Abstract
Purpose
This study explored demographic, clinical, and psychological moderators of the effect of a group-based physical exercise intervention on global quality of life (QoL) among cancer survivors who completed treatment.
Methods
Cancer survivors were assigned to a 12-week physical exercise (n = 147) or a wait-list control group (n = 62). The main outcome measure was global QoL, assessed with the EORTC QLQ-C30 at baseline and 12 weeks later. Potential moderators were age, gender, education level, marital status, employment status, type of treatment, time since treatment, the presence of comorbidities, fatigue, general self-efficacy, depression, and anxiety. Linear regression analyses were used to test effect modification of the intervention by each moderator variable using interaction tests (p ≤ 0.10).
Results
The physical exercise intervention effect on global QoL was larger for cancer survivors who received radiotherapy (β = 10.3, 95 % confidence interval (CI) = 4.4; 16.2) than for cancer survivors who did not receive radiotherapy (β = 1.8, 95 % CI = −5.9; 9.5, p interaction = 0.10), larger for cancer survivors who received a combination of chemoradiotherapy (β = 13.0, 95 % CI = 6.0; 20.1) than for those who did not receive this combination of treatments (β = 2.5, 95 % CI = −3.7; 8.7, p interaction = 0.02), and larger for cancer survivors with higher baseline levels of fatigue (β = 12.6, 95 % CI = 5.7; 19.6) than for those with lower levels (β = 2.4, 95 % CI = −3.9; 8.7, p interaction = 0.03). No other moderating effects were found.
Conclusions
This study suggests that cancer treatment modality and baseline fatigue levels moderate the effect of a physical exercise program on cancer survivors’global QoL.
Keywords: Cancer, Physical exercise, Fatigue, Quality of life, Treatment modality
Introduction
Due to advances in detection and treatment of cancer, the number of cancer survivors in Western countries has increased substantially over the last decades and is expected to rise in the years to come [1]. In Europe, the 5-year cancer survival rate has increased from 8 million in 2002 to 9.8 million in 2012 [2, 3]. Despite increased survival rates, however, many cancer survivors experience physical and psychological problems related to the disease and its treatment, such as increased fatigue, anxiety, depression, and decreased physical fitness and function [4]. These problems negatively affect the cancer survivors’ quality of life (QoL) [5].
Several meta-analyses have shown that physical exercise may improve their QoL, but reported effect sizes were small to moderate (range 0.29–0.48) [6–9]. In a physical exercise study performed in the Netherlands, we found a moderate effect (Cohen’s d = 0.51) of a 12-week group-based physical exercise program on global QoL of cancer survivors who completed cancer treatment compared to a wait-list control group (WLC). In addition, 53 % of cancer survivors who completed the program had a clinically relevant improvement (>10 points) in global QoL [10].
One possible explanation for the small to moderate effect sizes is that these interventions were offered to a heterogeneous group of cancer survivors and were not sufficiently targeted to the specific populationswith highest needs [11]. It is therefore important to investigate which subgroups of survivors are most likely to benefit from a physical exercise program. Insight into these moderators will help to determine which specific survivor group should be referred to the physical exercise program [12].
In previous studies among survivors undergoing treatment for breast cancer [13] and lymphoma [14], Courneya and colleagues showed that demographic and clinical variables, baseline health, and psychological function may moderate the physical exercise effects on QoL. Aerobic exercise had larger effects on QoL in survivors with breast cancer who were not married compared to those who were [13]. Compared to their counterparts, resistance exercise effects were larger for breast cancer survivors who were not married and had a preference for resistance exercises [13]. In cancer survivors with lymphoma, greater benefits of aerobic exercise on QoL were found in cancer survivors who were unmarried, had normal weight or were obese, or were in poor/fair health compared to cancer survivors who were married, overweight (but not obese), and in good health, respectively [14].
The current analyses used data from our previous trial that evaluated the effects of a 12-week group-based physical exercise program among cancer survivors who completed cancer treatment [10, 15, 16]. The aim of the present analyses was to explore which demographic (age, gender, education level, marital status, and employment status), clinical (type of treatment, time since treatment, presence of comorbidity), and psychological (fatigue, self-efficacy, symptoms of depression and anxiety) characteristics moderated the physical exercise effects on cancer survivors’ global QoL.
Materials and methods
Recruitment and allocation
This study is part of a multicenter randomized controlled trial evaluating the effects of group-based physical exercise on cancer survivors’ QoL [10, 15, 16]. Detailed descriptions of the study procedures are published elsewhere [10, 15, 16]. The trial was conducted at four rehabilitation or medical centers in the Netherlands [10, 15, 16]. The medical ethics committees of the University Medical Center Utrecht and the local centers approved the study.
