Abstract
The invasiveness of malignant gliomas is one of the major obstacles in glioma therapy and the reason for the poor survival of patients. Glioma cells infiltrate into the brain parenchyma and thereby escape surgical resection. Glioma associated microglia/macrophages support glioma infiltration into the brain parenchyma by increased expression and activation of extracellular matrix degrading proteases such as matrix-metalloprotease 2, matrix-metalloprotease 9 and membrane-type 1 matrix metalloprotease. In this work we demonstrate that, matrix-metalloprotease 9 is predominantly expressed by glioma associated microglia/macrophages in mouse and human glioma tissue but not by the glioma cells. Supernatant from glioma cells induced the expression of matrix-metalloprotease 9 in cultured microglial cells. Using mice deficient for different Toll-like receptors we identified Toll-like receptor 2/6 as the signalling pathway for the glioma induced upregulation of microglial matrix-metalloprotease 9. Also in an experimental mouse glioma model, Toll-like receptor 2 deficiency attenuated the upregulation of microglial matrix-metalloprotease 9. Moreover, glioma supernatant triggered an upregulation of Toll-like receptor 2 expression in microglia. Both, the upregulation of matrix-metalloprotease 9 and Toll-like receptor 2 were attenuated by the antibiotic minocycline and a p38 mitogen activated protein kinase antagonist in vitro. Minocycline also extended the survival rate of glioma bearing mice when given to the drinking water. Thus glioma cells change the phenotype of glioma associated microglia/macrophages in a complex fashion using Toll-like receptor 2 as an important signalling pathway and minocycline further proved to be a potential candidate for adjuvant glioma therapy.
Keywords: MMP9, TLR, glioma-associated microglia/brain macrophages, glioma, minocycline
Introduction
Gliomas are primary malignant brain tumors with poor prognosis. Glioblastoma multiforme (GBM), the most aggressive and malignant glioma, accounts for more than 50% of all gliomas with an average survival between 12 to 15 months1. Despite surgery, radiotherapy and chemotherapy malignant gliomas are characterized by fast recurrence2. The glioma cells are highly invasive and infiltrate into the brain parenchyma. Glioma invasion is promoted by the expression of MMPs in the glioma environment, which degrade the extracellular matrix3–5. Microglia/brain macrophages are one of the major cell populations infiltrating glioma and can contribute up to 30% of the tumor mass6, 7. Others and we have demonstrated that the glioma cells and the tumor stroma cells (e.g. microglia) are able to produce MMPs and thereby induce higher invasiveness of gliomas4, 8.
In our previous studies, we characterized the interaction between glioma and microglial cells in the context of MMP-2 activation. We found that the inactive proMMP-2 released from glioma cells can be activated by the membrane-bound MT1-MMP expressed by microglia. MT1-MMP expression in microglia is induced by factor(s) released from glioma cells and acts via TLR2 signalling and p38 MAPK pathway5, 9. The FDA approved antibiotic minocycline interfered with the p38 pathway and reduced the MT1-MMP expression in microglia. As a result, minocycline treatment of mice inoculated with glioma cells had a reduced tumor growth10.
MMP-9, also named as gelatinase B, is thought to play a key role in promoting tumor invasion as a member of the MMP family. MMP-9 levels were also significantly correlated with the histological grade of malignancy in gliomas11. Recent evidence indicated that MMP-9 has a distinct role in tumor angiogenesis mainly in regulating the bioactivity of vascular endothelial growth factor (VEGF), the most promising factor in interfering with tumor angiogenesis and thus a new therapeutic target12, 13. It is known that cancer cells release MMP-9 as well as the host tissue14. So far it has been shown that in the context of glioma MMP-9 is released from bone marrow derived myeloid cells12, 15.
TLRs are one of the most important pattern recognition receptors in the immune system. Ten TLR subtypes (TLR1–TLR10) have been identified in human and 12 (TLR1–TLR9 and TLR11–TLR13) in mice16. TLRs recognize not only pathogen-associated molecular patterns (PAMPs) like bacterial or viral components, but also endogenous mediators like proteins released by cancer cells17. We have previously shown that glioma induce the expression of MT1-MMP in microglia/brain macrophages thereby promoting tumor invasion through TLR2. In human glioma tissue (which contains a substantial proportion of microglia/brain macrophages) TLR2 is highly expressed and inversely correlates with patient survival9. Thus, TLR2 is a novel target for cancer therapy including gliomas18.
Although it has been shown that MMP-9 expression is related to TLR signalling, e.g. in influenza infection19, the correlation between MMP-9 expression and TLR signalling in the glioma context has not yet been explored. In the present study, we show that glioma induced microglial MMP-9 expression through TLR2 signalling and established the role of p38 MAPK blocker minocycline in regulating microglial TLR2 and MMP-9.
Materials and Methods
Human brain tumor tissue
Analysis of resected human brain tumors was performed according to the rules by the Ethical Committee (Charité, EA4/098/11). Briefly, tumor tissue was taken during surgery while patients were under a general anesthetic, and was placed immediately in either culture medium for establishment of cell culture or cell isolation or 4% paraformaldehyde for performing immunofluorescent staining. Resected brain tumors from 5 patients (3 GBMs, 2 anaplastic astrocytomas) were provided by the Department for Neurosurgery Charité University Hospital (Berlin, Germany).
Animals
C57Bl/6 WT mice (Charles River Laboratories, Sulzfeld, Germany) and MyD88 k.o. and TLRs 1, 2, 4, 6, 7 and 9 k.o. mice were used as described previously9, knock out efficiency is shown in supplementary Fig. 3. The mice were bred and maintained in the animal house facilities of the Max Delbrück Center and Charité University Hospital (Berlin, Germany) as per rules of the local governmental institutions.
Cell culture
GL261 murine glioma cells (isogenic to C57BL/6 mice, National Cancer Institute) were cultured in basic culture medium composed of DMEM with 200 mM glutamine, 50 units/ml penicillin, 50 µg/ml streptomycin, and 10% FBS (Invitrogen, Darmstadt, Germany). mCherry GL261 cells were generated as previously described and cultured as WT GL261 cells9. Mouse primary microglia was prepared from neonatal WT, TLR1, TLR2, TLR4, TLR6, TLR7, and TLR9 KO mice on C57BL/6 background as previously described9. The human glioblastoma cells (GBM 1 to 8) were derived from human tumor resections and cultured in RPMI 1640 with 20 µg/ml EGF, FGF (both from Cell Systems, Troisdorf, Germany) and supplements. GBM 1–8 were then established as primary cultures to exclude non-tumor cells. All cells mentioned above were maintained in a 37°C incubator with a 5% CO2 humidified atmosphere.
