Abstract
This quadrivalent human papillomavirus (qHPV) (HPV6, -11, -16, and -18) vaccine long-term follow-up (LTFU) study is an ongoing extension of a pivotal clinical study (FUTURE II) taking place in the Nordic region. The LTFU study was designed to evaluate the effectiveness, immunogenicity, and safety of the qHPV vaccine (Gardasil) for at least 10 years following completion of the base study. The current report presents immunogenicity data from testing samples of the year 5 LTFU visit (approximately 9 years after vaccination). FUTURE II vaccination arm subjects, who consented to being followed in the LTFU, donated serum at regular intervals and in 2012. Anti-HPV6, -11, -16, and -18 antibodies were detected by the competitive Luminex immunoassay (cLIA), and in addition, serum samples from 2012 were analyzed by the total IgG Luminex immunoassay (LIA) (n = 1,598). cLIA geometric mean titers (GMTs) remained between 70% and 93% of their month 48 value depending on HPV type. For all HPV types, the lower bound of the 95% confidence interval (CI) for the year 9 GMTs remained above the serostatus cutoff value. The proportion of subjects who remained seropositive based on the IgG LIA was higher than the proportion based on cLIA, especially for anti-HPV18. As expected, the anti-HPV serum IgG and cLIA responses were strongly correlated for all HPV types. Anti-HPV GMTs and the proportion of vaccinated individuals who are seropositive remain high for up to 9 years of follow-up after vaccination.
INTRODUCTION
Recently introduced prophylactic human papillomavirus (HPV) vaccines are based on combinations of naked icosahedral virus-like particles (VLPs) that resemble authentic virions and contain immunodominant neutralizing epitopes. Type-specific VLPs in the vaccines are composed of the respective major HPV virion protein L1 as well as adjuvant. These vaccines are currently delivered by intramuscular injection in 3 doses over 6 months and elicit immune responses that protect against incident and persistent viral infection and associated cervical intraepithelial neoplasia (CIN) as demonstrated by phase 3 trials (1). HPV vaccines have been publicly available since 2006 (2), and the World Health Organization guidelines for cervical cancer control have included HPV vaccination of preadolescent girls since 2009 (3). Mass-vaccination programs worldwide target young girls from 9 to 12 years of age, prior to the start of sexual activity and exposure to sexually transmitted HPV. By 2014, millions of vaccine doses were distributed through national vaccination programs worldwide (4).
The highest risk for HPV infection is seen in the years after sexual debut (5, 6). The development of preinvasive cervical intraepithelial neoplasia grades 2 and 3 (CIN2 and CIN3) to invasive cancer (from incident high-risk HPV infections) takes 10 to 15 years minimum and 20 to 25 years on average (7). Because of this, the ability of vaccine-induced immunity to prevent cervical preinvasive lesions and cancer in preadolescent girls is best measured years after immunization. Therefore, a serological correlate of protection would be helpful in assessing a minimum antibody threshold for vaccine-induced immunity over time. It has been shown that serum antibodies confer protection against cervico-vaginal HPV infection through pronounced transudation into genital tract mucus (8) and direct exudation of circulating antibodies at the site of trauma (9, 10). Together with an observed correlation between the type-restricted protection seen in clinical trials and a similar spectrum of type restriction in in vitro antibody neutralization assays (11), it confirms the current understanding that the protective effects of HPV vaccines are almost completely mediated through high concentrations of neutralizing antibodies. This underlines the importance of evaluating antibody responses in clinical trials.
Using the competitive Luminex immunoassay (cLIA), the quadrivalent human papillomavirus (qHPV) (HPV6, -11, -16, and -18) L1 VLP vaccine studies indicated that vaccination induced seroconversion to all four qHPV types, with peak titers achieved after dose 3 (12). Antibody titers then declined until reaching a plateau that was sustained with minimal change through 48 months postvaccination. A more pronounced decline in HPV18 titers was observed, as measured by cLIA, with a month 48 seropositivity rate of 65%. However, when the same serum samples were tested in the total IgG Luminex immunoassay (LIA), a pseudovirion neutralization assay that measures all IgG antibodies produced in response to vaccination, data showed that total HPV18 seropositivity was approximately 97% at month 48 (13).