Participants aged ≥18 years, who had completed cancer treatment at least 3 months before study entry and had an estimated life expectancy ≥1 year were recruited between February 2004 and December 2005. After a written consent, these cancer survivors completed baseline measurements and were randomized to physical training (PT) or PT plus cognitive behavioral therapy (PT + CBT). Randomization was conducted at group level by an independent researcher using a randomization list. Consecutive groups of 8 to 12 subjects were randomly assigned to each group. Both interventions were balanced in each center [10, 15, 16]. Eligible cancer survivors were invited to participate in the WLC group if, because of fully booked rehabilitation groups, they had to wait at least 3 months to start with a 12-week group-based multidisciplinary cancer rehabilitation program in other Dutch centers than the four recruiting centers [10, 15, 16].
In total, 209 cancer survivors participated in the study; 71 were allocated to PT, 76 to PT + CBT, and 62 to WLC. Measurements were performed at baseline and after 12 weeks. Participants’ adherence rates were 84 % of the total number of 24 PT sessions and 82 % of 12 CBT sessions. In total, 196 (94 %) cancer survivors completed the postintervention assessment [15]. No differences in changes from preintervention to postintervention in physical fitness, fatigue, distress, and QoL were found between PT and PT + CBT groups [10, 15, 16]. In the present study, we therefore combined the two intervention groups into one group. Because differences in moderating effects between the intervention groups may be present, we conducted a sensitivity analyses in which we separately examined moderation effects.
Interventions
Detailed descriptions of PT and CBT are provided elsewhere [16, 17]. PT was supervised by two physical therapists and CBT by a psychologist and a social worker, all experienced professionals in cancer rehabilitation. PT took place two times per week, for 12 weeks, in groups of 8–12 cancer survivors and included individual aerobic training (20–30 min), muscle strength training (20–30 min), and group sports (60 min). Intensity of the individual aerobic training was based on the maximum heart rate determined during baseline symptom-limited ergometry and the Karvonen formula. Exercise training was performed at a heart rate of [heart rate at rest + 40–50 % of (peak heart rate − heart rate at rest)] during the first 4 weeks and gradually increased to a heart rate of [heart rate at rest + 70–80 % of (peak heart rate − heart rate at rest)] in week 12.
Intensity of the muscle strength training was based on baseline individual 1 − repetition maximum (1-RM). Training intensity started at 30 % of the 1-RM during the first week and increased to 50–60 % of 1-RM in week 12. Group sports were included to promote enjoyment and adoption of a physically active lifestyle. Cancer survivors also received information about the benefits of exercise and, depending on their individual goals, also on coping with fatigue, restoration of the balance between demand and capacity during tasks and activities, exercise physiology, illness perceptions, and self-management to support them in regulating their physical activity.
The interventions were based on the principles of group-based self-management, i.e., goal selection, information collection, information processing and evaluation, decision making, action, and self-reaction [18]. These principles were incorporated by setting and monitoring personal training goals, and monitoring training progress using exercise logs, heart rate monitors, and the Borg Scale for dyspnea and fatigue. Self-efficacy improvement strategies included encouraging mastery experiences by starting at low intensity, improvements in physiological arousal by improving exercise capacity, verbal persuasion to perform training activities, and enhancing vicarious learning through the group format delivery [19].
CBT was conducted once a week for 12 weeks, in 2-h group sessions and aimed to train self-management skills using a cognitive behavioral problem-solving approach [20]. This approach aimed at finding effective and adaptive solutions to stressful problems and at changing dysfunctional cognition, emotions, and behaviors [21]. It included discussions on distress, exercise physiology, relaxation (sessions 1 to 4), and training self-management skills to realize personal goals (sessions 5 to 12). During this process, also problem orientation, problem definition and formulation and goal setting, generation of alternative solutions (brainstorming), decision making, and solution implementation and verification were discussed.
Measures and measurements
Outcome
Global QoL was assessed at baseline and 12 weeks later using the subscale of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) [22]. The EORTC QLQ-C30 is a reliable and valid instrument that has been used in many studies evaluating effects of clinical and psychosocial interventions on cancer survivors’ HRQoL [22]. The global QoL subscale includes two questions addressing perceptions of general health and overall QoL. After applying a linear transformation procedure according to the manual, the scores of the scale range from 0 to 100, with higher scores representing a higher global QoL.
Potential moderators
Demographic and clinical characteristics
Demographic characteristics including age, gender, education level, marital status, and employment status were collected at baseline using a self-reported questionnaire. We dichotomized education level into low (elementary and lower vocational education) versus middle and high (secondary and secondary vocational education, higher vocational and university education), marital status into single versus married and/or living together, and employment into employed versus unemployed at diagnosis. Because differences in moderating effects may appear when education level is dichotomized into low and middle versus high, we conducted a sensitivity analyses in which we examined this latter combination.
Clinical characteristics were collected using a self-report questionnaire including type of cancer, type of treatment received, time since completion of treatment, cancer recurrence, and presence of comorbidity. We dichotomized the treatment regimens surgery, radiotherapy, and chemotherapy into received versus not received. In addition, we dichotomized treatment regimens into having received both chemotherapy and radiotherapy versus one of the two or none. Disease recurrence was dichotomized into no or unknown versus yes and presence of comorbidity into none versus any. Cancer survivors with comorbidity reported to receive medical treatment for one or more of the following problems: cardiac problems, vascular problems, diabetes, asthma, rheumatic problems, musculoskeletal problems, psychological problems, or other complaints. Clinical characteristics were confirmed by the referring physicians.