Production of glioma conditioned medium (GCM)
GCM was prepared from the 80% confluent GL261 cultures by cultivation in basic culture medium overnight. The GCM was collected, briefly centrifuged and sterile-filtered through 0.2 µm filter mesh (Sartorius Stedim Biotech GmbH, Goettingen, Germany) and applied for further experiments.
Microglia stimulation with GCM and minocycline or SB202190
Primary cultured microglial cells were pretreated for 3 hours with either SB202190 (10uM, Sigma-Aldrich, Munich, Germany) or minocycline (25uM or 50uM, Sigma-Aldrich) in cultivation medium. GCM was added either with or without drugs to the microglial cells, after 24 hours stimulation, cells were further cultured for 24 hours in serum free medium. Cell supernatant was collected for western blot or gelatin zymography while cells were collected for RNA isolation, protein extraction or flow cytometry analysis.
Mouse glioma model
C57BL/6 (8–10 weeks old) mice were handled according to governmental (LaGeSo) and internal (MDC) rules and regulations. Briefly, after anesthetization, the mouse head was placed onto a stereotactic frame (David Kopf Instruments, Tujunga, USA). Through a midline incision, a burr hole was made by drilling at 1 mm anterior and 1.5 mm lateral to the bregma. Canals were created by inserting a 1 µL Hamilton syringe 4 mm ventral from dural mater and retracted to a depth of 3 mm from the dural surface. Total of 1 µL (2×104cells/µL) GL261 cell suspension was slowly injected. The needle was then slowly taken out from the injection canal and skin was sutured with a surgical sewing cone (Johnson & Johnson, Neuss, Germany).
Magnetic cell separation of human brain tumor tissue
Glioma associated brain microglia/macrophages were isolated from the human tumor resected tissues. Fresh tissue was dissociated immediately after resection using a neural tissue dissociation kit (MiltenyiBiotec, Bergisch Gladbach, Germany). Erythrocytes were lysed by adding 5 ml ammonium chloride solution. Thereafter, cells were resuspended in PBS containing 0.5% BSA and 2 mM EDTA. Magnetic sorting for CD11b+ cells was then performed by using CD11b MicroBead kit (MiltenyiBiotec) following the manufacturer´s instruction. Magnetic activated cell sorting (MACS) into CD11b negative (Flow-through) and CD11b positive (CD11b+) enriched cell populations was done using several MACS columns in series. Both CD11b negative and CD11b+ fractions were collected. A purity check was performed after MACS by flow cytometry analysis of a small fraction of the sorted populations.
Flow cytometry
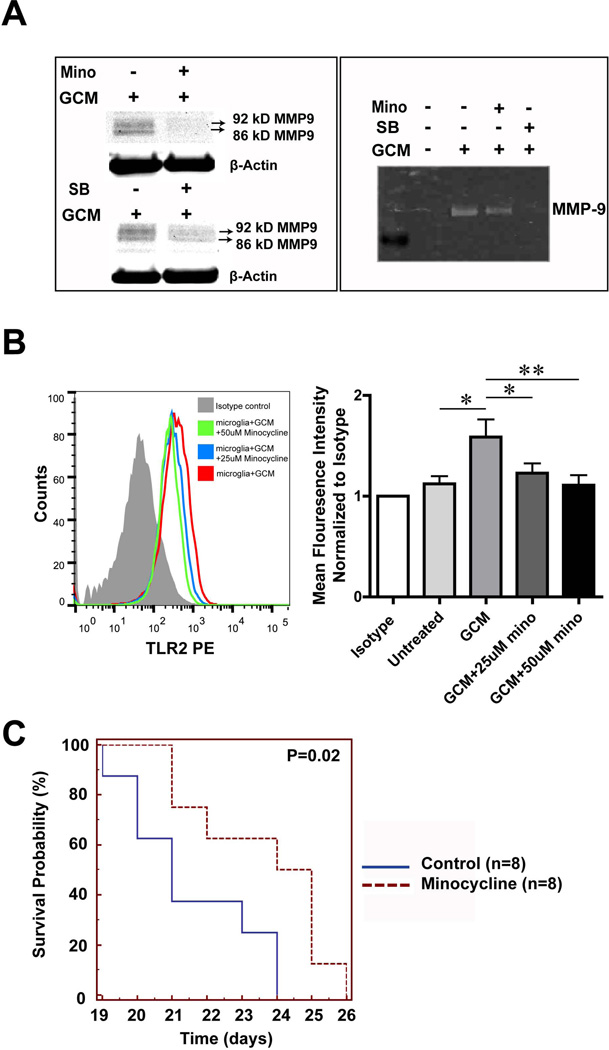
For all the flow cytometry analysis, after the treatment or isolation, cells were incubated for 30 min at 4 °C, using the following fluorescently conjugated antibodies: Phycoerythrin (PE)-conjugated anti-mouse TLR1, TLR2 or TLR6; fluorescein isothiocyanate (FITC)-conjugated CD11b (eBioscience, San Diego, CA) and their recommended isotype controls (Imegenex or eBioscience, San Diego, CA). Flow cytometry was done by BD FACS Aria and data were analyzed using FlowJo software (Treestar, Ashland, OR, USA). Flow cytometry data were quantified by mean fluorescence intensity (MFI) and presented in histograms.
Semi-quantitative and quantitative Real-Time Polymerase Chain Reaction
To assay MMP-9 expression in human tumor cells and mouse microglia, total cellular RNA was isolated with an RNeasy-kit (Qiagen, Hilden, Germany). First-strand cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) by using 50ng-250ng RNA and oligo-dT primer. PCR was performed with a High Fidelity kit (Invitrogen). Sequences for RT-PCR primer sets were as follow: for mouse MMP-9 forward 5’-CATTCGCGTGGATAAGGAGT-3’, reverse 5’–ACCTGGTTCACCTCATGGTC-3’, and for actin forward 5'-CCCTGAAGTACCCCATTGAA-3', reverse 5'-GTGGACAGTGAGGCCAAGAT'-3’. For mouse TLR and MyD88, specific primers were purchased from biomol (Hamburg, Germany) and described as previously20. Real-time quantitative RT-PCR was performed for 40 cycles on a Fast Real-Time PCR System (Applied Biosystems, Carlsbad, USA) using SYBR Green kit (Applied Biosystems). human MMP-9 primers are: forward 5’-AAGGCGCAGATGGTGGAT-3’, reverse 5'-TCAACTCACTCCGGGAACTC-3’.
