Abstract
Objective
To evaluate the effect of two different concentrations of sodium alendronate on the quantity of organic matrix in the femur of rats with estrogen suppression caused by ovariectomy.
Methods
Sixty-days-old Wistar rats (Rattus norvegicus) were subjected to bilateral laparotomy to remove the ovaries. The animals were divided into a control group, in which they only underwent laparotomy; an ovariectomized group (OVX); an ovariectomized group treated with 1 mg/kg of alendronate (OVX 1 mg); and an ovariectomized group treated with 2 mg/kg of alendronate (OVX 2 mg). The rats received alendronate twice a week for 90 days. The left femur was then removed, fixed and processed for embedding in paraffin. Semi-serial sections stained with hematoxylin and eosin were used to determine the area occupied by organic bone matrix, by means of image analysis software. The animals’ weights were obtained at the beginning and end of the experiment.
Results
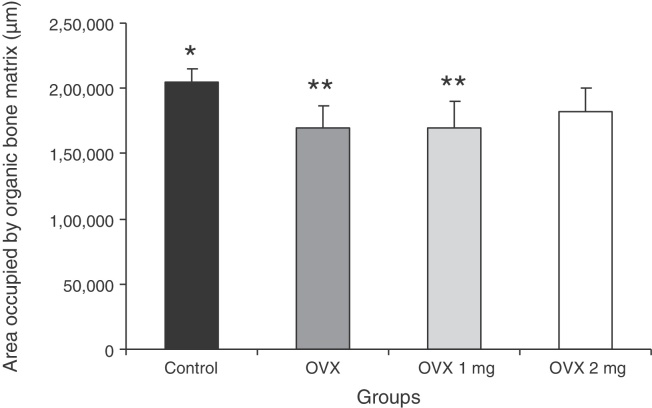
The ovariectomized animals and those treated with 1 mg/kg of alendronate presented significant increases in body weight (p < 0.05), in comparison with the control group. Histomorphometric analysis revealed that in the animals treated with 2 mg/kg of alendronate, the area (μm2) occupied by organic matrix (1,81,900 ± 18,130) was similar (p > 0.05) to that of the non-ovariectomized control animals (2,04,800 ± 9590), which indicates that this medication had a preventive effect with regard to bone mass loss.
Conclusion
The higher concentration of the medication, administered twice a week for 90 days, was more effective than the dose of 1 mg/kg over the same period.
Keywords: Ovariectomy, Alendronate, Osteoporosis, Bone matrix
Resumo
Objetivo
Avaliar a efeito de duas concentrações diferentes de alendronato de sódio (ALN) sobre a quantidade de matriz orgânica no fêmur de ratas com supressão estrogênica provocada por ovariectomia.
Métodos
Ratas Wistar (Rattus norvegicus) com 60 dias foram submetidas a laparotomia bilateral para remoção dos ovários. Os animais foram divididos em grupo controle, no qual os animais foram apenas laparotomizados; grupo ovariectomizado (OVX); grupo ovariectomizado tratado com 1 mg/kg de ALN (OVX 1 mg); e grupo ovariectomizado tratado com 2 mg/kg de ALN (OVX 2 mg). Receberam ALN duas vezes por semana durante 90 dias. O fêmur esquerdo foi coletado, fixado e processado para inclusão em parafina. Cortes semisseriados corados com H&E foram usados para a obtenção, com um software de análise de imagens, da área ocupada por matriz óssea orgânica. O peso dos animais foi obtido no inicio e no fim do experimento.
Resultados
Os animais ovariectomizados e aqueles tratados com 1 mg/kg de ALN tiveram um aumento significativo (p < 0,05) no peso corporal quando comparados com o grupo controle. A análise histomorfométrica revelou que nos animais tratados com 2 mg/kg de ALN a área (μm2) ocupada por matriz orgânica (181.900 ± 18.130) foi semelhante (p > 0,05) àquela dos animais controle não ovariectomizados (204.800 ± 9.590), o que indica um efeito preventivo desse medicamento sobre a perda de massa óssea.
Conclusão
A maior concentração do medicamento administrado duas vezes por semana por 90 dias foi mais eficaz do que a dose de 1 mg/kg no mesmo período.