Psychological characteristics
General fatigue
General fatigue was assessed at baseline with the four-item general fatigue subscale of the multidimensional fatigue inventory (MFI) [23]. The total MFI consists of 20 statements for which a person indicates the extent to which, during the previous few days, a particular statement applies to him or her on a five-point scale. The possible range for the general fatigue subscale is 4–20, with higher scores indicating higher levels of fatigue. The MFI has good internal consistency (average Cronbach’s alpha = 0.84) [23].
General self-efficacy
General self-efficacy was measured at baseline with the standardized Dutch version of the general self-efficacy scale [24]. This 16-item questionnaire yields a total score and three subscales: willingness to expend effort in completing a behavior, persistence in the face of adversity, and willingness to initiate behavior. The total score, with a possible range from 16 to 80, was used for further analysis with higher scores representing higher self-efficacy.
Anxiety and depression
Anxiety and depression were assessed at baseline with the 14-item Hospital Anxiety and Depression Scale (HADS) [25], validated for the Dutch population [26] and cancer survivors [27]. The HADS contains an anxiety and a depression subscale, both ranging from 0 to 21 points. A score ≥8 on the subscale was used to indicate possible anxiety or depression [28].
Statistical analysis
Baseline characteristics are presented as means and standard deviations (SD) or as numbers and percentages. Moderation analyses were conducted according to procedures proposed by Aguinis et al. [29]. First, we tested the underlying assumption of homoscedasticity among the moderator categories, indicating that the residual variances (i.e., the error variances that remain after predicting a dependent variable from the independent variables) are constant across the moderator categories. To test this assumption, we used the computer program ALTMMR. This program provides four tests: Deshon and Alexander’s rule for homogeneity, Bartlett’s test, James’s test, and Alexander’s test [30–33]. Homoscedasticity was assumed if three or more tests indicated homogeneous residual variances [29].
Second, we examined the achieved power for each moderator and the sample sizes required to be able to conduct subgroup analysis with a power of 80 %. For categorical moderators, we used the POWER computer program developed by Aguinis et al. [34]. For continuous moderators, we used the computer program GPower developed by Faul et al. [35].
Third, we used linear regression analysis to test effect modification of the intervention by each moderator variable in the form of an interaction test [36]. Global QoL was modeled to compare changes over time across intervention moderator groups. The analyses were adjusted for the baseline value of the outcome, marital status, educational level, and disease recurrence. The latter three variables were included because they differ significantly between the intervention and control group [10, 15, 16]. If homoscedasticity was not assumed, we used weighted least squares regression analyses. In this analysis, a weight factor was added in the analysis to adjust the residual error variance of the model [36]. The weighted factor was calculated for each moderator group by the number of degrees of freedom of the residual variation divided by the sum of squares of the residual variation.
We conducted stratified analysis to examine the intervention effect in the different moderator categories. In case of a continuous moderator, conditional effect of the intervention on global QoL after the exercise intervention was examined for the −1SD, mean, and +1SD values. A variable was considered a potential moderator when the p value of the interaction term was ≤0.10. To examine whether a significant moderating effect could be explained by differences in intervention adherence, we compared differences in adherence between moderator subgroups with an independent samples’ t test.
Finally, we calculated Cohen’s f 2 effect sizes [37] providing an estimate of the variance explained by the interaction term [37]. In case of continuous moderators or homoscedasticity in categorical moderators, effect sizes were calculated by f 2 = R 2 2 − R 1 2, where R 2 2 is the proportion of variance accounted for with all variables in the model (including the interaction term), and R 1 2 is the proportion of variance accounted for with all variables without the interaction term in the model. In case of heteroscedasticity in categorical moderators, effect sizes were calculated by f 2 = R 2 2 − R 1 2 / 1 − R 2 2. We used Cohen’s cutoff points for multiple regression modeling of f 2 = 0.02, f 2 = 0.15, and f 2 = 0.35 to indicate a small, medium, or large effect, respectively [37].
We used SPSS 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, version 20.0. Armonk, NY: IBM Corp.) to conduct the analyses.
Results
The mean age of cancer survivors in the exercise group was 48.8 years (SD = 10.9), 84 % were female and 56 % were diagnosed with breast cancer (Table 1). Cancer survivors in the WLC group were on average 51.3 years old (SD = 8.8), 90 % were female and 61 % were diagnosed with breast cancer.
Table 1.