Western blotting
Total cellular lysates from mouse microglia were obtained by RIPA buffer (Sigma-Aldrich) containing EDTA-free protease inhibitor cocktail tablets (Roche Diagnostics GmbH, Germany) at 4 °C. Cellular debris was removed by centrifugation, and protein quantification was performed using the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific,Rockford, USA). Proteins were resolved on 10% SDS-PAGE gels, and immunoblotting was performed using the following primary antibodies: rabbit polyclonal anti-MMP-9 (Abcam, Cambridge, United Kingdom) and mouse monoclonal anti-β-actin (Sigma-Aldrich). Anti-rabbit HRP-conjugated secondary antibody (Cell signaling, Danvers, USA) was used and visualized using enhanced chemiluminescence (Amersham Biosciences, Piscataway, USA).
Enzyme-linked immunosorbent assay (ELISA)
Supernatant collected from mouse primary cultured microglia (from WT, TLRs 1, 2, 4, 6, 7 and 9 KO mice) determined for total MMP-9 by ELISA kits according to the manufacturer’s protocols (R&D systems, Abingdon, United Kingdom). The colometric reaction was read on a Tecan Infinite F-500 photometer. Results were presented as picograms of MMP-9 per 1×106 cells.
Gelatine zymography
For activity of MMP-9 (gelatinase B) analysis, microglia conditioned medium was mixed with sample buffer and electrophoresed in 10 % SDS gels containing as substrate procaine gelatine. The detailed procedure was described previously4. Briefly, after electrophoresis, gels were washed in a solution of 2.5% Triton X-100, and incubated overnight at 37 °C in developing buffer. Gels were then stained with Coomassie Brilliant Blue R250 and destained in a solution of 10 % acetic acid and 40 % methanol. Recombinant human MMP-9 (R&D systems) was used as a positive control.
Immunofluorescent staining and image processing
Resected human glioma tissue and mouse brains were prepared as previously described21. After cryosection, 16 µm-thick brain sections were mounted onto glass slides. Sections were then washed with PBS (pH 7.4), followed by antigen retrieval using sodium citrate buffer, pH 6, at 95°C for 25 min in a water bath. Nonspecific staining in sections was blocked using 10% donkey serum in 0.1% Tween 20-PBS for 1 h at room temperature. Primary antibodies were added overnight at a dilution of 1:350 for goat Iba-1 (Abcam), 1:1000 for rabbit MMP-9 (Abcam) at 4°C. Alexa 488-conjugated donkey anti-goat IgG (1:200, Jackson Lab, Suffolk, United Kingdom) or Cy3-conjugated donkey anti-rabbit IgG (1:200, Jackson Lab) were subsequently applied. The nuclei were counterstained with DAPI (Sigma-Aldrich). Images were taken using a confocal microscope (TCS SP5, Leica, Wetzlar, Germany) with 20X, 40X, or 63X oil objectives. Iba-1 positive labeled cells were counted using Image J software (NIH, Bethesda, USA).
In vivo assessment of mouse survival with minocycline treatment
C57BL/6 mice bearing glioma were treated with minocycline (Sigma-Aldrich). Briefly, minocycline powder was dissolved in drinking water. The oral treatment started on the same day with tumor inoculation. Mice were kept under cautious observation and median survival time was also analyzed. Survival rate was calculated by the Kaplan-Meier method (MedCalc, Ostend, Belgium) by log rank analysis.
Statistical analysis
All data represent the average of at least 3 independent experiments. Error bars represent standard error of the mean. Data sets were analyzed statistically by SPSS11.5 software and tested for normality by Shapiro-Wilks test. For non-parametric analysis, the Mann-Whitney-U test was used. Parametric testing was done with Student-t test. Comparisons between multiple groups were done using one-way ANOVA with Scheffé post-hoc test. Statistical significance was determined at p values < 0.05 (*) and <0.01 (**) while n.s. implied a non-significant p value.
Results
Microglial MMP-9 is up-regulated by glioma supernatant and in the glioma environment
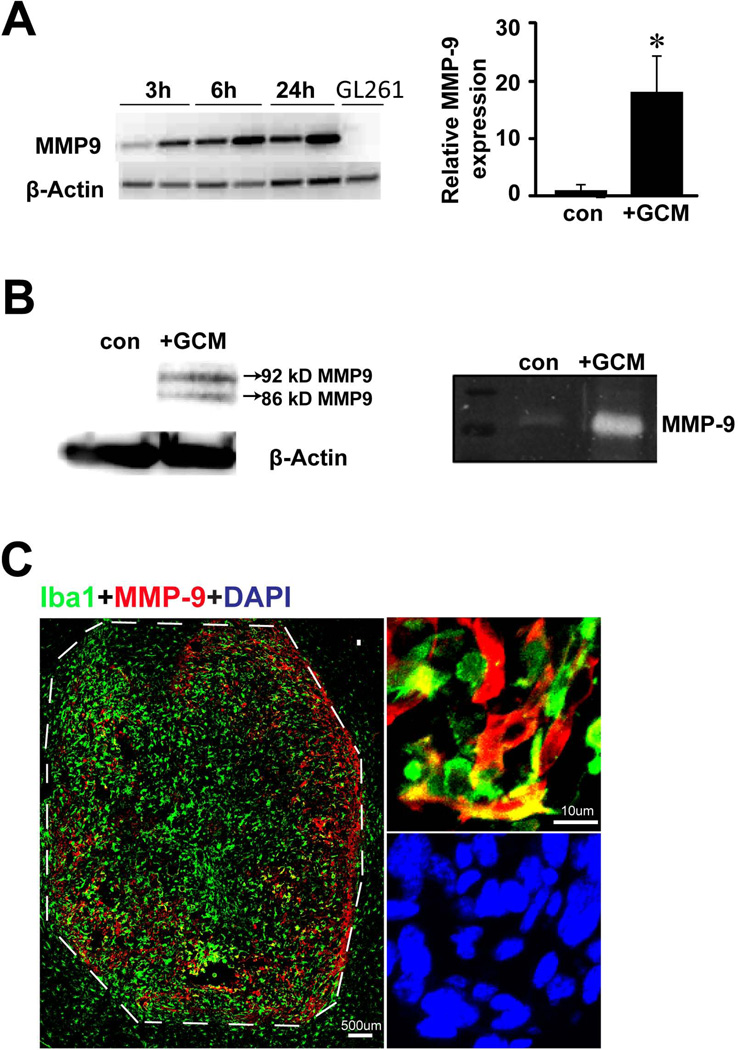
To test whether glioma cells trigger MMP-9 upregulation in microglia, mouse primary microglial cell cultures were treated with normal medium (as controls) or glioma conditioned medium (GCM). After 3, 6, or 24 h, cells were lysed and MMP-9 RT-PCR was performed. Compared to control medium, microglial MMP-9 mRNA was up-regulated upon GCM treatment already after 3 h and was further increased after 6 and 24 h (Fig. 1A). In GL261 cells, MMP-9 expression could not be detected. We further performed quantitative PCR and found that after 24 h of GCM treatment, microglial MMP-9 mRNA level was increased by 17 times (±6, p=0.02) when compared to non-treated controls (Fig. 1A). This effect was further verified by western blot and gelatin zymography where we observed a significant increase in MMP-9 production and secretion, respectively, upon GCM treatment (Fig. 1B). To examine the MMP-9 expression in an experimental glioma model, WT or mCherry GL261 cells were inoculated into mouse brains. Mice were sacrificed after two weeks of glioma growth, and brain sections were stained with Iba-1 and MMP-9 antibodies. As shown in Fig.1C, we detected strong immunolabelling for MMP-9 in the glioma tissue, but not in the tumor free area. The glioma associated Iba-1-positive microglia/brain macrophages, but not the tumor cells were the dominant cell population expressing MMP-9 (Supplementary Fig. 4).