We evaluated the serological immune response to the qHPV vaccine (Gardasil) in sera collected up to 9 years after vaccination in a randomized, placebo-controlled, double-blind study (FUTURE II). Antibody responses to HPV6, -11, -16, and -18 VLPs were assessed by cLIA at months 7, 24, 48, and 108 and by total IgG immunoassay at month 108. Ultimately, these results will be used to evaluate the relevance of different serology assays for measuring long-term HPV vaccine protection.
MATERIALS AND METHODS
This study is an ongoing extension of protocol V501-015 (also known as FUTURE II) in the Nordic region; the base study was completed on 31 July 2007. Protocol V501-015 was a randomized, worldwide, placebo-controlled, double-blind 4-year study that investigated the safety, immunogenicity, and efficacy of qHPV on the incidence of HPV16- and HPV18-related CIN2 and CIN3 or worse in 16- to 23-year-old women (14).
Subjects.
Among 12,167 subjects in FUTURE II, 5,493 women from Denmark, Iceland, Norway, and Sweden were randomized in a 1:1 ratio and received either qHPV vaccine or placebo in the base study (2002 to 2003) and consented to the Nordic long-term follow-up (LTFU) extension study (protocol V501-015-21) (Fig. 1). Of these, 4,847 were immunized with at least one dose of qHPV vaccine, either during the enrollment phase of the study or during the extension for vaccination of subjects who initially received placebo. Country-specific consents were obtained for serum collection, resulting in a total of 4,137 women who were eligible for the active follow-up blood collection for immunogenicity assessment in 2012. This paper focuses on 1,598 subjects who received the qHPV vaccine at the start of protocol V501-015 and donated blood samples approximately 9 years after vaccination.
FIG 1.
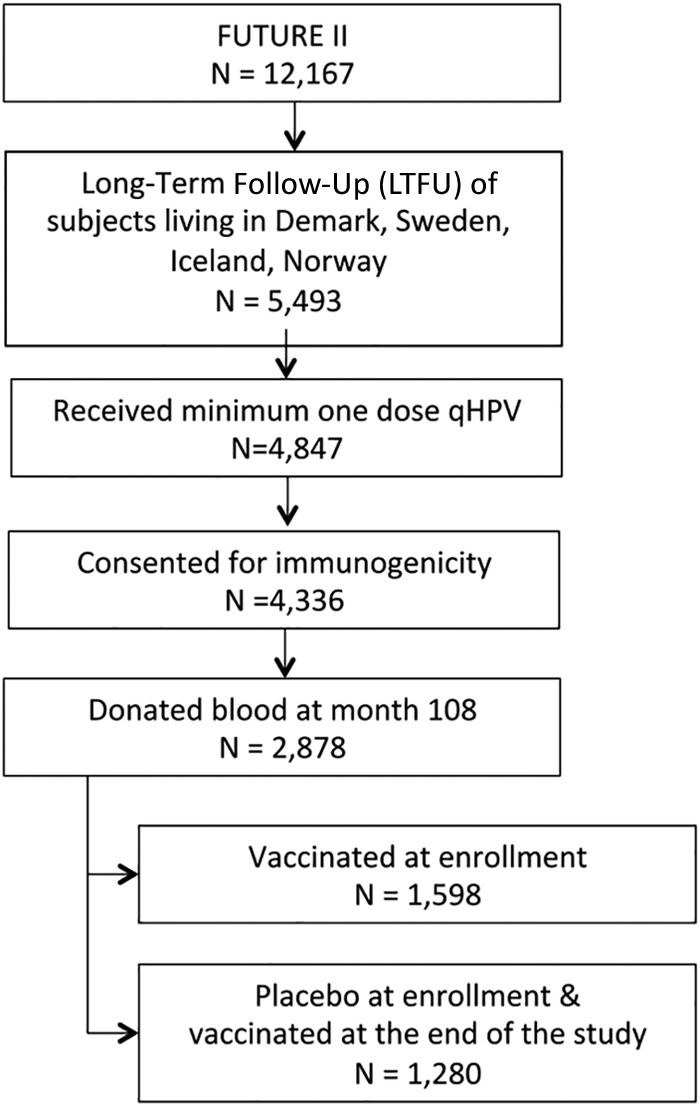
Flow chart of the sub-subjects from the FUTURE II study, who were enrolled in the Nordic follow-up and donated sera in 2012.
Collection of sera.
