Abstract
The outcome of coccidioidomycosis depends on a robust specific cellular immune response. A T-helper type 1 (Th1) cellular immune response has been previously associated with resolution of clinical illness. However, the precise elements of this response and whether cytokines not involved with the Th1 response play a role in coccidioidomycosis are not known. Whole-blood samples were obtained from subjects with active coccidioidomycosis and controls and incubated for 18 h with T27K, a coccidioidal antigen preparation. The supernatant was then assayed for gamma interferon (IFN-γ), interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), IL-4, IL-6, IL-10, and IL-17A. A total of 43 subjects, 16 with acute pneumonia, 9 with pulmonary sequelae of nodules and cavities, and 18 with nonmeningeal disseminated coccidioidomycosis, were studied. Compared to concentrations in healthy immune and nonimmune donors, the median concentration of IL-17A was significantly higher in those with active coccidioidomycosis (for both, P < 0.01). In addition, IL-6 concentrations were higher while IL-2 and IFN-γ concentrations were significantly lower in those with nonmeningeal disseminated disease diagnosed within 12 months than in those with acute pneumonia (for all, P < 0.05). The cytokine profile among patients with active coccidioidomycosis is distinct in that IL-17A is persistently present. In addition, those with nonmeningeal disseminated disease have an increased inflammatory cytokine response and diminished Th1 responses that modulate over time.
INTRODUCTION
Coccidioidomycosis is a fungal infection common in the San Joaquin Valley of California, in Arizona, and in other regions of the southwestern United States, as well as northern Mexico and areas of Central and South America (1). Most cases are acquired by inhalation of airborne fungal elements, called arthroconidia, from the soil or air. In approximately 60% of cases, infection is asymptomatic and results in a long-lived, protective cellular immune response. In the other 40%, a symptomatic pneumonia that usually resolves and also results in long-lived immunity occurs. In fewer than 1% of cases, clinical infection disseminates outside the thoracic cavity. The latter complication is associated with a poor outcome and a lack of a protective cellular immune response (2).
Smith and colleagues demonstrated many years ago that delayed-type dermal hypersensitivity (DTH), a manifestation of a cellular immune response, is predictive of a good outcome in coccidioidomycosis (3). Drutz and Catanzaro formalized this concept by relating the strength of the cellular immune response to control of disease and noted the similarity to the response to leprosy (4). Our laboratory has previously demonstrated that cytokines induced by incubation of the coccidioidal antigen preparation T27K with peripheral blood mononuclear cells (PBMC) (5, 6) or whole blood (7) results in a T helper type 1 (Th1) pattern, with high concentrations of interleukin-2 (IL-2) and gamma interferon (IFN-γ), in samples from subjects with limited or controlled coccidioidal infection. Moreover, these results parallel the response seen with coccidioidin skin testing (8).
Recently, murine studies have suggested that another response, associated with the release of IL-17A by T helper type 17 (Th17) lymphocytes, may be critical in inducing a protective immune response in coccidioidomycosis (9). Moreover, we have recently found that low concentrations of IL-17A were released by both PBMC and mononuclear cells obtained by bronchoalveolar lavage after incubation with T27K among subjects with acute pulmonary coccidioidomycosis (10).
In the present study, we explored the pattern of cytokine expression from samples of whole blood incubated with T27K among patients with pulmonary coccidioidomycosis and those with nonmeningeal disseminated disease. Patients with coccidioidal meningitis were not examined because of a limited number of subjects and because previous data have suggested that these subjects express DTH despite having disseminated disease (3). In addition to exploring the release of Th1 cytokines, we also examined the release of IL-17A and the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and IL-6. Through this work, we believe we have preliminarily identified cytokine biosignatures of immune responses during various types of active coccidioidomycosis.
MATERIALS AND METHODS
Subjects.