Fig. 1. Microglial cells are upregulating MMP-9 when associated with gliomas.
(A) MMP-9 gene expression in microglia stimulated with GCM for 3h, 6h and 24h was analyzed by RT-PCR (left). GL261 cells were also analyzed for MMP-9 expression. β-actin serves as a loading control. Microglial MMP-9 expression was quantified with qRT-PCR after 24h stimulation with GCM (right). Bars represent the mean±s.e.m. from 3 independent experiments. (B) Western blot from both cell lysate (left panel) and gelatin zymography from supernatant (right panel) showed MMP-9 induction in microglia upon GCM stimulation for 24h. (C) Mouse brains injected with GL261 glioma cells were stained for microglial marker Iba-1(green) and for MMP-9 (red). In the tumor free area the level of MMP-9 was low (left, out of the dash line) while within the tumor MMP-9 is expressed mainly in Iba-1 positive cells (right).
Microglia are main source of MMP-9 in the mouse and human glioma tissue
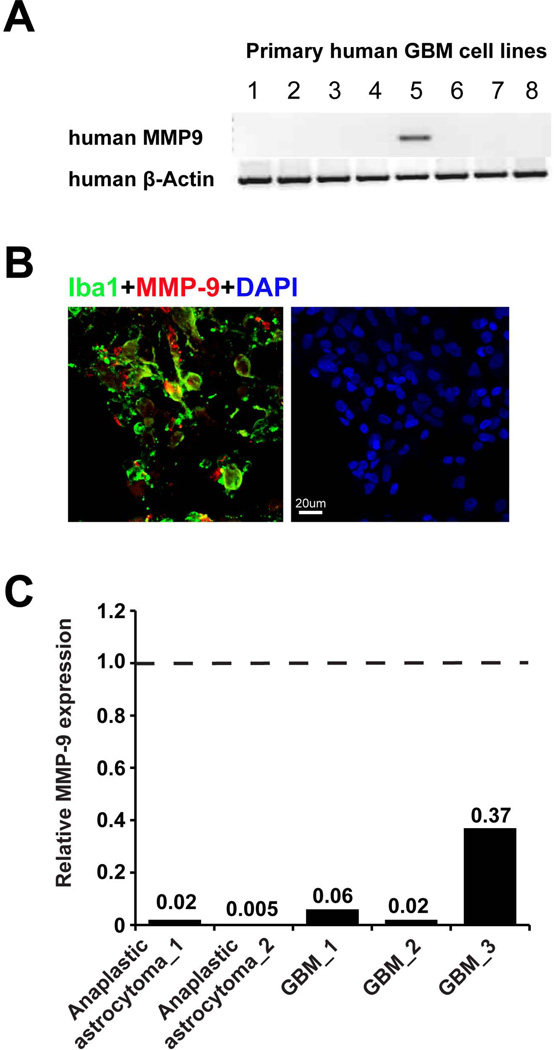
It has been reported that glioma cell lines as well as primary GBM tissue expressed MMP-922, 23, while for a mouse glioma model the main source of MMP-9 were glioma infiltrating CD45+ cells, which was essential and sufficient to initiate angiogenesis by increasing VEGF activity12. We analyzed the MMP-9 expression in human GBM patient tissue by immunohistochemistry. As seen in Fig. 2B, MMP-9 was predominantly expressed by Iba1+ cells, indicating glioma associated microglia/brain macrophages are the predominant cells expressing MMP-9. We also isolated these cells by MACS directly after tumor resection and analyzed MMP9 gene expression in CD11b+ cells (i.e. glioma associated microglia/macrophage) and flow-through cells (i.e. mainly glioma cells). CD11b+ cell purity was analyzed by flow cytometry (see Supplementary Fig. 1). The MMP-9 expression was normalized to the level of the expression in CD11b+ cells, and the level of MMP-9 expression in flow through cells was compared to that. In the human glioma samples MMP-9 expression was predominately from GAMs as determined by qPCR from MACS isolated cells and flow through cells (Fig. 2C). When we compared MMP-9 expression by RT-PCR in 8 different established primary human glioma cell lines, we found MMP-9 expression in only one (Fig. 2A). Moreover, the GL261 mouse glioma cell line also did not express MMP-9 (Fig. 1A).
Fig. 2. Glioma associated microglia/macrophages but not gliomas are the main MMP-9 producing cells.
(A) 8 primary cultured human GBM cell lines were analyzed for MMP-9 expression in mRNA level by RT-PCR (β-Actin serves as a loading control). In only one line MMP-9 was detected. (B) Human GBM tissues were analyzed by immunohistochemistry showing that Iba1 positive cells (green) express MMP-9 (red). (C) MACS freshly isolated CD11b+ cells and flow-through (i.e. mainly glioma cells) from 3 GBM and 2 anaplastic astrocytoma were analyzed for MMP-9 expression by qRT-PCR (Dash line represents MMP-9 expression in CD11b+ cells in each sample, solid bars represent folder changes of MMP-9 expression in flow through cells compared to CD11b+cells).