Approximately 6 months prior to the serum collection in 2012, subjects who had previously consented to providing a serum sample were contacted and invited to a blood collection center. Each country approached subjects slightly differently, according to national ethical committee requirements. At the blood collection centers, each subject's blood was collected in a 10-ml nonheparinized, nonserum separator, red-top tube and immediately separated to avoid hemolysis. Within 30 min of collection, the serum samples were placed in a freezer at −20°C (or lower) where they were stored until shipment on dry ice to the centralized lab where all analyses were performed. If the postvaccination immunogenicity data at months 7, 24, and 48 (from the V501-015 base study) were available, they were incorporated in the LTFU immunogenicity analyses.
Laboratory methods.
(i) Multiplexed competitive Luminex immunoassay. The purpose of the multiplexed HPV cLIA was to simultaneously quantify antibodies to a single neutralizing epitope on four separately manufactured VLPs for HPV6, -11, -16, and -18 (15, 16). cLIA was initially developed to evaluate the serological response to the vaccine by measuring vaccine-induced antibody production and to exclude subjects with evidence of a past HPV infection from the primary analysis (15). Shortly, yeast-derived VLPs were coupled to a set of 4 distinct fluorescent Luminex microspheres. Antibody titers were determined in a competitive format in which known, type-specific phycoerythrin-labeled, neutralizing monoclonal antibodies (MAbs) compete with the subject's serum antibodies to bind to conformationally sensitive, neutralizing epitopes on the VLPs. The fluorescent signals from the bound HPV-specific detection MAbs were inversely proportional to the subject's neutralizing antibody titers. Results for the assay were reported as the concentration of antibodies in milli-Merck units per milliliter (mMU/ml).
The seropositivity cutoffs of the HPV cLIA were assessed using a panel of serum samples from subjects highly likely to be HPV-naive (children) and from subjects who were highly likely to be HPV seropositive (15). Any sample with a value less than the cutoff was considered serostatus negative. Samples with values equal to or greater than the cutoff were considered serostatus positive. Serostatus cutoff values for HPV6, -11, -16, and -18 were 20 mMU/ml, 16 mMU/ml, 20 mMU/ml, and 24 mMU/ml, respectively.
(ii) Total IgG Luminex immunoassay.
The purpose of the HPV total IgG Luminex immunoassay (IgG LIA) was to measure a broader subset of the total antibody (Ab) concentrations to HPV VLPs 6, 11, 16, and 18. However, this assay does not distinguish between neutralizing and nonneutralizing antibodies (17). Shortly, yeast-derived VLPs are coupled to a set of nine distinct fluorescent Luminex microspheres. Antibody concentrations are determined in a multiplexed, direct binding format by measuring the amount of VLP-specific IgG bound to VLP microspheres. Following incubation with human serum, the fluorescent signal from an anti-human IgG detection antibody that binds directly to serum IgG and equally to each IgG subclass (1 to 4) is directly measured on the Luminex or Bio-Plex instrument. The fluorescent signal from the IgG-bound fluorescent detection antibody is proportional to the individual's anti-VLP IgG antibody levels. Results for the assay are reported as concentration of antibody in milli-Merck units per milliliter.
The HPV IgG LIA is performed in a 96-well microtiter filter plate. A 12-point standard reference serum pool from adult females vaccinated with 9-valent HPV vaccine, 4 controls, and 16 samples is added to the plate in duplicate. Samples are tested at a 1:100 and a 1:10,000 dilution. To each well, the VLP-microspheres for types 6, 11, 16, 18, 31, 33, 45, 52, and 58 are added. The plates are sealed with foil covers and incubated for 15 to 60 min. The contents of the filter plate are washed and incubated with mouse, anti-human IgG1 to 4 MAbs conjugated to phycoerythrin. The plates are covered with foil and incubated for an additional 30 to 60 min. Following the second incubation period, the plates are washed 3 times, and the samples are analyzed on a Bio-Plex (Luminex) instrument (13).
The high, medium, low, and negative controls used for this assay were collected from humans who were either HPV seronegative, had low antibody concentrations from natural infection, or had medium-to-high antibody concentrations to the nine HPV types following vaccination.
Statistical analysis: immunogenicity analysis for the per-protocol immunogenicity population.
Subjects were included in the per-protocol immunogenicity population (PPI) analysis if they were seronegative and PCR negative at baseline and PCR negative through month 7 to the appropriate HPV type(s), received 3 doses of qHPV vaccine within 1 year, did not violate the protocol, and consented to immunogenicity follow-up.