Palavras-chave: Ovariectomia, Alendronato, Osteoporose, Matriz óssea
Introduction
Postmenopausal osteoporosis is a disease characterized by deterioration of the microarchitecture and reduction of bone mass as a function of increased reabsorption by osteoclasts, with consequently greater susceptibility to fractures. This condition has very high prevalence and occurs more frequently than the sum of cases of myocardial infarct, breast cancer and stroke.1 Osteoporosis should be considered to be a public health problem,2 because it affects individuals with regard to their social, physical and work functions and, therefore has a socioeconomic impact.3 The main cause of loss of bone mass among women is the estrogen deficiency that arises at the menopause.4
Over the last decade, bisphosphonates have become the cornerstone of osteoporosis treatment.5 They are used for treating and preventing this disease and oncological diseases that result in increased bone remodeling.6 Bisphosphonates have a chemical structure that has a strong affinity with calcium phosphate, which facilitates bonding to bone. During bone reabsorption,7 the drug is absorbed by the osteoclasts and causes rupture of the cytoskeleton, loss of the pleated border, inhibition of lysosomal enzymes, loss of reabsorptive activity and death due to apoptosis.8 Thus, there is a diminution of osteoclastic activity, without direct interference with neoformation activity.9
Sodium alendronate (ALN) is a second-generation aminobisphosphonate that is a potent inhibitor of osteoclastic reabsorption.10 Use of ALN suppresses bone remodeling11, 12 and increases bone mineral density (BMD), thereby contributing toward avoiding vertebral and non-vertebral fractures. Through using ALN, bone turnover markers present lower levels.12 Use of a specific ALN dose for treating osteoporosis results in significant deceleration of disease progression.8
The animal model most used for studying postmenopausal osteoporosis comprises ovariectomy, because over a relatively short period after ovariectomy, a state of osteopenia very similar to the human condition is obtained.13, 14 By using animals, it is also possible to investigate the different forms of treatment and medications that exist on the market. However, there is no consensus regarding the appropriate dose for tests on animals. The aim of this study was to evaluate the bone tissue response in ovariectomized rats, to two different concentrations of ALN, which is one of the most widely available drugs on the market for osteoporosis treatment.
Materials and methods
All the procedures were approved by the Ethics Committee for Animal Experimentation of the State University of Maringá (protocol no. 033/2009).
Experimental procedure
Female Wistar rats (Rattus norvegicus) of 60 days of age were anesthetized with an intramuscular injection of 2-(2,6 xylidine)-5,6-dihydro-4H-1,3-thiazine hydrochloride (Ronpun®) and ketamine hydrochloride (Ketalar®) in a 1:1 ratio. This association was used at a rate of 1 mL/kg of body weight for laparotomy and ovary removal. The procedures were started only after the interdigital and ocular reflexes had disappeared. Pulmonary ventilation was maintained in a spontaneous manner. Until recovery from the anesthesia, the animals were observed with regard to their ocular reflex (by stimulating the upper eyelid using gauze), respiration rate (by direct observation of the thoracic-abdominal movements) and heart rate (checked using a pediatric stethoscope attached to the anterior region of the thorax).
The animals were divided into four groups of eight animals each: a control group, in which the animals only underwent laparotomy; an ovariectomized group (OVX); an ovariectomized group that was treated with 1 mg/kg of ALN (OVX 1 mg); and an ovariectomized group that was treated with 2 mg/kg of ALN (OVX 2 mg). After recovery from the anesthesia, the animals were kept in groups of four animals per cage, at a temperature of 20 °C, with dark/light cycles of 12 h and free access to water and food throughout the period of the experiment. All the animals were weighed at the start and end of the experiment.
During the week following the ovariectomy and for the subsequent 90 days, the treated groups were injected intramuscularly with ALN 1 mg/kg or 2 mg/kg, twice a week, always at the same time of day. After the treatment period, the animals were sacrificed by means of an overdose of ketamine. The left femur of each animal was removed and fixed in a solution of 4% paraformaldehyde for 48 h. The soft tissues were then dissected and, following this, the bone was demineralized in formic acid for 21 days. The samples were then washed in running water for 6 h and processed for embedding in paraffin. Semi-serial sections of thickness 7 μm were cut along the major axis of the femur. These sections were stained with hematoxylin and eosin (H&E) for analysis.
