Abstract
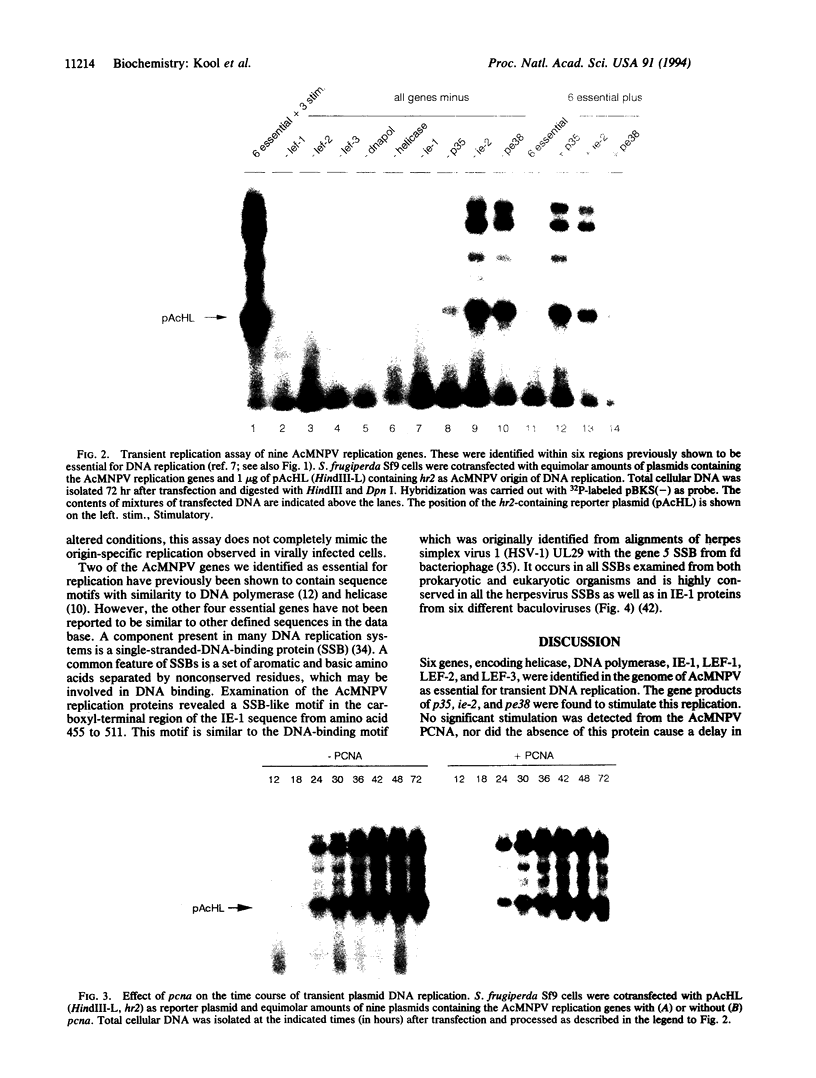
By use of a transient replication assay, nine genes involved in DNA replication were identified in the genome of the Autographa californica baculovirus. Six genes encoding helicase, DNA polymerase, IE-1, LEF-1, LEF-2, and LEF-3 are essential for DNA replication while three genes encoding P35, IE-2, and PE38 stimulate DNA replication. No stimulation by the AcMNPV pcna gene, encoding a protein with sequence homology to proliferating-cell nuclear antigen, was observed. A pattern of amino acids found in a number of single-stranded-DNA-binding proteins was identified in the carboxyl-terminal region of IE-1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blissard G. W., Rohrmann G. F. Baculovirus diversity and molecular biology. Annu Rev Entomol. 1990;35:127–155. doi: 10.1146/annurev.en.35.010190.001015. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Guarino L. A., Summers M. D. Functional mapping of an AcNPV immediately early gene which augments expression of the IE-1 trans-activated 39K gene. Virology. 1988 Feb;162(2):444–451. doi: 10.1016/0042-6822(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Summers M. D., Guarino L. A. Molecular analysis of a baculovirus regulatory gene. Virology. 1991 May;182(1):279–286. doi: 10.1016/0042-6822(91)90671-w. [DOI] [PubMed] [Google Scholar]
- Challberg M. D. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. S., Shenk T. The adenovirus DNA-binding protein stimulates the rate of transcription directed by adenovirus and adeno-associated virus promoters. J Virol. 1990 May;64(5):2103–2109. doi: 10.1128/jvi.64.5.2103-2109.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Clem R. J., Fechheimer M., Miller L. K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991 Nov 29;254(5036):1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- Crawford A. M., Miller L. K. Characterization of an early gene accelerating expression of late genes of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1988 Aug;62(8):2773–2781. doi: 10.1128/jvi.62.8.2773-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J. Channel catfish virus: a new type of herpesvirus. Virology. 1992 Jan;186(1):9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fixman E. D., Hayward G. S., Hayward S. D. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol. 1992 Aug;66(8):5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Miller L. K. Divergent transcription of early 35- and 94-kilodalton protein genes encoded by the HindIII K genome fragment of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1987 Jul;61(7):2264–2272. doi: 10.1128/jvi.61.7.2264-2272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Knipe D. M. Potential role for herpes simplex virus ICP8 DNA replication protein in stimulation of late gene expression. J Virol. 1991 May;65(5):2666–2675. doi: 10.1128/jvi.65.5.2666-2675.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss P., Krassa K. B., McPheeters D. S., Nelson M. A., Gold L. Zinc (II) and the single-stranded DNA binding protein of bacteriophage T4. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8515–8519. doi: 10.1073/pnas.84.23.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A. F., Blissard G. W., Rohrmann G. F. Characterization of the genetic organization of the HindIII M region of the multicapsid nuclear polyhedrosis virus of Orgyia pseudotsugata reveals major differences among baculoviruses. J Gen Virol. 1989 Jul;70(Pt 7):1815–1828. doi: 10.1099/0022-1317-70-7-1815. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Gonzalez M. A., Summers M. D. Complete Sequence and Enhancer Function of the Homologous DNA Regions of Autographa californica Nuclear Polyhedrosis Virus. J Virol. 