Anti-HPV responses for each of the 4 vaccine HPV types were summarized in terms of geometric mean titers (GMTs) with 95% confidence intervals (CIs) based on the time since the first vaccination with the qHPV vaccine. These CIs were constructed based on the assumption that log titers follow a normal distribution. Anti-HPV responses were investigated using longitudinal plots of the GMTs, incorporating results from the base study follow-up period, relative to the day of first vaccination. In all analyses, responses were summarized over intervals of time since the day of first vaccination.
RESULTS
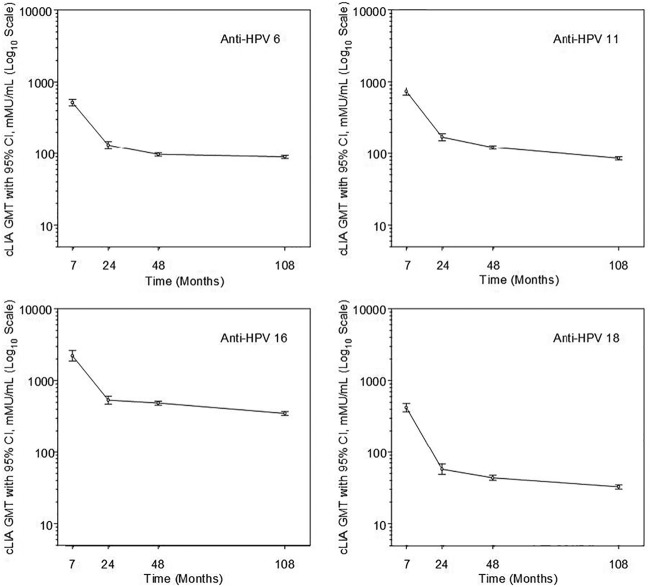
The number of per-protocol population subjects contributing to the immunogenicity analyses by cLIA in months 7, 24, 48, and 108 is presented in Table 1. At month 108, cLIA titers in milli-Merck units per milliliter among the PPI population for HPV6, -11, -16, and -18 were 89.3 (95% CI, 84.8 to 94.0), 85.2 (95% CI, 80.7 to 90.0), 348.3 (95% CI, 328.0 to 369.9) and 32.5 (95% CI, 30.3 to 34.9), respectively (Table 1). GMTs at month 108 remained between 70% and 93% of their month 48 values, depending on HPV type. For all HPV types, the lower bound of the 95% CI for year 9 GMTs remained above the serostatus cutoff value. Longitudinal plots of the GMTs along with 95% confidence intervals for each vaccine HPV type in the PPI analysis population are presented in Fig. 2.
TABLE 1.
Summary of anti-HPV cLIA GMTs in the per-protocol immunogenicity population
| Antibody type | Time point (mo)a | No. of subjects | GMT (mMU/ml) | 95% CI (mMU/ml) |
|---|---|---|---|---|
| Anti-HPV6 | 7 | 237 | 517.6 | 466.0, 574.9 |
| 24 | 246 | 131.2 | 116.4, 148.0 | |
| 48 | 1,121 | 96.5 | 91.2, 102.2 | |
| 108 | 1,233 | 89.3 | 84.8, 94.0 | |
| Anti-HPV11 | 7 | 238 | 736.3 | 656.2, 826.0 |
| 24 | 246 | 170.9 | 152.6, 191.5 | |
| 48 | 1,121 | 121.6 | 115.1, 128.4 | |
| 108 | 1,233 | 85.2 | 80.7, 90.0 | |
| Anti-HPV16 | 7 | 228 | 2,213.0 | 1,871.6, 2,616.8 |
| 24 | 237 | 532.2 | 468.6, 604.5 | |
| 48 | 1,206 | 485.0 | 455.3, 516.6 | |
| 108 | 1,178 | 348.3 | 328.0, 369.9 | |
| Anti-HPV18 | 7 | 259 | 420.9 | 368.6, 480.6 |
| 24 | 269 | 57.2 | 48.3, 67.8 | |
| 48 | 1,206 | 43.3 | 39.9, 47.1 | |
| 108 | 1,331 | 32.5 | 30.3, 34.9 |
Month 48 visits were generally scheduled earlier than month 48. This time point includes all visits occurring within 6 months of the approximate mean interval of 44 months. Month 108 visits were generally scheduled approximately 9 years after the first dose of qHPV vaccine. This time point also corresponds to 5 years after the start of the LTFU study.