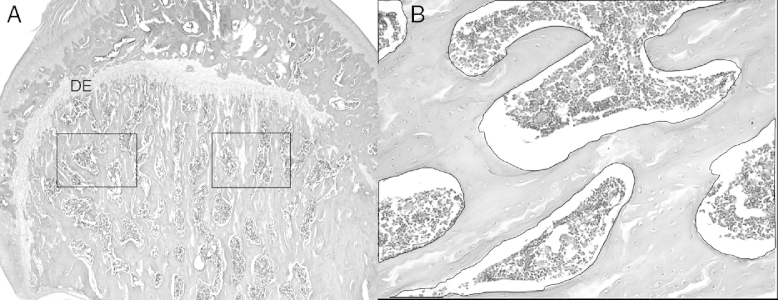
To determine the area occupied by the organic matrix, two images of a standardized area in the proximal diaphysis were captured, just below the epiphyseal disk of the left femur (Fig. 1A and B). The images were obtained from three sections per slide, on four slides per animal, thus totaling 24 images per animal. The image capture system used consisted of an Olympus BX41 microscope (Tokyo, Japan) with an Olympus Q Color 3 RT camera (Tokyo, Japan) coupled to it. The images were obtained using a 20× objective lens.
Fig. 1.
Photomicrograph of the left femur of a female Wistar rat. (A) The marked areas indicate the microscope fields that were captured by the Image-Pro Plus software for morphometric analysis on the area occupied by collagen. Original magnification 4×. (B) This represents the way in which the measurement of the area occupied by collagen was obtained: the total area was determined and the areas of the empty spaces were summed and then subtracted from the total area. Original magnification 20×. Hematoxylin and eosin.
The area occupied by collagen was assessed using image analysis software (Image-Pro Plus®, version 4.5, Media Cybernetics, Silver Spring, MD, USA), which obtained the area in square micrometers.
The area evaluated was composed of trabecular bone and to calculate the area occupied by the organic matrix, the following methodology was adopted: firstly, the total area of the image seen using the 20× objective lens was calculated; then, the empty areas on each slide (corresponding to trabeculated bone areas occupied by bone marrow) were measured and summed in order to subsequently subtract these areas from the total area (Fig. 2). The final result corresponded to the area occupied by the organic matrix. All the calculations were performed using the Excel software (Microsoft Corporation). The mean area in μm2 in each animal was obtained for statistical comparisons.
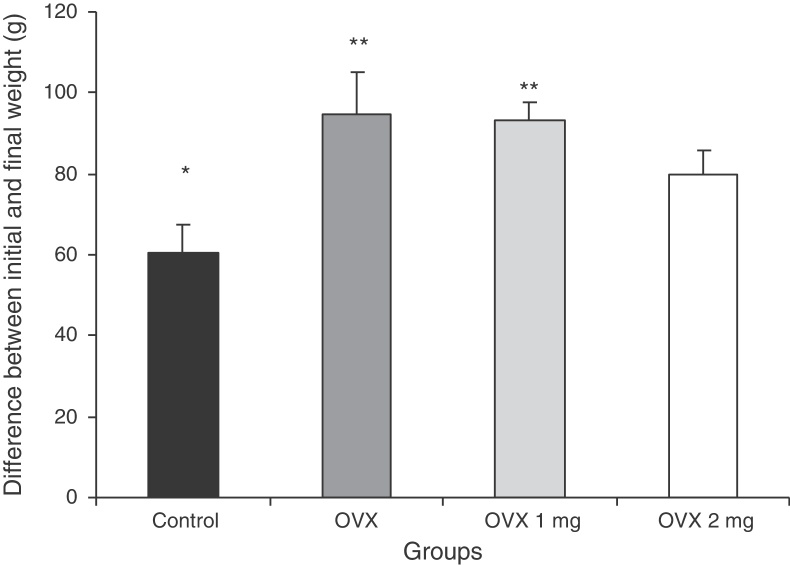
Fig. 2.
