of therapy with omeprazole, the PTH level had increased by 28% in relation to the baseline among these patients, accompanied by increases in osteocalcin, alkaline phosphatase and tartrate-resistant acid phosphatase, which are markers for bone formation. Thus, it was suggested that in that small group, there was an increase in bone formation, contrary to the results from the present study.
However, the urinary excretion of hydroxyproline and calcium among the patients in the study by Mizunashi et al.20 decreased, which may suggest that there was less calcium absorption. The possibility for comparison with the present study is limited, given that this was an experimental study, while the study by Mizunashi et al. was conducted among humans. Moreover, PTH was only measured at one time point, which may not reflect the dynamic effect of PTH over a 24-h period; and also, the omeprazole dose and calcium supplementation among those patients was questionable.
On the other hand, several previous studies have demonstrated associations between PPI use and a variety of fractures. Yang et al.4 stated that using PPIs could cause deleterious effects in bone tissue and could increase the risk of osteoporotic fractures, especially after four years of continual use. Vestergaard et al.21 corroborated the previous study and confirmed the relationship between PPI use and osteoporotic fractures. Furthermore, Gray et al.22 conducted a prospective analysis that included 161,806 women, with the aim of correlating PPI use with the risk of clinical fractures. The authors concluded that there were associations between PPI use and occurrences of clinical fractures of the wrist, vertebrae and forearm. The present study corroborates the previous findings, given that a relationship between omeprazole use and decreased bone mineral density was demonstrated. This can be taken to be a predictive sign indicating that fractures may occur as a result of long-term PPI use.
Kaye and Jick23 conducted a retrospective study in which PPI use and the risk of hip fractures were evaluated among patients without significant risk factors. These authors concluded that patients in the age group of 50–79 years who made use of PPIs did not have risk factors and so would not have higher frequency of hip fractures. In that study, the epidemiological data used came from the same data source that Yang et al.4 used in 2006. However, Yang et al.4 observed that the risks of hip fractures increased with PPI use, especially when used for four years or more. Kaye and Jick took the view that the results of Yang et al.4 referred only to patients with some form of risk factor for hip fractures, given that they did not exclude patients with significant risk factors.
Targowniket al.9 conducted a cross-sectional retrospective study followed by a longitudinal phase. In the cross-sectional study, cases of hip or lumbar vertebral osteoporosis were compared with controls presenting normal bone mineral density. In the longitudinal analysis, bone mineral density alterations among patients who either were or were not using PPIs were successively evaluated. The results from their study indicated that chronic use of PPI was not associated with an increased likelihood of occurrences of fractures. In addition, the authors concluded that higher intensity of exposure was not associated with increased risk of presenting osteoporosis. In other words, they believed that the risk of fractures was related to other variables that were independent of those correlated with osteoporosis.
Our study cannot be fully compared with that of Targowniket al.,9 since ours was experimental. Nonetheless, it is known that in addition to the mineral characteristics that can be assessed using DXA, bones have important viscoelastic properties that allow this tissue to fulfill its specific functions.24, 25 These properties are best evaluated through experimental studies, since these enable ex vivo analyses. To our knowledge, our study was the first to analyze the effects of PPIs on the viscoelastic properties of the bone tissue of animals subjected to different doses of omeprazole for a prolonged time.
Osteoporotic fractures are caused by diminished bone mineral content. When the bone mass is diminished, i.e. in situations of osteopenia or osteoporosis, it can be expected that the mechanical properties of the bone will be altered and thus, that the bone will break under smaller loads.26 Following this reasoning, Peng et al.27 analyzed different experimental models for bone loss and changes to mechanical resistance such that the bones only withstood smaller breaking loads, especially among ovariectomized female rats. It can therefore be concluded that the bones of osteopenic female rats present altered mechanical properties.
Despite the high dosages of medications and the long period over which the animals remained exposed to omeprazole intake, the mechanism of action of the PPIs did not influence any of the mechanical properties of the bones in our study. We believe that the highest dose stipulated or the length of use was insufficient to alter the mechanical properties of the bones, even though these factors diminished the bone mineralization.
We did not determine the dose of PPIs in these animals that could be stipulated for simulating their use in humans. The dose of this medication that is generally administered to humans is 20 mg per day, although it may reach up to 90 mg. However, it is not known what the equivalent dose in animals would be. It is known that omeprazole binds to around 95% of the plasma in humans28 and, according to Regårdh et al.,29 the proportion of binding would be lower in dogs and rats (90% and 87%, respectively). This suggests that a simple calculation of dose per kilogram would not reflect the dose used in humans. Moreover, it has been seen that while the mean half-life of this drug is 1 h in humans and dogs, half-lives in the range of 5–15 min have been recorded in rats.29
Some dose–response studies on PPIs in animals have been conducted, but with objectives that differed from those of our study. Londonget al.30 conducted a dose–response study using the doses of 30, 60 and 90 mg/kg and a second study used a dose of 1–10 mg/kg.31 Adhikay et al.32 used a dose of 3 mg/kg in rats with a view to curing gastric ulcers that had been induced. In 2003, Melo et al.33 subjected Wistar rats to intake 0.2 mg/kg, with the aim of evaluating the effect of this intake on partial hepatectomy. Even though many dosages have been observed, there is no consensus in the literature on animal experiments regarding the PPI dose that would be equivalent to those used in humans, for the purpose of long-term testing.