FIG 2.
Longitudinal anti-HPV6, -11, -16, and -18 cLIA GMTs (per-protocol immunogenicity population). Anti-HPV6, -11, -16, and -18 cLIA results are presented in separate panels with assessments at 7, 24, 48, and 108 months since dose 1. The y axis refers to the cLIA GMTs on a log scale. Bars represent 95% confidence intervals.
Table 2 summarizes anti-HPV cLIA seropositivity percentages along with 95% confidence intervals in the PPI population for each HPV vaccine type and time point. Seropositivity rates for the PPI population measured at 7 months were close to 100% for all HPV types. The proportion of subjects remaining seropositive through approximately 108 months following the first dose of vaccine remained above 94% for HPV6, -11, and -16; the proportion of those seropositive to HPV18 was 60%.
TABLE 2.
Summary of anti-HPV seropositivity in the per-protocol immunogenicity population
| Antibody type | Time point (mo)a | No. of subjects | Seropositivity (%) | 95% CI (%) |
|---|---|---|---|---|
| Anti-HPV6 | 7 | 237 | 99.6 | 97.7, 100 |
| 24 | 246 | 97.6 | 94.8, 99.1 | |
| 48 | 1,121 | 94.1 | 92.6, 95.4 | |
| 108 | 1,233 | 94.4 | 93.0, 95.6 | |
| Anti-HPV11 | 7 | 238 | 99.6 | 97.7, 100 |
| 24 | 246 | 98.4 | 95.9, 99.6 | |
| 48 | 1,121 | 97.5 | 96.4, 98.3 | |
| 108 | 1,233 | 95.5 | 94.1, 96.6 | |
| Anti-HPV16 | 7 | 228 | 100 | 98.4, 100 |
| 24 | 237 | 98.7 | 96.3, 99.7 | |
| 48 | 1,067 | 98.9 | 98.0, 99.4 | |
| 108 | 1,178 | 99.1 | 98.3, 99.5 | |
| Anti-HPV18 | 7 | 259 | 98.1 | 95.6, 99.4 |
| 24 | 269 | 73.6 | 67.9, 78.8 | |
| 48 | 1,206 | 67.1 | 64.3, 69.7 | |
| 108 | 1,331 | 60.0 | 57.3, 62.6 |
Month 48 visits were generally scheduled earlier than month 48. This time point includes all visits occurring within 6 months of the approximate mean interval of 44 months. Month 108 visits were generally scheduled approximately 9 years after the first dose of qHPV vaccine. This time point also corresponds to 5 years after the start of the LTFU study.
Summaries of the anti-HPV6, -11, -16, and -18 serum IgG GMTs and seropositivity at month 108 in the PPI population are presented in Tables 3 and 4. For all HPV types, the lower bound of the 95% CI for the end of study GMTs remained above the serostatus cutoff value. Anti-HPV6, -11, -16, and -18 IgG seropositivity percentages at month 108 in the PPI population were 97.6% (95% CI, 96.6% to 98.4%), 96.3% (95% CI, 95.1% to 97.3%), 100% (95% CI, 99.7% to 100%), and 91.4% (95% CI, 89.7% to 92.8%), respectively (Table 3).
TABLE 3.
Summary of anti-HPV IgG GMTs in the per-protocol immunogenicity population at month 108a
| Antibody type | No. of subjects | GMT (mMU/ml) | 95% CI (mMU/ml) |
|---|---|---|---|
| Anti-HPV6 | 1,235 | 95.2 | 90.5, 100.1 |
| Anti-HPV11 | 1,235 | 67.4 | 64.3, 70.8 |
| Anti-HPV16 | 1,181 | 346.1 | 327.3, 365.9 |
| Anti-HPV18 | 1,333 | 46.1 | 43.3, 49.2 |
Month 108 visits were generally scheduled approximately 9 years after the first dose of qHPV vaccine. This time point also corresponds to 5 years after the start of the LTFU study.
TABLE 4.