Difference between the initial and final body weight of the rats in the different groups: control; ovariectomized (OVX); ovariectomized and treated with 1 mg/kg of sodium alendronate (OVX 1 mg); and ovariectomized and treated with 2 mg/kg of sodium alendronate (OVX 2 mg). Results are expressed as mean ± standard deviation. P < 0.05, comparing * with **.
Statistical analysis
The statistical analysis was performed using the GraphPad Prism R 3.1 software. The data obtained were presented as the mean ± standard deviation. The variance analysis test used was ANOVA. Tukey's test was used as a post-test for comparisons between the means. The significance level used was 5%.
Results
The animals’ initial and final weights in grams are shown in Fig. 2. Fig. 3 presents the results from the analysis on the area (μm2) occupied by the organic bone matrix.
Fig. 3.
Area (μm2) occupied by organic bone matrix in the different groups: control; ovariectomized (OVX); ovariectomized and treated with 1 mg/kg of sodium alendronate (OVX 1 mg); and ovariectomized and treated with 2 mg/kg of sodium alendronate (OVX 2 mg). Results are expressed as mean ± standard deviation. P < 0.05, comparing * with **.
Discussion
Experimental ovariectomy is a study model that makes it possible to evaluate the consequences, in animals, of loss of bone mass in a variety of situations.15 The commonest study topic is the efficacy of medications available on the market for treating osteoporosis.4, 8, 10, 16 In this model, a period of three months after removal of the ovaries is sufficient for a significant loss of bone mass in the femurs of ovariectomized rats to have occurred.17
Ovariectomy leads to a significant progressive increase in body weight.18 In the present study, the ovariectomized rats were treated with two different concentrations of ALN for 90 days. There was an increase in body weight in the untreated ovariectomized rats and in those that received ALN at the rate of 1 mg/kg. The animals that received 2 mg/kg did not present any change in body weight, in comparison with the control group. These results demonstrate that the dose of 2 mg/kg was more efficient in preventing gains in body mass.
Estrogen deficiency may be related to decreased numbers of leptin receptors in the hypothalamus, thereby causing diminished satiety,19 greater food intake and consequent weight gain. On the other hand, there is also the possibility of diminished energy expenditure in females with estrogen deficiency, which would facilitate the gain in body mass.20
The ovariectomized rats received treatment with 1 or 2 mg/kg of ALN twice a week. It is important to emphasize that the treatment started one day after ovariectomy and, therefore, these female rats did not already present a condition of bone loss. Through histomorphometric analysis on the organic matrix, it was observed that the treatment with 2 mg/kg of ALN had a preventive effect on the loss of bone mass. The animals that received 1 mg/kg presented a reduction in bone matrix similar to that of the ovariectomized animals.
Bisphosphonates decrease the degree of bone growth but without interfering with the quality of the mechanical resistance of the bone.21 In other words, ALN enables prevention of reabsorption, but the bone maintains its normal structural and mechanical characteristics.22 Bisphosphonates make the bone turnover slower and provide more time for bone structural organization, without impairing or altering the mechanical properties of the tissue.23
In humans, the recommended dose of ALN is 1 mg/kg of body weight, once a week. However, rats have a metabolism that is twice as fast as that of humans and for this reason, the drug was administered twice a week. The dose of 1 mg/kg was chosen to be similar to that applied to humans. On the other hand, the dose of 2 mg/kg was chosen to be the test dose.
There is no consensus between different authors regarding the ALN dose that should be administered to animals. Ito et al.16 observed, in a study on ovariectomized rats, that daily administration of 0.2 mg and 1 mg/kg, for 12 weeks, caused a significant increase in the quantity of organic matrix, in relation to the untreated ovariectomized rats; the bone mineral density (BMD) of the ovariectomized group was 24.2% lower than that of the control group. Allen24 evaluated the concentrations of 0.1, 0.2 and 1 mg/kg/day of ALN during a one-year treatment period. It was observed that the best results were obtained with higher concentrations of the medication.