Glioma released factors induced microglial MMP-9 expression through MyD88-TLR2 signalling
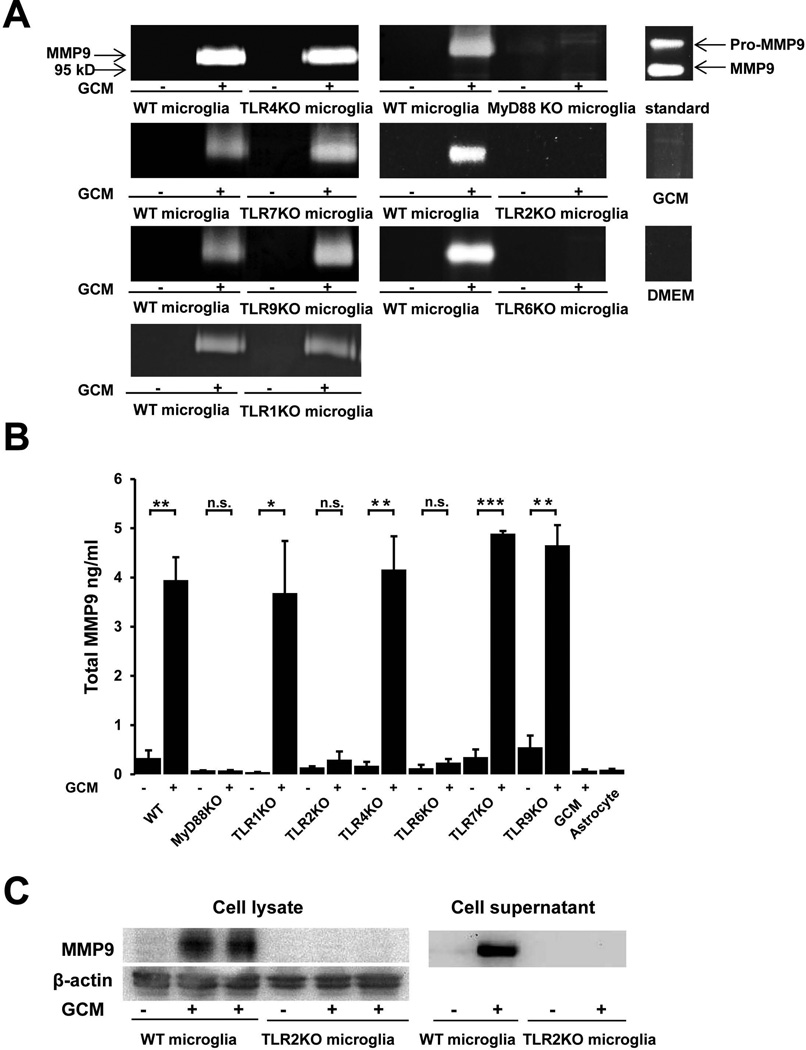
We have previously shown that glioma-released factor(s) induced microglial MT1-MMP production mediated through the TLR2 signalling pathway9. To check if microglial MMP-9 induction is also regulated by TLRs, we cultured microglial cells from WT mice and from mice deficient for TLR1, TLR2, TLR4, TLR6, TLR7, TLR9 and the intracellular adaptor molecule MyD88. The microglial cells were stimulated for 24 h with GCM and incubated for another 24 hours with serum free cultivation medium. MMP-9 expression in the cell supernatant was analyzed by gelatin zymography, Elisa and Western blot. As seen in Fig. 3A, GCM induced MMP-9 functional expression as revealed by zymograpy in WT and TLR1KO, TLR4 KO, TLR7 KO, TLR9 KO, but not in the Myd88, TLR2 and TLR6 deficient microglia. These effects were further verified by Elisa (Fig. 3B); GCM stimulated microglia released significant higher amount of MMP-9 compared to the untreated control (Control: 0.33±0.17ng/ml, GCM: 3.95±0.46ng/ml, p=0.002), and this expression level was not altered when TLR1 (control: 0.05±0.003ng/ml, GCM: 3.68±1.06ng/ml, p=0.026), TLR4 (control: 0.17±0.08ng/ml, GCM: 4.16±0.68ng/ml, p=0.004), TLR7 (control: 0.35±0.15ng/ml, GCM: 4.89±0.06ng/ml, p<0.001) or TLR9 (control: 0.55±0.24ng/ml, GCM: 4.65±0.41ng/ml, p=0.001) was absent. In contrast, microglial MMP-9 induction by GCM was impaired from mice deficient for MyD88 (control: 0.08±0.001ng/ml, GCM: 0.08±0.006ng/ml, p=0.378), TLR2 (control: 0.14±0.023ng/ml, GCM: 0.3±0.17ng/ml, p=0.409) or TLR6 (control: 0.12±0.07ng/ml, GCM: 0.238±0.08ng/ml, p=0.107). Cell lysate analyzed by Western blot confirmed that the GCM induced microglial MMP-9 up-regulation depends on TLR2 signaling since increased protein level was observed in WT microglia, but not in TLR2 KO microglia (Fig. 3C). To confirm that microglial MMP-9 secretion can be induced by TLR2 activation, primary microglia from WT mice were stimulated for 24 h with the TLR2 agonists Pam3CSK4, PG-LPS from P. gingivalis and HKLM (heat-killed Listeria monocytogenes). Subsequently cell supernatant was collected and gelatin zymography was performed. As shown in Fig. 4A, all three agonists induced the expression of MMP-9.
Fig. 3. TLR2 signalling triggers glioma associated microglial MMP-9 up-regulation.
(A) Microglia from WT and MyD88 or TLR 1, 2, 4, 6, 7 and 9 deficient mice were stimulated with GCM for 24h, cell supernatant was collected for gelatin zymography. Recombinant MMP-9 was used as a standard, GCM and DMEM were loaded as controls. Images were representative from 3 independent experiments. (B) Elisa was performed on cell supernatant for quantification of total MMP9 release. GCM and conditioned medium from primary cultured astrocytes were used as controls. Bars represent the mean±s.e.m. from 3 independent experiments. (C) Cell lysate and supernatant from WT and TLR2 KO microglia treated with GCM for 24h were further analyzed by western blot and compared to an untreated control (β-actin served as a loading control).