Summary of anti-HPV IgG seropositivity in the per-protocol immunogenicity population at month 108a
| Antibody type | No. of subjects | Seropositivity (%) | 95% CI (%) |
|---|---|---|---|
| Anti-HPV6 | 1,235 | 97.6 | 96.6, 98.4 |
| Anti-HPV11 | 1,235 | 96.3 | 95.1, 97.3 |
| Anti-HPV16 | 1,181 | 100 | 99.7, 100 |
| Anti-HPV18 | 1,333 | 91.4 | 89.7, 92.8 |
Month 108 visits were generally scheduled approximately 9 years after the first dose of qHPV vaccine. This time point also corresponds to 5 years after the start of the LTFU study.
DISCUSSION
We observed high levels of anti-HPV6, -11, -16, and -18 GMTs in sera from subjects who received the qHPV vaccine approximately 9 years earlier, implying a sustainable and strong immunologic response elicited by the qHPV vaccine. All subjects tested negative to HPV6, -11, -16, and -18 infections by serology and HPV DNA in cervical samples during the course of vaccine administration. The proportion of vaccinated individuals who were seropositive to vaccine HPV types was higher with the total IgG LIA compared to the cLIA. Our observation is consistent with the observed efficacy of the HPV vaccine that provides continued protection against HPV16 and -18 CIN2 or worse through at least 8 years following vaccination (18).
The current report describes the long-term immunologic response among a subset, 45% (5,493/12,167), of subjects who received the qHPV vaccine within a phase III clinical study between December 2001 and May 2003. Antibody responses measured by the same method, cLIA, in a large population of qHPV vaccine recipients have been reported previously (19). We observed similar GMTs of anti-HPV antibody levels at different time points in our subsample compared to those of the qHPV clinical studies: (i) anti-HPV6 was 526 versus 543 mMU/ml at month 7, 131 versus 113 mMU/ml at month 24, and 97 versus 75 mMU/ml at month 48, respectively; (ii) anti-HPV11 was 738 versus 762 mMU/ml at month 7, 173 versus 145 mMU/ml at month 24, and 122 versus 90 mMU/ml at month 48, respectively; (iii) anti-HPV16 was 2,263 versus 2,294 mMU/ml at month 7, 544 versus 460 mMU/ml at month 24, and 484 versus 335 mMU/ml at months 48, respectively; and (iv) anti-HPV18 was 420 versus 462 mMU/ml at month 7, 57 versus 52 mMU/ml at month 24, and 43 versus 34 mMU/ml at month 48, respectively. These observations suggest good generalizability of the results from the current substudy to the overall clinical study and further to all women who are immunized prior to exposure to vaccine HPV types.
The peak antibody response in PPI populations after immunization with three doses has been observed at 1 month after the third dose (12). This was the first measurement, which was available for a subsample of our subjects. We observed a decrease in antibody concentrations over the subsequent 12 to 18 months, which eventually plateaued. These data are the first to show empirical evidence for the sustained plateau of four anti-HPV antibodies over a 9-year period. Compared to those at month 48, GMTs at month 108 were reduced by 7% to 30% as judged by the cLIA assay, depending on HPV type (Fig. 2). However, the lower bounds of the 95% CIs for the year 9 GMTs remained above the serostatus cutoff value.
Antiviral vaccines are known to induce a lasting protection through stimulation of antigen-specific memory B cells or long-lived plasma cells that then secrete large amounts of antibodies. Peaks in type-specific antibody concentrations were measured 1 month after the third dose of the qHPV vaccine in the current study, suggesting a large population of short- and long-lived anti-L1 antibody-secreting plasma cells in response to qHPV vaccination (20–22). Antibody concentrations declined rapidly over the subsequent 12 to 18 months and then stabilized, with anti-HPV16 GMTs more than 10 times greater than in the placebo group. Anti-HPV6 and -11 GMTs remained about 5 times greater than in the placebo group. Further, antibody levels did not decline remarkably at year 9 and remained virtually the same as at months 18 and 48. Olsson et al. suggested, based on observed rapid and strong immune responses to the antigen challenge, that systemic antibodies measured in the blood about 60 months after immunization were most likely produced by long-lived plasma cells (12, 23). It is likely that the plateau phase we observed over 108 months (Fig. 2) represents the same type of long-lived plasma cell-produced antibodies. Nevertheless, despite the observed decrease in anti-HPV18 GMTs, our concomitant clinical study with 8 years of follow-up cannot detect any HPV18-related breakthrough cases (18), which underlines that the drop of anti-HPV18 GMTs as measured by cLIA is most likely not predictive for vaccine effectiveness.