1986 Oct;60(1):224–229. doi: 10.1128/jvi.60.1.224-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Summers M. D. Functional mapping of a trans-activating gene required for expression of a baculovirus delayed-early gene. J Virol. 1986 Feb;57(2):563–571. doi: 10.1128/jvi.57.2.563-571.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Summers M. D. Nucleotide sequence and temporal expression of a baculovirus regulatory gene. J Virol. 1987 Jul;61(7):2091–2099. doi: 10.1128/jvi.61.7.2091-2099.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. L., Rothman-Denes L. B. N4 virion RNA polymerase sites of transcription initiation. Cell. 1985 Jun;41(2):597–605. doi: 10.1016/s0092-8674(85)80032-5. [DOI] [PubMed] [Google Scholar]
- Hershberger P. A., Dickson J. A., Friesen P. D. Site-specific mutagenesis of the 35-kilodalton protein gene encoded by Autographa californica nuclear polyhedrosis virus: cell line-specific effects on virus replication. J Virol. 1992 Sep;66(9):5525–5533. doi: 10.1128/jvi.66.9.5525-5533.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink W. F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970 May 2;226(5244):466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- Hoheisel J. D. A cassette with seven unique restriction sites, including octanucleotide sequences: extension of multiple-cloning-site plasmids. Gene. 1989 Aug 1;80(1):151–154. doi: 10.1016/0378-1119(89)90260-6. [DOI] [PubMed] [Google Scholar]
- Huybrechts R., Guarino L., Van Brussel M., Vulsteke V. Nucleotide sequence of a transactivating Bombyx mori nuclear polyhedrosis virus immediate early gene. Biochim Biophys Acta. 1992 Feb 11;1129(3):328–330. doi: 10.1016/0167-4781(92)90511-w. [DOI] [PubMed] [Google Scholar]
- Koetsier P. A., Schorr J., Doerfler W. A rapid optimized protocol for downward alkaline Southern blotting of DNA. Biotechniques. 1993 Aug;15(2):260–262. [PubMed] [Google Scholar]
- Kool M., Vlak J. M. The structural and functional organization of the Autographa californica nuclear polyhedrosis virus genome. Arch Virol. 1993;130(1-2):1–16. doi: 10.1007/BF01318992. [DOI] [PubMed] [Google Scholar]
- Kool M., Voeten J. T., Goldbach R. W., Tramper J., Vlak J. M. Identification of seven putative origins of Autographa californica multiple nucleocapsid nuclear polyhedrosis virus DNA replication. J Gen Virol. 1993 Dec;74(Pt 12):2661–2668. doi: 10.1099/0022-1317-74-12-2661. [DOI] [PubMed] [Google Scholar]
- Kool M., Voeten J. T., Goldbach R. W., Vlak J. M. Functional mapping of regions of the Autographa californica nuclear polyhedrosis viral genome required for DNA replication. Virology. 1994 Feb;198(2):680–689. doi: 10.1006/viro.1994.1080. [DOI] [PubMed] [Google Scholar]
- Kool M., van den Berg P. M., Tramper J., Goldbach R. W., Vlak J. M. Location of two putative origins of DNA replication of Autographa californica nuclear polyhedrosis virus. Virology. 1993 Jan;192(1):94–101. doi: 10.1006/viro.1993.1011. [DOI] [PubMed] [Google Scholar]
- Krappa R., Knebel-Mörsdorf D. Identification of the very early transcribed baculovirus gene PE-38. J Virol. 1991 Feb;65(2):805–812. doi: 10.1128/jvi.65.2.805-812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisy D. J., Rohrmann G. F. Characterization of the replication of plasmids containing hr sequences in baculovirus-infected Spodoptera frugiperda cells. Virology. 1993 Oct;196(2):722–730. doi: 10.1006/viro.1993.1529. [DOI] [PubMed] [Google Scholar]
- Li Y., Passarelli A. L., Miller L. K. Identification, sequence, and transcriptional mapping of lef-3, a baculovirus gene involved in late and very late gene expression. J Virol. 1993 Sep;67(9):5260–5268. doi: 10.1128/jvi.67.9.5260-5268.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A., Carstens E. B. Immediate-early baculovirus genes transactivate the p143 gene promoter of Autographa californica nuclear polyhedrosis virus. Virology. 1993 Aug;195(2):710–718. doi: 10.1006/viro.1993.1422. [DOI] [PubMed] [Google Scholar]