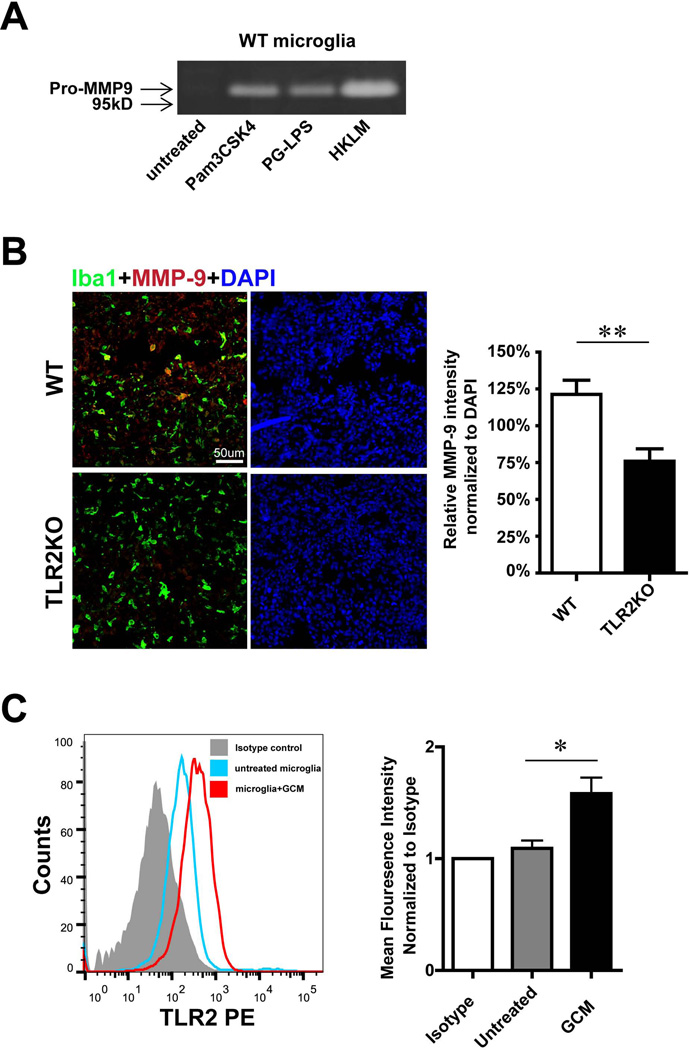
Fig. 4. Microglial MMP-9 as well as TLR2 is regulated by GCM stimulation.
(A) The influence of TLR2 ligands on MMP-9 expression was determined in primary microglia from WT mice. Microglial cells were stimulated with 3 different TLR2 agonists for 24h and levels of MMP-9 in the supernatant were analyzed by gelatin zymography. (B) Slices from glioma inoculated WT and TLR2 KO mice were immunohistologically labeled. Microglia/brain macrophages were identified by the expression of Iba1 (green) and MMP-9 by immunolabelling in red (left), fluorescence intensity was quantified by ImagJ and normalized to DAPI (right). (C) TLR2 expression of microglia upon GCM stimulation for 24 h was analyzed by flow cytometry. Representative histogram of flow cytometry was shown (left panel), mean fluorescence intensity (normalized to isotype control) was used to quantify the expression of TLR2 (right panel). Bars represent the mean±s.e.m. from 6 independent experiments.
TLR2-deficient mice show reduced MMP-9 in vivo
We used an experimental mouse glioma model to study MMP-9 expression in GAMs in vivo. Cells of the mouse glioma cell line GL261 were inoculated into WT and TLR2 KO mice. After 2 weeks of glioma growth, brain tissue was analyzed by immunohistochemistry for MMP-9 expression. MMP-9 fluorescence intensity was quantified and normalized to DAPI with Imag J software. As shown in Fig. 4B, we observed an increase in MMP-9 immunoreactivity within the tumor in WT mice, whereas in TLR2 KO mice, the immunoreactivity of MMP-9 was significantly lower compared to the WT controls (WT 121%±10%, TLR2KO 76%±8%, p=0.008).
TLR2 expression is upregulated by GCM
We addressed the question whether microglial TLR2 expression is affected by GCM. Primary cultured microglia were incubated with GCM for 24 hours, and microglial TLR2 expression levels were analyzed by flow cytometry. GCM stimulated microglia had higher levels of TLR2 as compared to the unstimulated controls (Fig.4C). We quantified the data by analyzing the mean fluorescence intensity (normalized to isotype); microglial TLR2 expression is significantly (1.58±0.13 folder) up-regulated after GCM treatment (n=6). Since TLR1 and TLR6 are heterodimers of TLR2, we also determined TLR1 and TLR6 expression under GCM stimulation. We could not see any significant difference between control and GCM stimulated microglia (see supplementary Fig. 2).
Minocycline attenuated glioma-induced MMP-9 and TLR2 expression in vitro
Our previous data have shown that minocycline and the p38 MAPK inhibitor SB202190 inhibit MT1-MMP expression in glioma associated microglia5, 10. We therefore tested whether minocycline and the p38 MAPK inhibitor SB202190 can also interfere with the induction of MMP-9 expression. Mouse microglial cells were first pretreated with either minocycline or SB202190 for 3h, following 24 hours treatment either with GCM alone or GCM together with SB202190 or minocycline. Cells were further incubated for another 24 hours with serum free medium. Cell lysate was then collected to perform Western blot and supernatant to perform gelatin zymography. 50 µM minocycline and 10 µM p38 MAPK inhibitor (SB202190) reduced MMP-9 production from GCM treated microglia as analyzed in Western blot and gelatin zymography (Fig. 5A).
Fig. 5. Minocycline interfers with glioma associated microglial MMP9 and TLR2 induction.
(A) MMP-9 expression is shown by Western blot in the lysate (left) and Gelatin zymography in the supernatant (right) obtained from primary cultured microglia. On the left, cells were stimulated with GCM alone or with GCM combined with either 50 uM minocycline (Mino, upper panel) or 10uM MAPK inhibitor SB202190 (SB, lower panel) for 24h. Similarly on the right panel MMP-9 expression is shown for the supernatant obtained from cells stimulated with GBM alone or combined with minocycline and SB. (B) Microglia was either treated with GCM or GCM together with 25uM or 50uM minocycline for 24 h and TLR2 in microglia was analyzed by flow cytometry (left panel: representative histogram). On the right panel, mean fluorescence intensity was quantified. Bars represent the mean±s.e.m. from 5 independent experiments. (C) The Kaplan-Meier survival curve represents the survival rate of mice bearing tumor with or without minocycline treatment through drinking water (30mg/kg/day).
We also tested whether minocycline would affect the up regulation of TLR2 by GCM. As shown in Fig. 5B, we detected significantly lower levels of microglial TLR2 expression when GCM-stimulated cells were treated with minocycline (Mean fluorescence intensity was normalized to isotype, 25uM: 1.23±0.1 p=0.02; 50uM: 1.11±0.1 p<0.001) compared to the cells treated with GCM only (GCM: 1.59±0.18).
Minocycline prolonged survival time of glioma bearing mice
We showed previously that minocycline could reduce glioma growth in an experimental mouse glioma model10. We now analyzed survival time comparing minocycline-treated mice with untreated controls. After tumor inoculation into mice brains, the mice received minocycline (30mg/kg/day) in the drinking water from the day of implantation. We could demonstrate that treatment with minocycline significantly improved the mouse survival by Kaplan Meier analysis, p=0.02 (Fig. 5C).
Discussion
In this study we demonstrate that soluble factors released from glioma cells induce MMP-9 expression in microglial cells/macrophages via a MyD88/TLR2 signalling pathway using mouse cell culture, mouse in vivo glioma model and human glioblastoma samples. Moreover we show that the FDA-approved drug minocycline attenuates the glioma induced MMP-9 as well as TLR2 expression in microglia and thereby qualifies as possible adjuvant therapy for gliomas.