In fact, measured GMTs seem to be highly dependent on the type of assay used. In our analysis, the proportion of subjects who remained seropositive to the four vaccine HPV types approximately 9 years after vaccination based on the IgG LIA was higher than the proportion based on the cLIA, especially for anti-HPV18. We measured by cLIA 60% of subjects seropositive to anti-HPV18 compared to 91% of subjects as measured by IgG. This finding was expected, as the anti-HPV18 antibody used in the cLIA has been optimized for its specificity against a neutralizing epitope through displacing a monoclonal antibody directed at a single neutralizing epitope for each HPV type (15). The IgG LIA, however, measures a wider antibody profile than that of the cLIA (13). If that highly specific neutralizing epitope used by cLIA happens to be highly immunodominant, then antibody responses are long lasting in the cLIA, like those seen in anti-HPV16. If the highly specific neutralizing epitope happens to be less immunodominant, then the loss of antibodies over time does not reflect loss of protection, as we see with HPV18. The IgG LIA functions to show that a cLIA-negative result does not equate to unprotected. This was further confirmed by a recent publication that used a current gold standard method, a pseudovirion-based neutralization assay (PBNA) to establish the accuracy of the IgG LIA and cLIA in measuring neutralizing antibody responses against HPV16, -18, -31, -45, -52, and -58. PBNA confirmed the presence of neutralizing antibodies in the cLIA HPV18-negative serum samples (24).
Organized HPV vaccination programs to vaccinate young women have been launched in many countries worldwide, most commonly starting at 12 years of age (25). Protection from HPV is most needed about 8 to 10 years later when exposure to HPV and infection rates are highest. Our results show sustained antibody titers at 9 years postvaccination, which suggest that vaccine-induced protection against HPV6, -11, -16, and -18 infection will be present without revaccination. However, there is currently no global standard for HPV antibody assays. A conversion factor has been suggested by Brown et al. to translate milli-Merck units per milliliter to international units for HPV16 and -18 antibody levels (24), allowing unified reporting of the antibody titers. For HPV16, 1 mMU/ml equals about 0.14 to 0.18 IU/ml, and for HPV18, 1 mMU/ml equals about 0.18 to 0.19 IU/ml (24). Nevertheless, the critical level of anti-HPV antibodies representing an immunological correlate for vaccine effectiveness remains to be assessed. Recent reports from the clinical long-term follow-up study in Nordic countries did not indicate breakthrough cases of HPV18-related CIN at year 6 postvaccination (18). However, a longer follow-up study is ongoing (26).
Anti-HPV GMTs and the proportion of vaccinated individuals who are seropositive remain high up to 9 years after vaccination. While continued effectiveness is excellent support for continued immunogenicity, the IgG LIA shows that the lower rate of seropositivity to HPV18 in the cLIA is likely a function of the assay itself and does not necessarily reflect lower immunogenicity. This observation also suggests the important role of immune memory for long-term protection.
ACKNOWLEDGMENTS
Support for this study was provided by Merck & Co., Inc. (Whitehouse Station, NJ, USA). We thank Karyn Davis (Merck) for help with formatting and editing for submission.
C.M. has received support for conference participation and speaker's fees from Sanofi Pasteur MSD. S.K.K. received advisory board, speaker's fees, and unrestricted research grants through her institution from Sanofi Pasteur MSD and Merck. M.N. and J.D. report receiving funding from Merck & Co., Inc. through their respective institutions to conduct clinical trials of this vaccine. M.H. is working with clinical trials of this vaccine, sponsored by Merck & Co. A.S., Karyn Davis, and S.V. are employees of Merck & Co., Inc. and may own stock and or stock options. No other potential conflicts of interest are reported.