Patients with a diagnosis of coccidioidomycosis seen at the Southern Arizona Veterans Affairs Medical Center (SAVAHCS) were asked to participate. Subjects with underlying immunodeficiency, including HIV infection, those undergoing cancer chemotherapy or treatment with corticosteroids or biological immune modulators, and those with severe renal insufficiency and uncontrolled diabetes were excluded, as were patients with coccidioidal meningitis. Clinical data were collected, including the time from diagnosis to assay, the type of coccidioidomycosis, and the results of the serologic coccidioidal complement fixation titer by immunodiffusion (IDCF titer). IDCF titration was performed by the clinical laboratory of SAVAHCS (11). In addition, healthy subjects with known prior cellular immunity to coccidioidomycosis and healthy subjects without demonstrable coccidioidal cellular immunity served as control donors. The study was approved by the Human Subjects Protection Committee of SAVAHCS.
Whole-blood assay.
Approximately 5 ml of blood was drawn by venipuncture and placed into a sterile tube containing sodium heparin. The aliquots were dispensed and incubated at 37°C in 5% CO2, 95% air with 20 μg/ml of the coccidioidal antigen preparation T27K or with nothing to serve as an unstimulated control. After 18 h, the supernatant plasma sample was aspirated and frozen at −80°C until assayed for cytokine concentrations.
Determination of cytokine concentrations.
A flow cytometric cytokine bead array assay was used to determine the concentration of the following cytokines: IL-2, IFN-γ, TNF-α, IL-6, IL-4, and IL-10 (BD Biosciences, San Jose, CA). A highly sensitive enzyme-linked immunosorbent assay (ELISA) was used to measure IL-17A levels to 0.5 pg/ml (eBioscience, San Diego, CA). In all cases, the assays were performed according to the manufacturer's directions.
Calculation of a cytokine reactivity score.
A score was calculated for those subjects with either pulmonary or nonmeningeal disseminated coccidioidomycosis based on the concentrations of released cytokines to reflect whether a cellular or Th1 response or an inflammatory cytokine response was predominant. One point each was given for a concentration of IL-2 or IFN-γ of >100 pg/ml. In addition, a point was awarded if the released concentration of TNF-α was <100 pg/ml and an additional point if the IL-6 concentration was <10,000 pg/ml.
Statistical analysis.
Summarized data are expressed as medians with interquartile ranges. Continuous variables were analyzed by the nonparametric Mann-Whitney rank sum test and the Kruskal-Wallace test. Categorical variables were assessed using the χ2 test. A P value of <0.05 was considered to be statistically significant. All data were analyzed using Stata version 14 (Stata Corp., College Station, TX).
RESULTS
Overview of subjects.
A total of 43 subjects with active coccidioidomycosis were enrolled in the study. These included 18 with nonmeningeal extrathoracic dissemination and 25 with pulmonary disease only. Among the latter subjects, 16 had acute primary coccidioidal pneumonia, and 9 had a sequela of pulmonary coccidioidomycosis, either a nodule or a cavity. In addition, whole-blood samples were obtained from 6 healthy subjects with previously known coccidioidal immunity and from 6 healthy coccidioidal nonimmune subjects based on previous in vitro cytokine testing using T27K.
Demographic information for the subjects is depicted in Table 1. The subjects with active coccidioidomycosis were significantly older and more likely to be male than the healthy control subjects. In addition, there was a predominance of African-American men among the subjects with extrathoracic dissemination, and these subjects had been diagnosed for a significantly longer time than the subjects with acute pulmonary disease and those with pulmonary sequelae.
TABLE 1.