There are several studies which highlight the importance of MMP-9 for glioma growth and invasiveness24. It has been reported, that silencing of MMP-9 either by shRNA or antisense RNA approach in the human glioma cell lines diminishes its proliferation, tumor growth and neovascularisation both in vitro and in vivo25, 26. There is also evidence that glioma associated microglia or infiltrated myeloid cells are major MMP-9 producers12, 27. Treatment with a microglia inhibitor (propentophyline) down regulated microglial MMP-9 expression and decreased glioma growth and invasiveness in a rat glioma model27. Hagemann showed that human GBM samples expressed high level of MMP-9 in passage 1, while the expression successively decreased and almost disappeared at passage 1028. This could indicate that primary samples contain MMP-9 expressing microglia, which are diluted with each passage until they disappear at late passages. Our data support the statement that MMP-9 is predominantly expressed in glioma-associated microglia/macrophages and considerably less in glioma cells. Gene regulation (e.g. MMPs) in microglia might be species-dependent29, however, MMP-9 expression pattern was similar in mouse and human glioma tissue from which we were able to isolate and separate the two cell populations, microglia and glioma cells.
Microglia/macrophages associated with human gliomas express higher levels of TLR1, 2, 3 and 4 as compared to control microglia, but the activation of these receptors did not lead to the secretion of pro-inflammatory cytokines such as tumor necrosis factor alpha, interleukin 1, or interleukin 630. Nevertheless, experimental TLR stimulation was employed for inducing anti-tumoral immunity in mouse gliomas and TLR9 stimulation lead to decreased tumor growth31. We have recently demonstrated that TLR2 is an important signalling pathway for inducing MT1-MMP in GAMs9. Here we demonstrate that TLR2 is equally important for the induction of MMP-9 in these cells thus sharing a similar pathway. While for the induction of MT1-MMP both heterodimers TLR1/TLR2 and TLR2/TLR6 play a role, we show in the present study that the induction of MMP-9 predominantly requires the presence of TLR2/TLR6 only. TLR1, 4, 7 and 9 did not play a significant role as in cells deficient for these TLRs the induction of MMP-9 by GCM was not different compared to control cells. We could confirm the importance of TLR2 since we could mimic the effect of GCM on the induction of MMP-9 by specific TLR2 agonists. Moreover, these results were also supported by in vivo data, since we observed lower levels of MMP-9 in TLR2KO animals injected with glioma cells compared to wild type animals. We showed that glioma cells increase the expression of TLR2 on GAMS and utilize this signalling pathway to upregulate MMP-9 and thus provide the substrate of extracellular matrix degradation to promote glioma cell invasion. In our gel zymography we could only detect the latent form (pro-form) of MMP-9 while in western blot both latent and active form of MMP-9 were found. The activation of MMP-9 involves other molecules like MMP-3 and TIMP-1, these could be provided by tumor cells or microglia32.
In this study, we used minocycline to attenuate the pro-tumorigenic phenotype of GAMs. Minocycline is a tetracycline based antibiotic and an FDA approved drug. It has been demonstrated to inhibit microglial activation in different disease models including glioma33. Minocycline down-regulates MMP-9 expression in T lymphocytes34 and other MMPs such as MT1-MMP in microglia35. It has also been shown that minocycline could impede microglial TLR2 during infections. Minocycline even attenuated LPS or bacteria induced TLR2 up-regulation in microglial cells36, 37. We now could show that minocycline attenuated the deleterious upregulation of TLR2 and subsequently MMP-9 in GAMs. Moreover, recent studies also demonstrated that minocycline reduced glioma growth by inducing glioma autophagy38, 39.
Minocycline is currently used in many experimental and clinical studies showing beneficial effects on chronic inflammatory diseases such as Huntington´s and Alzheimer´s disease34, 40, 41, but also in cancer. In an ovarian cancer mouse model the authors demonstrated the inhibitory effect of minocycline on ovarian cancer growth42. In a study of combined treatment with minocycline and Celecoxib (COX-2 inhibitor) in a mouse model for breast cancer metastasis into bone the authors showed that MMP-9 was downregulated in the tumor tissue next to the metastasis site43. Finally, the University of Utah has started a clinical study using repeated radiation, minocycline and VEGF monoclonal blocking-antibody Bevacizumab (Avastin®) in treating recurrent glioblastomas (http://clinicaltrials.gov/ct2/show/NCT01580969?term=minocycline+glioma&rank=1). Thus minocycline has the potential to become a standard element inglioma therapy.
Supplementary Material
Novelty and Impact Statements.
Glioma converts microglia into a tumor-supportive phenotype via Toll-like receptor (TLR) signaling. We now demonstrate that activation of the TLR2/6 pathway in microglia induces the expression of MMP-9, a member of the matrix metalloprotease family important for tumor invasion as well as angiogenesis. In parallel, TLR2 receptors are upregulated. Both mechanisms, MMP-9 and TLR2 upregulation, are attenuated by the antibiotic minocycline qualifying it as an adjuvant for glioma therapies.
Acknowledgments
We sincerely thank Karen Rosenberger and Katja Drekow for their extensive help in providing MyD88, TLRs 4, 7 and 9 KO mice from Charité, Berlin. Many thanks to Irene Haupt, Regina Piske and Nadine Scharek for excellent technical assistance. This work was supported by China Scholarship Council stipend (CSC, China) to Feng Hu and by the Deutsche Forschungsgemeinschaft (SFB-TR 43, KE 329/30-1), NeuroCure and NIC Grant (U01CA160882-01A1).