Distribution of potential moderators by group assignment
| Variable | Physical exercise group (n = 147) | Wait-list control group (n = 62) |
|---|---|---|
| Demographic | ||
| Age, mean (SD) years | 48.8 (10.9) | 51.3 (8.8) |
| Gender, n (%) | ||
| Male | 24 (16) | 6 (10) |
| Female | 123 (84) | 56 (90) |
| Education level, n (%)* | ||
| Low | 20 (14) | 16 (26) |
| Middle | 72 (49) | 32 (52) |
| High | 55 (37) | 14 (22) |
| Marital status, n (%)* | ||
| Single | 43 (29) | 7 (11) |
| Married | 104 (71) | 55 (89) |
| Employment status, n (%) | ||
| Not employed at diagnosis | 40 (28) | 16 (26) |
| Employed at diagnosis | 107 (73) | 46 (74) |
| Clinical | ||
| Type of cancer, n (%) | ||
| Breast | 82 (56) | 38 (61) |
| Hematological | 23 (16) | 10 (16) |
| Gynecological | 18 (12) | 7 (11) |
| Urologic | 9 (6) | 0 (0) |
| Lung | 4 (3) | 4 (7) |
| Colon | 3 (2) | 2 (3) |
| Other | 8 (5) | 1 (2) |
| Radiotherapy, n (%) | ||
| No | 63 (43) | 23 (37) |
| Yes | 84 (57) | 39 (63) |
| Chemotherapy, n (%) | ||
| No | 47 (32) | 21 (34) |
| Yes | 100 (68) | 41 (66) |
| Radiotherapy and chemotherapy, n (%) | ||
| No | 87 (59) | 35 (56) |
| Yes | 60 (41) | 27 (44) |
| Time since treatment, mean (SD) years | 1.3 (1.7) | 1.9 (2.7) |
| Recurrence >3 months ago* | ||
| No | 133 (90) | 47 (76) |
| Yes | 14 (10) | 15 (24) |
| Presence of comorbidity, n (%) | ||
| No comorbidity | 79 (54) | 34 (55) |
| Comorbidity | 68 (46) | 27 (43) |
| Psychological | ||
| General fatigue (MFI), mean (SD) | 15.6 (3.4) | 15.0 (3.3) |
| General self-efficacy (ALCOS), mean (SD) | 44.0 (8.8) | 42.6 (8.5) |
| Depression (HADS), n (%) | ||
| No (<8) | 104 (71) | 35 (57) |
| Yes ≥8) | 43 (29) | 27 (43) |
| Anxiety (HADS), n (%) | ||
| No (<8) | 77 (52) | 34 (55) |
| Yes (≥8) | 70 (48) | 28 (45) |
| Global QoL (EORTC QLQ-C30), mean (SD) | 57.1 (17.6) | 60.1 (18.4) |
ALCOS general self-efficacy scale, CT chemotherapy, EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, HADS Hospital Anxiety and Depression Scale, MFI multidimensional fatigue inventory, RT radiotherapy, QoL quality of life, SD standard deviation
*p < 0.05, significant differences between exercise and wait-list control groups using chi-squared tests
Homoscedasticity was found for gender, marital status, employment status, presence of comorbidity, and anxiety (Table 2). Heteroscedasticity was found for education level, radiotherapy, chemotherapy, combination chemoradiotherapy, and depression. The achieved power for the categorical variables varied between 0.2 % for marital status and 54 % for combination chemoradiotherapy (Table 2).
Table 2.
Exercise intervention effects on global quality of life (QoL), stratified by potential moderator subgroups
| Intervention effect on global QoL | ||||||
|---|---|---|---|---|---|---|
| Moderator | Number | β (95 % CI)b | p interaction b | f 2 | ESP | n (P80 %) |
| Demographic | ||||||
| Age, years | 196 | 0.13 | 0.01 | 0.29 | 787 | |
| −1SD (39.4) | 11.0 (3.7; 18.3) | |||||
| Mean (49.7 ) | 7.1 (2.3; 11.9) | |||||
| +1SD (60.0) | 3.2 (−3.4; 9.8) | |||||
| Gendera | 0.73 | 0.001 | 0.16 | |||
| Male | 28 | 8.9 (−4.9; 22.6) | 154 | |||
| Female | 168 | 6.3 (1.2; 11.4) | 924 | |||
| Education levela | 0.78 | 0.0003 | 0.03 | |||
| Low | 34 | 8.2 (−2.0; 18.5) | 374 | |||
| Middle or high | 162 | 6.7 (1.3; 12.0) | 1782 | |||
| Marital statusa | 0.56 | 0.001 | 0.02 | |||
| Single | 48 | 10.3 (−1.9; 22.5) | 360 | |||
| Married | 148 | 6.4 (1.2; 11.6) | 1110 | |||
| Employment statusa | 0.50 | 0.002 | 0.12 | |||
| Not employed at diagnosis | 52 | 4.50 (−4.6; 13.6) | 260 | |||
| Employed at diagnosis | 144 | 8.1 (2.6; 13.6) | 720 | |||
| Clinical | ||||||
| Radiotherapya | 0.10 | 0.02 | 0.23 | |||
| No | 81 | 1.8 (−5.9; 9.5) | 230 | |||
| Yes | 115 | 10.3 (4.4; 16.2) | 330 | |||
| Chemotherapya | 0.14 | 0.02 | 0.37 | |||
| No | 62 | 2.0 (−6.2; 10.1) | 155 | |||
| Yes | 134 | 9.8 (3.9; 15.7) | 335 | |||
| Radiotherapy and chemotherapy | 0.02 | 0.03 | 0.54 | |||