Several other authors have tested different concentrations of ALN, at different times, either in or not in association with other substances such as vitamin K,25 vitamin D3,16 calcium26 and estradiol.4 The results from these studies demonstrated in a general manner that ALN has an anabolic effect on loss of bone mass in ovariectomized animals.
Our results showed that the dose of 1 mg/kg twice a week was insufficient to stimulate an anabolic effect. However, Masaya et al.16 and Allen24 demonstrated that this same concentration, given as daily treatment, produced an increase in bone mineral density after 12 weeks and one year, respectively.
Conclusion
At least three parameters need to be taken into consideration when ALN is used in animal models: the dose, treatment duration and posology. Thus, for lower concentrations of the medication, the doses should be more frequent and the treatment should be for a longer period, in order to achieve the desired results; whereas for higher concentrations, the frequency of administration can be lower.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Work developed at the Animal Histotechnical Laboratory, State University of Maringá (UEM), Paraná, Brazil.
References
- 1.Perez-López F.R. Postmenopausal osteoporosis and alendronate. Maturitas. 2004;48(3):179–192. doi: 10.1016/j.maturitas.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 3.Anbinder A.L., Prado F., Prado M. de A., Balducci I., Rocha R.F. The influence of ovariectomy, simvastatin, and sodium alendronate on alveolar bone in rats. Braz Oral Res. 2007;21(3):247–252. doi: 10.1590/s1806-83242007000300010. [DOI] [PubMed] [Google Scholar]
- 4.Da Paz L.H., de Falco V., Teng N.C., dos Reis L.M., Pereira R.M., Jorgetti V. Effect of 17beta-estradiol or alendronate on the bone densitometry, bone histomorphometry, and bone metabolism of ovariectomized rats. Braz J Med Biol Res. 2001;34(8):1015–1022. doi: 10.1590/s0100-879x2001000800007. [DOI] [PubMed] [Google Scholar]
- 5.Fisher A., Martin J., Srikusalanukul W., Davis M. Bisphosphonate use and hip fracture epidemiology: ecologic proof from the contrary. Clin Interv Aging. 2010;5:355–362. doi: 10.2147/CIA.S13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inzerillo A.M., Zaidi M. Osteoporosis: trends and intervention. Mt Sinai J Med. 2002;69(4):220–231. [PubMed] [Google Scholar]
- 7.Skripitz R., Johansson H.R., Ulrich S.D., Werner A., Aspenberg P. Effect of alendronate and intermittent parathyroid hormone on implant fixation in ovariectomized rats. J Orthop Sci. 2009;14(2):138–143. doi: 10.1007/s00776-008-1311-x. [DOI] [PubMed] [Google Scholar]
- 8.Christopoulou G.E., Stavropoulou A., Anastassopoulos G., Panteliou S.D., Papadaki E., Karamanos N.K. Evaluation of modal damping factor as a diagnostic tool for osteoporosis and its relation with serum osteocalcin and collagen I N-telopeptide for monitoring the efficacy of alendronate in ovariectomized rats. J Pharm Biomed Anal. 2006;41(3):891–897. doi: 10.1016/j.jpba.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay R., Silverman S.L., Cooper C., Hanley D.A., Barton I., Broy S.B. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 10.Azuma Y., Sato H., Oue Y., Okabe K., Ohta T., Tsuchimoto M. Alendronate distributed on bone surfaces inhibits osteoclastic bone resorption in vitro and in experimental hypercalcemia models. Bone. 1995;16(2):235–245. doi: 10.1016/8756-3282(94)00035-x. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto J., Miyata A., Sato Y., Takeda T., Matsumoto H. Five-year alendronate treatment outcome in older postmenopausal Japanese women with osteoporosis or osteopenia and clinical risk factors for fractures. Ther Clin Risk Manage. 2009;5:773–779. doi: 10.2147/tcrm.s6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orwoll E., Ettinger M., Weiss S., Miller P., Kendler D., Graham J. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9):604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 13.Li M., Shen Y., Wronski T.J. Time course of femoral neck osteopenia in ovariectomizd rats. Bone. 1997;20(1):55–61. doi: 10.1016/s8756-3282(96)00317-1. [DOI] [PubMed] [Google Scholar]