Abbreviations
- GAM
glioma-associated microglia/brain macrophages
- GCM
glioma conditioned medium
- KO
knockout
- MAPK
mitogen activated protein kinase
- MMP
matrix metalloprotease
- MT1-MMP
membrane-type 1 matrix metalloprotease
- MyD88
myeloid differentiation primary response gene (88)
- TLR
Toll-like receptor
- WT
wild-type
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markovic DS, Glass R, Synowitz M, Rooijen N, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64:754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- 5.Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kalin R, van Rooijen N, Holmbeck K, Heppner FL, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci U S A. 2009;106:12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech. 2001;54:106–113. doi: 10.1002/jemt.1125. [DOI] [PubMed] [Google Scholar]
- 7.Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002;40:252–259. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs VL, Landry RP, Liu Y, Romero-Sandoval EA, De Leo JA. Propentofylline decreases tumor growth in a rodent model of glioblastoma multiforme by a direct mechanism on microglia. Neuro-oncology. 2012;14:119–131. doi: 10.1093/neuonc/nor194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinnakota K, Hu F, Ku MC, Georgieva PB, Szulzewsky F, Pohlmann A, Waiczies S, Waiczies H, Niendorf T, Lehnardt S, Hanisch UK, Synowitz M, et al. Toll-like receptor 2 mediates microglia/brain macrophage MT1-MMP expression and glioma expansion. Neurooncology. 2013 doi: 10.1093/neuonc/not115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markovic DS, Vinnakota K, van Rooijen N, Kiwit J, Synowitz M, Glass R, Kettenmann H. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun. 2011;25:624–628. doi: 10.1016/j.bbi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Rao JS, Steck PA, Mohanam S, Stetler-Stevenson WG, Liotta LA, Sawaya R. Elevated levels of M(r) 92,000 type IV collagenase in human brain tumors. Cancer Res. 1993;53:2208–2211. [PubMed] [Google Scholar]
- 12.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raithatha SA, Muzik H, Muzik H, Rewcastle NB, Johnston RN, Edwards DR, Forsyth PA. Localization of gelatinase-A and gelatinase-B mRNA and protein in human gliomas. Neuro-oncology. 2000;2:145–150. doi: 10.1093/neuonc/2.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 17.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, Dev A, Sievert W, Ooi CH, Ishikawa TO, Oshima H, Bhathal PS, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–478. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJ. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog. 2012;8:e1002641. doi: 10.1371/journal.ppat.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaul D, Habbel P, Derkow K, Kruger C, Franzoni E, Wulczyn FG, Bereswill S, Nitsch R, Schott E, Veh R, Naumann T, Lehnardt S. Expression of Toll-like receptors in the developing brain. PloS one. 2012;7:e37767. doi: 10.1371/journal.pone.0037767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ku MC, Wolf SA, Respondek D, Matyash V, Pohlmann A, Waiczies S, Waiczies H, Niendorf T, Synowitz M, Glass R, Kettenmann H. GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol. 2013;125:609–620. doi: 10.1007/s00401-013-1079-8. [DOI] [PubMed] [Google Scholar]
- 22.Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 23.Song H, Li Y, Lee J, Schwartz AL, Bu G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009;69:879–886. doi: 10.1158/0008-5472.CAN-08-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagemann C, Anacker J, Ernestus RI, Vince GH. A complete compilation of matrix metalloproteinase expression in human malignant gliomas. World J Clin Oncol. 2012;3:67–79. doi: 10.5306/wjco.v3.i5.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun C, Wang Q, Zhou H, Yu S, Simard AR, Kang C, Li Y, Kong Y, An T, Wen Y, Shi F, Hao J. Antisense MMP-9 RNA inhibits malignant glioma cell growth in vitro and in vivo. Neurosci Bull. 29:83–93. doi: 10.1007/s12264-012-1296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Xiao A, diPierro CG, Carpenter JE, Abdel-Fattah R, Redpath GT, Lopes MB, Hussaini IM. An extensive invasive intracranial human glioblastoma xenograft model: role of high level matrix metalloproteinase 9. Am J Pathol. 176:3032–3049. doi: 10.2353/ajpath.2010.090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs VL, Landry RP, Liu Y, Romero-Sandoval EA, De Leo JA. Propentofylline decreases tumor growth in a rodent model of glioblastoma multiforme by a direct mechanism on microglia. Neuro-oncology. 14:119–131. doi: 10.1093/neuonc/nor194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagemann C, Anacker J, Haas S, Riesner D, Schomig B, Ernestus RI, Vince GH. Comparative expression pattern of Matrix-Metalloproteinases in human glioblastoma cell-lines and primary cultures. BMC Res Notes. 2010;3:293. doi: 10.1186/1756-0500-3-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitek MP, Brown C, Xu Q, Dawson H, Mitsuda N, Colton CA. Characterization of NO and cytokine production in immune-activated microglia and peritoneal macrophages derived from a mouse model expressing the human NOS2 gene on a mouse NOS2 knockout background. Antioxidants & redox signaling. 2006;8:893–901. doi: 10.1089/ars.2006.8.893. [DOI] [PubMed] [Google Scholar]
- 30.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-oncology. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grauer OM, Molling JW, Bennink E, Toonen LW, Sutmuller RP, Nierkens S, Adema GJ. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008;181:6720–6729. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- 32.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. The Journal of biological chemistry. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 33.Suk K. Minocycline suppresses hypoxic activation of rodent microglia in culture. Neurosci Lett. 2004;366:167–171. doi: 10.1016/j.neulet.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 34.Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- 35.Markovic DS, Vinnakota K, van Rooijen N, Kiwit J, Synowitz M, Glass R, Kettenmann H. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain, behavior, and immunity. 25:624–628. doi: 10.1016/j.bbi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kielian T, Esen N, Liu S, Phulwani NK, Syed MM, Phillips N, Nishina K, Cheung AL, Schwartzman JD, Ruhe JJ. Minocycline modulates neuroinflammation independently of its antimicrobial activity in staphylococcus aureus-induced brain abscess. Am J Pathol. 2007;171:1199–1214. doi: 10.2353/ajpath.2007.070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu WT, Lin CH, Hsiao M, Gean PW. Minocycline inhibits the growth of glioma by inducing autophagy. Autophagy. 2011;7:166–175. doi: 10.4161/auto.7.2.14043. [DOI] [PubMed] [Google Scholar]
- 39.Liu WT, Huang CY, Lu IC, Gean PW. Inhibition of glioma growth by minocycline is mediated through endoplasmic reticulum stress-induced apoptosis and autophagic cell death. Neuro-oncology. 2013;15:1127–1141. doi: 10.1093/neuonc/not073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer's disease. Neurodegener Dis. 9:187–198. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sancho M, Herrera AE, Gortat A, Carbajo RJ, Pineda-Lucena A, Orzaez M, Perez-Paya E. Minocycline inhibits cell death and decreases mutant Huntingtin aggregation by targeting Apaf-1. Hum Mol Genet. 20:3545–3553. doi: 10.1093/hmg/ddr271. [DOI] [PubMed] [Google Scholar]
- 42.Pourgholami MH, Mekkawy AH, Badar S, Morris DL. Minocycline inhibits growth of epithelial ovarian cancer. Gynecol Oncol. 125:433–440. doi: 10.1016/j.ygyno.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Niu G, Liao Z, Cai L, Wei R, Sun L. The combined effects of celecoxib and minocycline hydrochloride on inhibiting the osseous metastasis of breast cancer in nude mice. Cancer Biother Radiopharm. 2008;23:469–476. doi: 10.1089/cbr.2008.0475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.