| No | 87 | 2.5 (−3.7; 8.7) | 180 | |||
| Yes | 122 | 13.1 (6.0; 20.1) | 140 | |||
| Time since treatment in years | 196 | 0.14 | 0.01 | 0.29 | 787 | |
| −1SD (0.1 ) | 4.8 (−0.8; 10.5) | |||||
| Mean (1.5) | 6.9 (2.1; 11.7) | |||||
| +1SD (3.6) | 10.1 (3.8; 16.4) | |||||
| Presence of comorbiditya | 0.88 | 0.0001 | 0.15 | |||
| No comorbidity | 102 | 6.7 (0.2; 13.1) | 273 | |||
| Comorbidity | 93 | 7.4 (0.4; 14.4) | 253 | |||
| Psychological | ||||||
| General fatigue | 196 | 0.03 | 0.02 | 0.50 | 395 | |
| −1SD (12.1) | 2.4 (−3.9; 8.7) | |||||
| Mean (15.4) | 7.5 (2.7; 12.3) | |||||
| +1SD (18.8) | 12.7 (5.7; 19.6) | |||||
| General self-efficacy | 196 | 0.20 | 0.01 | 0.29 | 787 | |
| −1SD (35.1) | 9.9 (3.1; 16.6) | |||||
| Mean (43.9) | 6.7 (1.9; 11.5) | |||||
| +1SD (52.6) | 3.6 (−3.2; 10.4) | |||||
| Depression | 0.67 | 0.002 | 0.30 | |||
| No (<8) | 131 | 5.9 (−0.3; 12.0) | 262 | |||
| Yes (≥8) | 65 | 8.0 (0.4; 15.6) | 130 | |||
| Anxietya | 0.64 | 0.001 | 0.22 | |||
| No (<8) | 105 | 6.0 (−0.3; 12.3) | 258 | |||
| Yes (≥8) | 91 | 8.3 (1.1; 15.5) | 223 | |||
Regression coefficients (β), 95 % confidence intervals (CI), and p value of the interaction test (pinteraction) are presented as well as Cohen’s effect size (f 2) and estimated statistical power (ESP) for the interaction effect, and the number of cancer survivors needed for estimated statistical power of 80 % (n (p80 %))
SD standard deviation
aNo violation of homogeneity of error variances was assumed
bAdjusted for marital status, education level, disease recurrence, and global quality of life measured at baseline
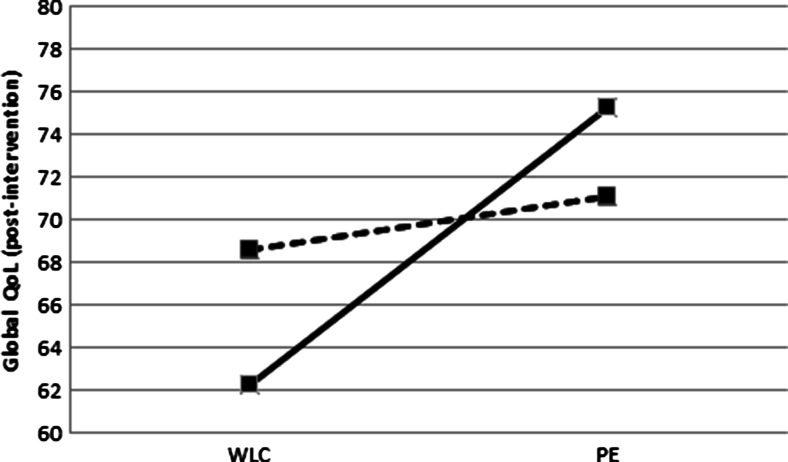
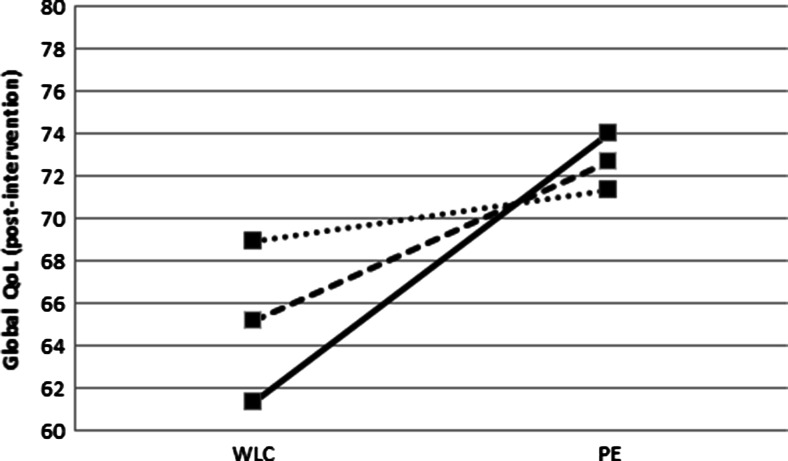
We found a small (f 2 = 0.02, p interaction = 0.10) interaction effect of radiotherapy, indicating that the exercise intervention effect on global QoL was larger for cancer survivors who received radiotherapy (β = 10.3, 95 % confidence interval (CI) = 4.4; 16.2) than for those who did not (β = 1.8, 95 % CI = −5.9; 9.5). No statistical significant (p = 0.14) moderating effect was found for chemotherapy. However, sensitivity analyses showed a significant (p = 0.02) moderating effect of chemotherapy for cancer survivors in the PT + CBT group (β = 10.1, 95 % CI = 3.5; 16.8), but not for survivors in the PT group (β = 9.3, 95 % CI = 2.2; 16.3, p = 0.71). Comparing cancer survivors who received a combination of chemoradiotherapy and those who received one or none of these treatments, we found a significant interaction effect (p interaction = 0.02) in favor of cancer survivors who received a combination of chemoradiotherapy (β = 13.1, 95 % CI = 6.0; 20.1) than for those who did not (β = 2.5, 95 % CI = −3.7; 8.7) (Fig. 1). In addition, we found a small (f 2 = 0.02) but significant (p interaction = 0.03) interaction effect of fatigue, indicating that the exercise intervention effect on global QoL is larger for cancer survivors with higher baseline levels of fatigue (β = 12.7, 95 % CI = 5.7; 19.6) than for those with lower baseline fatigue levels (β = 2.4, 95 % CI = −3.9; 8.7, Fig. 2). No differences in adherence were found for all subgroups.
Fig. 1.
Differences in mean global quality of life (Global QoL) post-intervention between wait-list control group (WLC) and physical exercise group (PE) according to having received a combination of chemoradiotherapy (solid line) or one or none of these treatments (dotted line)
Fig. 2.
Differences in mean global quality of life (Global QoL) post-intervention between wait-list control group (WLC) and physical exercise group (PE) according to low general fatigue (one standard deviation (SD) below the mean of general fatigue; dotted line), mean general fatigue (dashed line), and high general fatigue (one SD above the mean of general fatigue; solid line)
No moderating effects were found for age, gender, education level, marital status, employment status, time since treatment, presence of comorbidity, self-efficacy, depression, and anxiety (Table 2). Sensitivity analysis showed no significant moderating effect of education level when education level is dichotomized into low and medium versus high.