Demographic information for the subjects
| Group (no. of subjects) | Age (yr)a | No. M/no. Fb | Race/ethnicity (no. of subjects)c | No. of mo since diagnosisa |
|---|---|---|---|---|
| Acute pulmonary coccidioidomycosis (n = 16) | 66 (58–73) | 14/2 | WNH (11), WH (3), AA (2) | 7.0 (1.7–12.3) |
| Pulmonary sequelae (n = 9) | 66 (61–71) | 8/1 | WNH (7), WH (1), AA (1) | 8.5 (0–17.1) |
| Disseminated coccidioidomycosis (n = 18) | 65 (58–72) | 18/0 | WNH (7), WH (1), AA (10) | 115.5 (36.5–194.5) |
| Healthy donors (n = 12) | 35 (28–42) | 6/6 | WNH (11), WH (1) | NAd |
| P value | <0.001 | 0.050 | 0.565 | 0.006 |
Results are medians, with interquartile range.
M, male; F, female.
WNH, white non-Hispanic; WH, white Hispanic; AA, African-American.
NA, not applicable.
Comparison of cytokine results among the different groups of subjects.
The median concentration of IL-2, IFN-γ, and TNF-α in unstimulated control samples was 0.0 pg/ml; it was 19.2 pg/ml for IL-6 and <0.5 pg/ml for IL-17A. The results in Table 2 display the median cytokine concentrations of the T27K-stimulated sample above those of the paired unstimulated control in subjects with acute pulmonary coccidioidomycosis, pulmonary sequelae, and nonmeningeal disseminated coccidioidomycosis as well as among the healthy immune and nonimmune donors. For these samples, significantly higher concentrations of IL-2 and IFN-γ were observed in those with acute pulmonary coccidioidomycosis, in those with pulmonary sequelae and nonmeningeal disseminated disease, and in healthy immune donors than in healthy, nonimmune adults (for all, P < 0.05). In addition, significantly elevated concentrations of IL-17A compared to those in nonimmune donors were seen in subjects with acute pulmonary coccidioidomycosis, pulmonary sequelae, and nonmeningeal disseminated coccidioidomycosis (for all, P < 0.05), but not in healthy, immune donors. The concentrations of IL-6 were increased in samples from subjects with disseminated coccidioidomycosis compared to those in samples from healthy immune and nonimmune donors (for both, P < 0.05). The median concentrations of IL-4 and IL-10 were zero for all groups studied, and the results are not displayed.
TABLE 2.
Concentrations of IL-2, IFN-γ, TNF-α, IL-6, and IL-17A among donors with acute pulmonary coccidioidomycosis, the pulmonary sequelae of nodules and cavities, and nonmeningeal disseminated coccidioidomycosis and healthy coccidioidal immune and nonimmune donors
| Group (no. of subjects) | Concn, pg/ml (IQRa) |
||||
|---|---|---|---|---|---|
| IL-2 | IFN-γ | TNF-α | IL-6 | IL-17A | |
| Acute pulmonary coccidioidomycosis (n =16) | 1,559 (468–2,650)b | 518 (0–1,037)b | 33 (0–111)b | 1,485 (0–4,689)b | 14.7 (9.1–20.6)b,c |
| Pulmonary sequelae (n = 9) | 845 (510–1,198)b | 248 (99–398)b | 17 (0–150)b | 1,485 (486–2,485)b | 3.8 (0–11.4)b,c |
| Disseminated coccidioidomycosis (n = 18) | 541 (0–1,470)b | 173 (0–609)b | 341 (0–875)b | 4,584 (0–9,775)b,c | 6.9 (3.8–10.0)b,c |
| Immune (n = 6) | 315 (52–579)b | 194 (55–334)b | 41 (0–124)b | 691 (313–1,069)b | 0.8 (0.3–1.3) |
| Nonimmune (n = 6) | 0.0 (0–3.1) | 4.4 (0–9.5) | 0.0 (0) | 54 (17–92) | 0.0 (0–0.3) |
IQR, interquartile range.
P < 0.05 compared to healthy, nonimmune donors.
P < 0.01 compared to healthy, immune donors.
Effect of time from diagnosis on the cytokine pattern among those with nonmeningeal disseminated disease.