The present study was based on the work by Cui et al.,1 in which the objectives were similar to ours, i.e. to study the long-term effects of PPIs. Cui et al.1 treated their animals for 77 days, at a dose of 400 μmol. The highest dose of the present study (300 μmol) was stipulated as slightly lower than the dose used by those authors, but was used for 90 days, i.e. for slightly longer than in the study by Cui et al.1 It could be seen that the effects were similar, since those authors also identified decreased bone mineral density among their animals.
In summary, the present study demonstrated that long-term PPI use did not alter the mechanical properties of the femurs of adult rats. However, it was observed that the femurs of the rats subjected to daily administration of omeprazole at a dose of 300 μmol/kg/day developed bone demineralization, which may suggest that this gives rise to predisposition to bone fractures. Further experimental studies using protocols similar to ours would be useful for confirming these results.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Our sincere thanks to Messrs. Lúcio Ivan de Melo, José Lopes and Marcos Martins for their assistance in caring for the animals, and to Mrs. Nelsi Nara Vieira Marchette for her contribution relating to the medications. Also to the postgraduate student Bruna Rezende, for her help in the densitometry analyses.
Footnotes
Work developed at the Pouso Alegre School of Medical Sciences, Universidade do Vale do Sapucaí, Pouso Alegre, MG, Brazil.
References
- 1.Cui G.L., Syversen C.M., Zhao D., Chen H.L. Long-term omeprazole treatment suppresses body weight gain and bone mineralization in young male rats. Scand J Gastroenterol. 2001;36(10):1011–1015. doi: 10.1080/003655201750422585. [DOI] [PubMed] [Google Scholar]
- 2.Guimarães E.V., Marguet C., Camargos P.A.M. Tratamento da doença do refluxo gastroesofágico. J Pediatr. 2006;82(Suppl 5):133–145. [Google Scholar]
- 3.Hyun J.J., Chun H.J., Keum B., Seo Y.S., Kim Y.S., Jeen Y.T. Effect of omeprazole on the expression of transcription factors in osteoclasts and osteoblasts. Int J Mol Med. 2010;26(6):877–883. doi: 10.3892/ijmm_00000537. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y.X., Lewis J.D., Epstein S., Metz D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y. Chronic proton pump inhibitor therapy and calcium metabolism. Curr Gastroenterol Rep. 2012;14(6):473–479. doi: 10.1007/s11894-012-0290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner C. Pontifícia Universidade Católica do Rio Grande do Sul. Faculdade de Odontologia; Porto Alegre: 2011. Eficácia da associação de inibidores da bomba de prótons com pasta de hidróxido de cálcio como medicação intracanal em dentes de ratos com lesões periapicais [dissertação] [Google Scholar]
- 7.Kakehasi A.M. Universidade Federal de Minas Gerais: Faculdade de Medicina; Belo Horizonte: 2008. Gastrite autoimune e gastrite associada à infecção pelo helicobacterpylori– Estudo histológico e imuno-histoquímico da mucosa gástrica oxíntica e correlação com a densidade mineral óssea [tese] [Google Scholar]
- 8.Targownik L.E., Lix L.m., Metge C.J., Prior H.J.P., Leung S., Leslie W.D. Use of proton pump inhibitors and risk of osteoporosis related. CMAJ. 2008;179(4):319–326. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targownik L.E., Lix L.M., Leung S., Leslie W.D. Proton-pump inhibitors use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138(3):896–904. doi: 10.1053/j.gastro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Wandall J.H. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome. GUT. 1992;33(5):617–621. doi: 10.1136/gut.33.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scaringi L., Cornacchione P., Fettucciari K., Rosati E., Rossi R., Marconti P. Activity inhibition of cytolytic lymphocytes by omeprazole. Scand J Immunol. 1996;44(3):204–214. doi: 10.1046/j.1365-3083.1996.d01-300.x. [DOI] [PubMed] [Google Scholar]
- 12.International Guiding Principles for Biomedical Research Involving Animals . National Academy Press; Washington, DC: 1985. Council for International Organizations of Medical Sciences (Cioms) [Google Scholar]
- 13.Larsson H., Carlsson E., Jungggren U., Olbe L., Sjöstrand S.E., Skanberg I. Inhibition of gastric acid secretion by omeprazole in the dog and rat. Gastroenterology. 1983;85(4):900–907. [PubMed] [Google Scholar]