Discussion
In the current analyses, we explored moderators of physical exercise effects on global QoL. We found larger intervention effectsfor cancer survivors who received radiotherapy, and particularly for survivors who received the combination chemoradiotherapy compared to those who did not.
Cancer survivors who received the combination chemoradiotherapy improved 13 points (95 % CI = 6; 20) on the global QoL scale, which is larger than the clinically meaningful difference of 10 points [38]. In contrast, the intervention effect on global QoL was 2 points (95 % CI = −6; 10) for cancer survivors who were treated with one of these treatments or none. Differences in intervention effects across moderator subgroups do not seem to be explained by exercise adherence, since no significant differences in adherence were found across moderator subgroups. A recent longitudinal study showed that cancer survivors who received both types of treatments had lower QoL and fatigue levels compared to radiotherapy or surgery alone [39]. These differences in QoL may consequently give more room for improvement by physical exercise. However, we found in our study no statistically significant differences in baseline values of QoL between cancer survivors who received both radiotherapy and chemotherapy and those who received one or none of these treatments. Therefore, and due to the relatively small sample size and the exploratory nature of our analysis, our findings should be interpreted with caution. Future studies should examine whether cancer survivors who received different treatment regimens respond differently to physical exercise.
Baseline fatigue also moderated the exercise intervention effects on global QoL. Improvements in global QoL were 12 points higher in cancer survivors with high baseline levels of fatigue (≥1SD above the mean) compared to those with low baseline levels of fatigue (≥1SD below the mean), which was a clinically meaningful difference [38]. Differences in effects could not be explained by differences in adherence to the intervention. It is known that higher levels of fatigue negatively affect a cancer survivors’ QoL [5]. Exercise may reduce fatigue and consequently improve a cancer survivors’ QoL [40]. Cancer survivors with higher levels of fatigue at baseline may have more room for reducing their fatigue and consequently have larger improvements in global QoL.
We found no moderating effect of marital status. This is in contrast with the studies of Courneya et al. [13, 14], who found a larger effect of exercise during cancer treatment on QoL in unmarried cancer survivors with breast cancer or lymphoma than in their counterparts. It has been suggested that unmarried cancer survivors may have less social support at home than married cancer survivors and consequently benefit more from the social group effect of the intervention [41], resulting in larger improvements in global QoL [42]. In contrast with the previous mentioned studies, cancer survivors in our intervention were included from 3 months after treatment. Perhaps, social support from a partner may be more important during treatment than after treatment. Cancer survivors who participated in our group-based rehabilitation program reported the support of fellow cancer survivors and the sharing of experiences to be an important part of the rehabilitation [43]. It should also be noted that our results were based on a comparison between married and unmarried cancer survivors where 70 % in the exercise group and 90 % in the WLC group had a partner. This may bias our results, and therefore, our findings should be used with caution. Future studies should investigate the moderating role of social support from a partner or fellow cancer survivors of the physical exercise effect on global QoL.