Based on previous observations (12, 13), it was surprising that Th1 cytokines in subjects with nonmeningeal disseminated coccidioidomycosis were similar to those in subjects with pulmonary disease and healthy immune donors. To explore this further, we examined cytokine concentrations based on the time from diagnosis to the time of assay. The released concentrations of IL-2 increased significantly with increasing time from diagnosis in those with nonmeningeal disseminated coccidioidomycosis (R2 = 0.197; P = 0.037), suggesting that a cellular immune response develops over time in these patients. Similar analyses for IFN-γ, TNF-α, IL-6, and IL-17A did not reveal a significant relationship (data not shown).
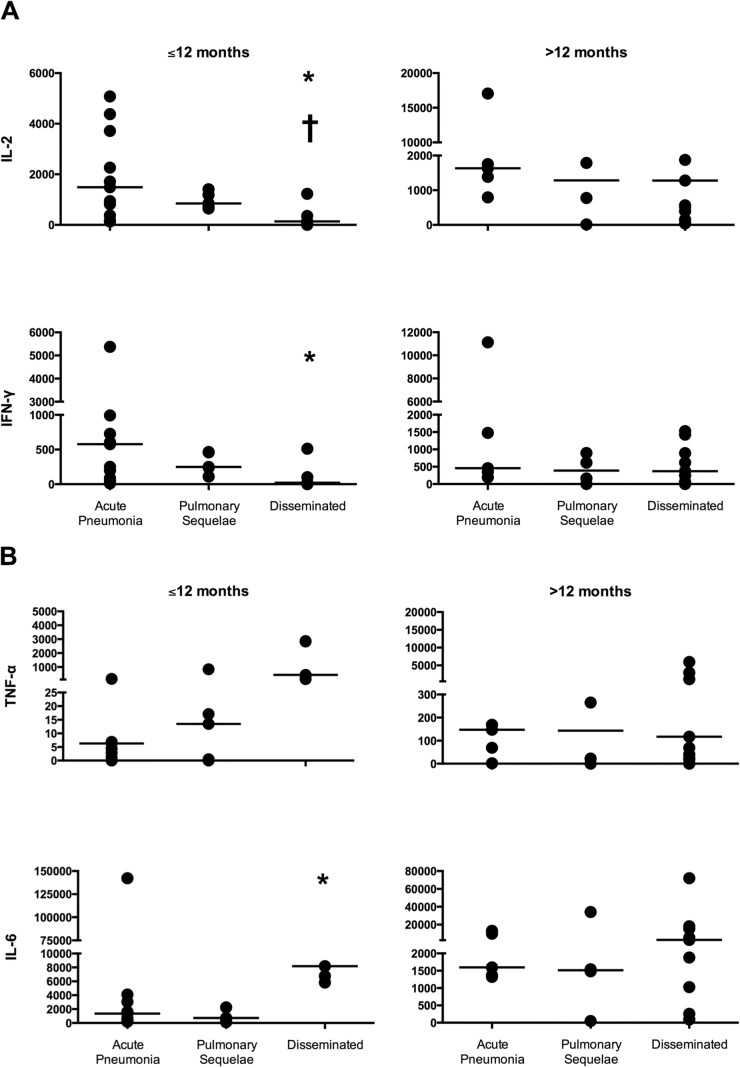
These data were further examined by comparing the concentrations of released cytokines in samples from those with acute pulmonary disease, pulmonary sequelae, and nonmeningeal disseminated disease who were diagnosed ≤12 months and >12 months from the time of the assay. Twelve months was used as a cutoff since observations in the era before treatment of coccidioidal disease suggest that the clinical and cellular immune response has stabilized by then (3). As shown in Fig. 1A, in those subjects diagnosed ≤12 months from the time of assay, released concentrations of both IL-2 and IFN-γ were significantly lower in samples from those with disseminated disease than in samples from those with acute pulmonary disease (for both, P < 0.05). In addition, concentrations of IL-2 were also lower in those with disseminated disease diagnosed ≤12 months from the time of the assay than in those diagnosed >12 months from the time of the assay (P = 0.034). However, this was not the case for IFN-γ (P = 0.115).