- 14.Wronski T.J., Dann L.M., Horner S.L. Time course of vertebral osteopenia in ovariectomized rats. Bone. 1990;10(4):295–301. doi: 10.1016/8756-3282(89)90067-7. [DOI] [PubMed] [Google Scholar]
- 15.Bagi C.M., Ammann P., Rizzoli R., Miller S.C. Effect of estrogen deficiency on cancellous and cortical bone structure and strength of the femoral neck in rats. Calcif Tissue Int. 1997;61(4):336–344. doi: 10.1007/s002239900344. [DOI] [PubMed] [Google Scholar]
- 16.Ito M., Azuma Y., Takagi H., Komoriya K., Ohta T., Kawaguchi H. Curative effect of combined treatment with alendronate and 1 alpha-hydroxyvitamin D3 on bone loss by ovariectomy in aged rats. Jpn J Pharmacol. 2002;89(3):255–266. doi: 10.1254/jjp.89.255. [DOI] [PubMed] [Google Scholar]
- 17.Hernandes L., Ramos A.L., Micheletti K.R., Santi A.P., Cuoghi O.A., Salazar M. Densitometry, radiography, and histological assessment of collagen as methods to evaluate femoral bones in an experimental model of osteoporosis. Osteoporos Int. 2012;23(2):467–473. doi: 10.1007/s00198-011-1539-8. [DOI] [PubMed] [Google Scholar]
- 18.Ignacio D.L., Frankenfeld T.G., Fortunato R.S., Vaisman M., Werneck-de-Castro J.P., Carvalho D.P. Body mass regulation by estrogen and physical activity. Arq Bras Endocrinol Metabol. 2009;53(3):310–317. doi: 10.1590/s0004-27302009000300003. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M., Irahara M., Yasui T., Saito S., Tezuka M., Yamano S. The obesity in bilateral ovariectomized rats is related to a decrease in the expression of leptin receptors in the brain. Biochem Biophys Res Commun. 2002;290(4):1349–1353. doi: 10.1006/bbrc.2002.6355. [DOI] [PubMed] [Google Scholar]
- 20.Richard D. Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Am J Physiol. 1986;250(2 Pt 2):R245–R249. doi: 10.1152/ajpregu.1986.250.2.R245. [DOI] [PubMed] [Google Scholar]
- 21.Lepola V.T., Kippo K., Hannuniemi R., Lauren L., Virtamo T., Osterman T. Bisphosphonates clodronate and etidronate in the prevention of ovariectomy-induced osteopenia in growing rats. J Bone Miner Res. 1996;11(10):1508–1517. doi: 10.1002/jbmr.5650111018. [DOI] [PubMed] [Google Scholar]
- 22.Giavaresi G., Fini M., Torricelli P., Martini L., Giardino R. The ovariectomized ewe model in the evaluation of biomaterials for prosthetic devices in spinal fixation. Int J Artif Organs. 2001;24(11):814–820. [PubMed] [Google Scholar]
- 23.Rodan G.A. Alendronate preclinical studies. Rev Rhum Engl Ed. 1997;64(6 Suppl):50S–52S. [PubMed] [Google Scholar]
- 24.Allen M.R. Morphologycal assessment of basic multicellular unit resorption parameters in dogs shows additional mechanisms of biphosphonate effects on bone. Calcif Tissue. 2010;86(1):67–71. doi: 10.1007/s00223-009-9315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki H., Miyakoshi N., Kasukawa Y., Maekawa S., Noguchi H., Kamo K. Effects of combination treatment with alendronate and vitamin K(2) on bone mineral density and strength in ovariectomized mice. J Bone Miner Metab. 2010;28(4):403–409. doi: 10.1007/s00774-009-0148-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen B.L., Xie D.H., Zheng Z.M., Lu W., Ning C.Y., Li Y.Q. Comparison of the effects of alendronate sodium and calcitonin on bone-prosthesis osseointegration in osteoporotic rats. Osteoporos Int. 2011;22(1):265–270. doi: 10.1007/s00198-010-1186-5. [DOI] [PubMed] [Google Scholar]