Strengths of the present study are the supervised, standardized and theory-based exercise interventions, the high attendance rates, and the low dropout rates. However, this study had some limitations. First, participants were not randomly assigned to the WLC group. Nevertheless, the groups were well balanced in baseline physical and psychological outcomes, and we adjusted for relevant sociodemographic and clinical variables in all analyses. Second, although the sample size was relatively large for an exercise trial among cancer survivors, the original study was not powered to investigate moderators of intervention effect.This study showed that the sample size should be at least 395 to be able to adequately conduct stratified analyses with a power of 80 %. Therefore, our analyses of moderating effects should be interpreted as exploratory (hypothesis generating) post hoc analyses. Identifying for whom and under what circumstances specific exercise interventions improve the QoL is an important step toward the development of personalized exercise interventions [44]. Future studies with large sample sizes are needed to confirm the moderating effects of being treated with radiotherapy or a combination chemoradiotherapy and fatigue and to provide insight into whether people with different demographic, clinical, and psychological characteristics indeed respond differently [11, 45].
In conclusion, this study suggests that the physical exercise effects immediately after intervention on global QoL were larger in cancer survivors who received radiotherapy, and in particular, those who received a combination of chemoradiotherapy, and cancer survivors with higher baseline levels of fatigue compared to those who received no radiotherapy, no combination of chemoradiotherapy, and had lower baseline fatigue levels, respectively. Future studies with larger sample sizes and thus more power are needed to confirm the moderating effects of treatment regimens and fatigue.
Acknowledgments
We thank all participants and professional staff of participating institutes. This study was supported by a grant from the Dutch Cancer Society (UU-2000-2585), the Comprehensive Cancer Center, location Groningen and the Maastricht University. The contribution of L.M. Buffart and J. Kalter was further supported by the “Bas Mulder Award” granted to L.M. Buffart by the Alpe d’HuZes foundation, part of the Dutch Cancer Society.
Conflict of interest
The authors declare to have no conflicts of interest.
References
- 1.Rowland JH, Kent EE, Forsythe LP, Loge JH, Hjorth L, Glaser A, Mattioli V, Fossa SD. Cancer survivorship research in Europe and the United States: where have we been, where are we going, and what can we learn from each other? Cancer. 2013;119(Suppl 11):2094–2108. doi: 10.1002/cncr.28060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. (2014). http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 24 Feb 2014
- 4.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35:1846–1852. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 5.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, Vogelzang NJ. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 6.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;8:CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors—a meta-analysis. Psychooncology. 2011;20:115–126. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 9.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 10.May AM, Korstjens I, van Weert E, van den Borne B, Hoekstra-Weebers JE, van der Schans CP, Mesters I, Passchier J, Grobbee DE, Ros WJ. Long-term effects on cancer survivors' quality of life of physical training versus physical training combined with cognitive-behavioral therapy: results from a randomized trial. Support Care Cancer. 2009;17:653–663. doi: 10.1007/s00520-008-0519-9. [DOI] [PubMed] [Google Scholar]
- 11.Buffart LM, Kalter J, Chinapaw MJ, Heymans MW, Aaronson NK, Courneya KS, Jacobsen PB, Newton RU, Verdonck-de Leeuw IM, Brug J. Predicting Optimal Cancer Rehabilitation and Supportive Care (POLARIS): rationale and design for meta-analyses of individual patient data of randomized controlled trials that evaluate the effect of physical activity and psychosocial interventions on health-related quality of life in cancer survivors. Syst Rev. 2013;2:75. doi: 10.1186/2046-4053-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 13.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, Segal RJ. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy: a randomized controlled trial. Cancer. 2008;112:1845–1853. doi: 10.1002/cncr.23379. [DOI] [PubMed] [Google Scholar]
- 14.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Friedenreich CM, Peddle CJ, Basi S, Chua N, Tankel K, Mazurek A, Reiman T. Moderator effects in a randomized controlled trial of exercise training in lymphoma patients. Cancer Epidemiol Biomarkers Prev. 2009;18:2600–2607. doi: 10.1158/1055-9965.EPI-09-0504. [DOI] [PubMed] [Google Scholar]
- 15.Korstjens I, May AM, van Weert E, Mesters I, Tan F, Ros WJ, Hoekstra-Weebers JE, van der Schans CP, van den Borne B. Quality of life after self-management cancer rehabilitation: a randomized controlled trial comparing physical and cognitive-behavioral training versus physical training. Psychosom Med. 2008;70:422–429. doi: 10.1097/PSY.0b013e31816e038f. [DOI] [PubMed] [Google Scholar]