FIG 1.
Concentrations (picograms per milliliter) of IL-2 and IFN-γ (A) and TNF-α and IL-6 (B) for subjects diagnosed ≤12 months and >12 months from the time of the assay. Each value is depicted as a circle with the horizontal line representing the median. Note that the y axes differ for each cytokine. *, P < 0.05 comparing samples from subjects with acute pulmonary disease to samples from subjects with disseminated coccidioidomycosis diagnosed ≤12 months from the time of the assay. †, P < 0.05 comparing samples from those with disseminated coccidioidomycosis with a diagnosis ≤12 months compared to >12 months from the time of the assay.
In Fig. 1B, released concentrations of TNF-α and IL-6 based on the time of diagnosis are shown. The concentrations of IL-6 were significantly higher in those with disseminated disease than in those with acute pulmonary disease when diagnosed ≤12 months from the time of the assay (P = 0.034). There was also a trend toward higher TNF-α concentrations (P = 0.061). In addition, for samples from subjects with disseminated disease, the concentrations of IL-6 trended toward a higher value for samples from patients who were diagnosed at ≤12 months from the time of the assay than for samples from patients who were diagnosed at >12 months from the time of the assay (P = 0.068). No changes in IL-17A concentrations were observed over time for any of the subject groups (data not shown).
Visual inspection of these data indicated that in those with disseminated disease, the concentrations of IL-2 and IFN-γ appeared to be higher after 12 months, while the concentrations of TNF-α and IL-6 appeared to decline and approximate those in subjects with acute pulmonary disease and healthy immune donors. In order to examine this further, we calculated a cytokine reactivity score, where a low number indicates a diminished Th1 response and increased inflammatory response and a higher value suggests a heightened Th1 response with a lessened inflammatory response. When the diagnosis was ≤12 months from the time of the assay, the median cytokine reactivity score was 1 for those 5 subjects with disseminated disease, which was significantly lower than the median score of 3 for the 13 subjects with acute pulmonary coccidioidomycosis (P = 0.008) and the 6 healthy immune subjects (P = 0.012). At a time from diagnosis of >12 months, the median reactivity score for the 13 subjects with disseminated disease was 3 and was not significantly different from that for the 5 subjects with pulmonary disease (P = 0.377) or the 6 healthy immune subjects (P = 0.187).
Effects of antifungal therapy and coccidioidal serologic titer on the released cytokine concentration pattern.
We compared the released cytokine concentrations of the 21 subjects receiving antifungal therapy (in all cases a triazole antifungal) to those of the 22 who were not. No significant differences in any of the released cytokine concentrations were observed (data not shown). However, because none of the 9 subjects with pulmonary sequelae were receiving antifungal therapy and 17 of the 18 subjects in the nonmeningeal disseminated group were receiving antifungal therapy, we elected to analyze only the 16 subjects with acute pulmonary coccidioidomycosis, 12 of whom were receiving antifungal therapy. Again, no significant differences in any of the released cytokine concentrations were seen (data not shown).
The coccidioidal serologic titer is a measure of disease activity (14) but may not reflect the cellular immune response to infection. We compared the released cytokine concentrations in the 36 subjects whose complement-fixing titer was ≤1:4 to those in the 7 subjects in whom it was higher. This differentiation was used because IDCF titers of >1:4 are considered by many clinicians to be suggestive of active disease and because of the paucity of subjects available with higher titers. Both the IL-2 and IFN-γ concentrations were lower in those with elevated serologic titers, while all of the other released cytokine concentrations were not different from those in subjects with lower titers (data not shown). Because the number of subjects with pulmonary disease and IDCF titers of >1:4 was low, the subjects with acute pneumonia and pulmonary sequelae were combined and their results were then compared to the results for subjects with nonmeningeal disseminated disease. These results are shown in Table 3 and demonstrate that the depressed IL-2 and IFN-γ concentrations are only seen in subjects with disseminated disease with IDCF titers of >1:4.