- 14.Mello J.R.B., Mello F.B., Etges R.N., Hollenbach C., Rodrigues J.M., Hirtz L. Toxicidade pré-clínica de fitoterápico contendo gossypiumherbaceum (algodoeiro) em ratos Wistar. Lat Am J Pharm. 2008;27(1):46–55. [Google Scholar]
- 15.Silva A.S., Fernandes E.S., Pinto R.M., Reis J.E.P., Guerra M.O., Peters V.M. Avaliação hematológica e bioquímica em ratas prenhes tratadas com extrato de ginkgobiloba. Rev Interdiscipl Est Experiment. 2010;2(3):81–88. [Google Scholar]
- 16.Matheus J.P.C., Milani J.G.P.O., Gomide L.B., Volpon J.B., Shimano A.C. Análise biomecânica dos efeitos da crioterapia no tratamento da lesão muscular aguda. Rev Bras Med Esporte. 2008;14(4):372–375. [Google Scholar]
- 17.Frateschi M.E.M.J.M. Universidade de São Paulo, Bioengenharia; São Carlos: 2002. Efeitos da imobilização e remobilização em algumas propriedades mecânicas do osso [dissertação] [Google Scholar]
- 18.Pugliesi H.B., Moro C.A., Paccola C.A.J.J. Estudo da resistência mecânica do ligamento cruzado anterior em ratos que praticaram natação. Rev Bras Ortop. 2005;40(5):260–269. [Google Scholar]
- 19.Szejnfeld V.L., Jennings F., Castro C.H.M., Pinheiro M.M., Lopes A.C. Conhecimento dos médicos clínicos do Brasil sobre as estratégias de prevenção e tratamento da osteoporose. Rev Bras Reumatol. 2007;47(4):251–257. [Google Scholar]
- 20.Mizunashi K., Furukawa Y., Katano K., Abe K. Effect of omeprazole, an inhibitor of H+,K+-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53(1):21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 21.Vestergaard P., Rejnmark L., Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79(2):76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 22.Gray S.L., LaCroix A.Z., Larson J., Robbins J., Cauley J.A., Manson J.A. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women – results from the women's health initiative. Arch Intern Med. 2010;170(9):765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye J.A., Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28(8):951–959. doi: 10.1592/phco.28.8.951. [DOI] [PubMed] [Google Scholar]
- 24.Nordin M., Frankel V.H. 3ª. ed. Guanabara Koogan; Rio de Janeiro: 2003. Biomecânica básica do sistema musculoesquelético. [Google Scholar]
- 25.Shimano A.C., Shimano M.M. VII Congresso Brasileiro de Engenharia Biomédica. Santa Catarina. Anais do VIII Cebeb; Florianópolis: 2000. Ensaios tecnológicos de matérias biológicos. [Google Scholar]
- 26.Kodama A.C. Universidade de São Paulo, Bioengenharia; São Carlos: 2003. Efeitos do ultrassom pulsado de baixa intensidade em um modelo ósseo de ratas ovarectomizadas analisadas por meio do ensaio de flexocompressão [dissertação] [Google Scholar]
- 27.Peng Z., Tuukkanen J., Zhang H., Jämsä T., Väänänen H.K. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone. 1994;15(5):523–532. doi: 10.1016/8756-3282(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 28.BRASIL . Ministério da Saúde; Brasília: 2008. Ministério da Saúde. Formulário terapêutico nacional 2008: Rename 2006. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/formulario_terapeutico_nacional_2008.pdf. [Google Scholar]
- 29.Regårdh C.G., Gabrielsson M., Hoffman K.J., Löfberg I., Skånberg I. Pharmacokinetics and metabolism of omeprazole in animals and man – an overview. Scand J Gastroenterol. 1985;108(Suppl):79–94. doi: 10.3109/00365528509095821. [DOI] [PubMed] [Google Scholar]
- 30.Londong W., Londong V., Cederberg C., Steffen H. Dose–response study of omeprazole on meal-stimulated gastric acid secretion and gastrin release. Gastroenterology. 1983;85(6):1373–1378. [PubMed] [Google Scholar]
- 31.Takeuchi K., Konaka A., Nishijima M., Kato S., Yasuhiro T. Effects of pantoprazole, a novel H+/K+-ATPase inhibitor, on duodenal ulcerogenic and healing responses in rats: a comparative study with omeprazole and lansoprazole. J Gastroenterol Hepatol. 1999;14(3):251–257. doi: 10.1046/j.1440-1746.1999.01843.x. [DOI] [PubMed] [Google Scholar]
- 32.Adhikary B., Yadav S.K., Bandyopadhyay S.K., Chattopadhyay S. Epigallocatechin gallate accelerates healing of indomethacin-induced stomach ulcers in mice. Pharmacol Rep. 2011;63(2):527–536. doi: 10.1016/s1734-1140(11)70519-9. [DOI] [PubMed] [Google Scholar]
- 33.Melo G.B., Silva R.L., Fakhouri R., Melo V.A., Lima S.O. Efeito do omeprazol e do pantoprazol sobre a regeneração hepática após hepatectomia parcial em ratos. Acta Cir Bras. 2003;18(6):542–544. [Google Scholar]