- Lu A., Carstens E. B. Nucleotide sequence of a gene essential for viral DNA replication in the baculovirus Autographa californica nuclear polyhedrosis virus. Virology. 1991 Mar;181(1):336–347. doi: 10.1016/0042-6822(91)90500-b. [DOI] [PubMed] [Google Scholar]
- Messerle M., Keil G. M., Schneider K., Koszinowski U. H. Characterization of the murine cytomegalovirus genes encoding the major DNA binding protein and the ICP18.5 homolog. Virology. 1992 Nov;191(1):355–367. doi: 10.1016/0042-6822(92)90198-x. [DOI] [PubMed] [Google Scholar]
- O'Reilly D. R., Crawford A. M., Miller L. K. Viral proliferating cell nuclear antigen. Nature. 1989 Feb 16;337(6208):606–606. doi: 10.1038/337606a0. [DOI] [PubMed] [Google Scholar]
- Pari G. S., Anders D. G. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J Virol. 1993 Dec;67(12):6979–6988. doi: 10.1128/jvi.67.12.6979-6988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pari G. S., Kacica M. A., Anders D. G. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J Virol. 1993 May;67(5):2575–2582. doi: 10.1128/jvi.67.5.2575-2582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarelli A. L., Miller L. K. Identification and characterization of lef-1, a baculovirus gene involved in late and very late gene expression. J Virol. 1993 Jun;67(6):3481–3488. doi: 10.1128/jvi.67.6.3481-3488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarelli A. L., Miller L. K. Three baculovirus genes involved in late and very late gene expression: ie-1, ie-n, and lef-2. J Virol. 1993 Apr;67(4):2149–2158. doi: 10.1128/jvi.67.4.2149-2158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M. N., Bjornson R. M., Ahrens C., Rohrmann G. F. Identification and characterization of a putative origin of DNA replication in the genome of a baculovirus pathogenic for Orgyia pseudotsugata. Virology. 1993 Dec;197(2):715–725. doi: 10.1006/viro.1993.1647. [DOI] [PubMed] [Google Scholar]
- Pearson M., Bjornson R., Pearson G., Rohrmann G. The Autographa californica baculovirus genome: evidence for multiple replication origins. Science. 1992 Sep 4;257(5075):1382–1384. doi: 10.1126/science.1529337. [DOI] [PubMed] [Google Scholar]
- Possee R. D., Sun T. P., Howard S. C., Ayres M. D., Hill-Perkins M., Gearing K. L. Nucleotide sequence of the Autographa californica nuclear polyhedrosis 9.4 kbp EcoRI-I and -R (polyhedrin gene) region. Virology. 1991 Nov;185(1):229–241. doi: 10.1016/0042-6822(91)90770-c. [DOI] [PubMed] [Google Scholar]
- Rice W. C., Miller L. K. Baculovirus transcription in the presence of inhibitors and in nonpermissive Drosophila cells. Virus Res. 1986 Nov;6(2):155–172. doi: 10.1016/0168-1702(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Telford E. A., Watson M. S., McBride K., Davison A. J. The DNA sequence of equine herpesvirus-1. Virology. 1992 Jul;189(1):304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Theilmann D. A., Stewart S. Identification and characterization of the IE-1 gene of Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology. 1991 Feb;180(2):492–508. doi: 10.1016/0042-6822(91)90063-h. [DOI] [PubMed] [Google Scholar]
- Tomalski M. D., Wu J. G., Miller L. K. The location, sequence, transcription, and regulation of a baculovirus DNA polymerase gene. Virology. 1988 Dec;167(2):591–600. [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Wang Y. S., Hall J. D. Characterization of a major DNA-binding domain in the herpes simplex virus type 1 DNA-binding protein (ICP8). J Virol. 1990 May;64(5):2082–2089. doi: 10.1128/jvi.64.5.2082-2089.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Stillman B. Expression of adenovirus E1B mutant phenotypes is dependent on the host cell and on synthesis of E1A proteins. J Virol. 1987 Feb;61(2):426–435. doi: 10.1128/jvi.61.2.426-435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]