- 16.van Weert E, May AM, Korstjens I, Post WJ, van der Schans CP, van den Borne B, Mesters I, Ros WJ, Hoekstra-Weebers JE. Cancer-related fatigue and rehabilitation: a randomized controlled multicenter trial comparing physical training combined with cognitive-behavioral therapy with physical training only and with no intervention. Phys Ther. 2010;90:1413–1425. doi: 10.2522/ptj.20090212. [DOI] [PubMed] [Google Scholar]
- 17.May AM, Van Weert E, Korstjens I, Hoekstra-Weebers JE, Van Der Schans CP, Zonderland ML, Mesters I, Van Den Borne B, Ros WD. Improved physical fitness of cancer survivors: a randomised controlled trial comparing physical training with physical and cognitive-behavioural training. Acta Oncol. 2008;47:825–834. doi: 10.1080/02841860701666063. [DOI] [PubMed] [Google Scholar]
- 18.Creer TL. Self-management of chronic illness. Handbook of self regulation. San Diego: Academic; 2002. [Google Scholar]
- 19.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 20.Nezu AM, Nezu CM, Felgoise SH, McClure KS, Houts PS. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. J Consult Clin Psychol. 2003;71:1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- 21.D'Zurilla TJ, Nezu AM. Problem-solving therapy: a positive approach to clinical intervention. New York: Springer; 2007. [Google Scholar]
- 22.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 23.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- 24.Bosscher RJ, Smit JH, Kempen GI. Algemene competentieverwachtingen bij ouderen; een onderzoek naar de psychometrische kenmerken van de algemene competentieschaal (ALCOS) [in Dutch: general competency expectations in elderly; a study into the psychometric properties of the general competency scale] Ned Tijdschr Psychol. 1997;52:239–248. [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363–370. doi: 10.1017/S0033291796004382. [DOI] [PubMed] [Google Scholar]
- 27.Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464–1488. doi: 10.1093/jnci/djp336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 29.Aguinis H, Petersen SA, Pierce CA. Appraisal of the homogeneity of error variance assumption and alternatives to multiple regression for estimating moderating effects of categorical variables. Organ Res Methods. 1999;2:315–339. doi: 10.1177/109442819924001. [DOI] [Google Scholar]
- 30.DeShon RP, Alexander RA. Alternative procedures for testing regression slope homogeneity when group error variances are unequal. Psychol Methods. 1996;1:261–277. doi: 10.1037/1082-989X.1.3.261. [DOI] [Google Scholar]
- 31.Snedecor GW, Cochran WG. Statistical methods. 8. Ames: Iowa State University Press; 1989. [Google Scholar]
- 32.James GS. Tests of linear hypotheses in univariate and multivariate analysis when the ratios of the population variances are unknown. Biometrika. 1954;41:19–43. [Google Scholar]
- 33.Alexander RA, Govern DM. A new and simpler approximation for Anova under variance heterogeneity. J Educ Stat. 1994;19:91–101. doi: 10.2307/1165140. [DOI] [Google Scholar]
- 34.Aguinis H, Pierce CA, Stoneromero EF. Estimating the power to detect dichotomous moderators with moderated multiple-regression. Educ Psychol Meas. 1994;54:690–692. doi: 10.1177/0013164494054003013. [DOI] [Google Scholar]
- 35.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 36.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of longitudinal data. Oxford: Oxford University Press; 2002. [Google Scholar]
- 37.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 38.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 39.Yucel B, Akkas EA, Okur Y, Eren AA, Eren MF, Karapinar H, Babacan NA, Kilickap S. The impact of radiotherapy on quality of life for cancer patients: a longitudinal study. Support Care Cancer. 2014;22:2479–2487. doi: 10.1007/s00520-014-2235-y. [DOI] [PubMed] [Google Scholar]
- 40.Buffart LM, Ros WJ, Chinapaw MJ, Brug J, Knol DL, Korstjens I, van Weert E, Mesters I, van den Borne B, Hoekstra-Weebers JE, May AM. Mediators of physical exercise for improvement in cancer survivors’ quality of life. Psychooncology. 2014;23:330–338. doi: 10.1002/pon.3428. [DOI] [PubMed] [Google Scholar]
- 41.Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 42.Graves KD. Social cognitive theory and cancer patients’ quality of life: a meta-analysis of psychosocial intervention components. Health Psychol. 2003;22:210–219. doi: 10.1037/0278-6133.22.2.210. [DOI] [PubMed] [Google Scholar]
- 43.Korstjens I, Mesters I, Gijsen B, van den Borne B. Cancer patients’ view on rehabilitation and quality of life: a programme audit. Eur J Cancer Care (Engl) 2008;17:290–297. doi: 10.1111/j.1365-2354.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 44.Buffart LM, Galvao DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40:327–340. doi: 10.1016/j.ctrv.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Courneya KS. Physical activity and cancer survivorship: a simple framework for a complex field. Exerc Sport Sci Rev. 2014;42:102–109. doi: 10.1249/JES.0000000000000011. [DOI] [PubMed] [Google Scholar]