REFERENCES
- 1.Ault KA. 2007. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. 2006. Approval letter: human papillomavirus quadrivalent (types 6, 11, 16, 18) vaccine, recombinant. U.S. Food and Drug Administration, Washington, DC. [Google Scholar]
- 3.Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, Brotons M, Mena M, Cosano R, Munoz J, Bosch J, de Sanjosé S, Castellsagué X. Human papillomavirus and related diseases in the world. Summary Report 2015-04-08. ICO Information Centre on HPV and Cancer (HPV Information Centre). Accessed 24 June 2015. http://www.hpvcentre.net/statistics/reports/XWX.pdf. [Google Scholar]
- 4.Mariani L, Vici P, Suligoi B, Checcucci-Lisi G, Drury R. 2015. Early direct and indirect impact of quadrivalent HPV (4HPV) vaccine on genital warts: a systematic review. Adv Ther 32:10–30. doi: 10.1007/s12325-015-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. 2003. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 6.Moscicki AB, Schiffman M, Kjaer S, Villa LL. 2006. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 24(Suppl):S3, S42–S51. doi: 10.1016/j.vaccine.2005.01.101. [DOI] [PubMed] [Google Scholar]
- 7.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. 2007. Human papillomavirus and cervical cancer. Lancet 370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 8.Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, De Grandi P. 2003. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst 95:1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 10.Lowy DR, Schiller JT. 2006. Prophylactic human papillomavirus vaccines. J Clin Invest 116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley M. 2008. Immunobiology of HPV and HPV vaccines. Gynecol Oncol 109:S15–S21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Hoye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, von Krogh G, Lehtinen M, Paavonen J, Tamms GM, Giacoletti K, Lupinacci L, Esser MT, Vuocolo SC, Saah AJ, Barr E. 2007. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Brown DR, Garland SM, Ferris DG, Joura E, Steben M, James M, Radley D, Vuocolo S, Garner EI, Haupt RM, Bryan JT. 2011. The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccin 7:230–238. doi: 10.4161/hv.7.2.13948. [DOI] [PubMed] [Google Scholar]
- 14.Villa LL. 2007. Overview of the clinical development and results of a quadrivalent HPV (types 6, 11, 16, 18) vaccine. Int J Infect Dis 11(Suppl):S17–S25. doi: 10.1016/S1201-9712(07)60017-4. [DOI] [PubMed] [Google Scholar]
- 15.Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, Brown M, Smith J, Chirmule N, Barr E, Jansen KU, Esser MT. 2005. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 12:959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed Luminex assay. Clin Diagn Lab Immunol 10:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opalka D, Matys K, Bojczuk P, Green T, Gesser R, Saah A, Haupt R, Dutko F, Esser MT. 2010. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin Vaccine Immunol 17:818–827. doi: 10.1128/CVI.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjaer S, Nygård M, Dillner J, Marshall B, Hansen B, Sigurdardottir L, Hortlund M, Tryggvadóttir L, Saah A. 2015. HPV infection and related cancers: translating research innovation into improved practice, p 15, 19 In Eurogin 2015 International Multidisciplinary Congress, Sevilla, Spain. Eurogin, Paris, France. [Google Scholar]
- 19.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Lehtinen M, Steben M, Bosch X, Dillner J, Kurman RJ, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, Lu S, Vuocolo S, Hesley TM, Haupt RM, Barr E. 2008. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 26:6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 20.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsague X, Rusche SA, Lukac S, Bryan JT, Cavanaugh PF Jr, Reisinger KS. 2006. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 118:2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 21.Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, Bryan J, Vuocolo S, Haupt RM, Saah A. 2011. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br J Cancer 105:28–37. doi: 10.1038/bjc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, Brown DR, Ferenczy A, Harper DM, Koutsky LA, Kurman RJ, Lehtinen M, Malm C, Olsson SE, Ronnett BM, Skjeldestad FE, Steinwall M, Stoler MH, Wheeler CM, Taddeo FJ, Yu J, Lupinacci L, Railkar R, Marchese R, Esser MT, Bryan J, Jansen KU, Sings HL, Tamms GM, Saah AJ, Barr E. 2006. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 23.Stanley M. 2010. Potential mechanisms for HPV vaccine-induced long-term protection. Gynecol Oncol 118:S2–S7. doi: 10.1016/j.ygyno.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Brown D, Muller M, Sehr P, Pawlita M, Seitz H, Rubio I, Antonello J, Radley D, Roberts C, Saah A. 2014. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomaviruse types 16 and 18. Vaccine 32:5880–5887. doi: 10.1016/j.vaccine.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, Brotherton JM. 2012. Human papillomavirus vaccine introduction—the first five years. Vaccine 30(Suppl):F139–F148. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 26.Nygård M, Kjaer S, Dillner J, Marshall JB, Hansen BH, Sigurdardottir LG, Hortlund M, Tryggvadottir L, Saah A. 2013. Long-term immunogenicity, safety and effectiveness of Gardasil in the Nordic countries, p OC 6-3. In Eurogin 2013 International Multidisciplinary Conference, Florence, Italy; Eurogin, Paris, France. [Google Scholar]