TABLE 3.
Concentrations of released cytokines among 25 subjects with pulmonary disease, both acute pneumonia and pulmonary sequelae, compared to those among 18 subjects with nonmeningeal disseminated disease, based on whether the clinically obtained IDCF serologic titer was ≤1:4 or >1:4a
| Cytokine | Serologic titer | Subjects with pulmonary disease |
Subjects with disseminated disease |
||
|---|---|---|---|---|---|
| Cytokine concn, pg/ml (IQRb) | No. of subjects | Cytokine concn, pg/ml (IQR) | No. of subjects | ||
| IL-2 | ≤1:4 | 1,387 (648–2,127) | 21 | 1,227 (367–2,088) | 15 |
| >1:4 | 830 (88–1,572) | 4 | 61 (0–131) | 3 | |
| P value | 0.335 | 0.015 | |||
| IFN-γ | ≤1:4 | 458 (194–723) | 21 | 370 (323–3,101) | 15 |
| >1:4 | 331 (0–675) | 4 | 10 (0–1,058) | 3 | |
| P value | 0.4585 | 0.033 | |||
| TNF-α | ≤1:4 | 59 (0–190) | 21 | 132 (0–343) | 15 |
| >1:4 | 3 (0–9) | 4 | 2834 (1,580–4,088) | 3 | |
| P value | 0.128 | 0.110 | |||
| IL-6 | ≤1:4 | 1,598 (0–6,119) | 21 | 3,629 (0–10,856) | 15 |
| >1:4 | 525 (110–940) | 4 | 8,184 (5,432–10,936) | 3 | |
| P value | 0.138 | 0.214 | |||
| IL-17A | ≤1:4 | 14.6 (8.6–20.5) | 21 | 7.0 (3.8–10.1) | 15 |
| >1:4 | 2.5 (0–5.9) | 4 | 5.5 (2.3–8.7) | 3 | |
| P value | 0.075 | 0.953 | |||
P values of <0.05, indicating statistical significance, are shown in boldface.
IQR, interquartile range.
DISCUSSION
Cytokine analysis of antigen-stimulated whole blood not only defines those who have developed a cellular immune response to coccidioidal infection but also allows dissection of the specific elements of that response. In this report, we found that individuals with active coccidioidomycosis, including those with pulmonary and nonmeningeal disseminated disease, expressed a Th1 response and also a persistent Th17 response that distinguishes them from both nonimmune healthy donors and asymptomatic healthy immune donors. While this is a novel observation in coccidioidomycosis, there have been similar observations in studies of patients with active tuberculosis (15–17), and elevated expression levels of IFN-γ and IL-17A have been observed in memory CD4+ T lymphocytes from patients with sarcoidosis (18). The results suggest that antigen-induced release of IL-17A may be a marker for active disease during granulomatous infections. The role of the persistently elevated IL-17A concentration among subjects with active illness is not clear, and the fact that it is not seen in healthy immune donors without active illness suggests that it might reflect a lack of ability to control infection. Moreover, the levels of IL-17A in response to antigen stimulation did not appear to change over time among subjects with active coccidioidomycosis. The reason for this fixed response is unclear and deserves further study.
We also observed changes in the Th1 response with other changes in coccidioidal disease, including increased release of IL-2 associated with prolonged follow-up among patients with nonmeningeal disseminated disease and decreased IL-2 and IFN-γ associated with increased serologic responses in those with nonmeningeal disseminated disease. These data suggest that there is a relationship between the clinical outcome and cytokine response. While a direct correlation between IL-2 release and time was seen, this was not observed for IFN-γ, likely because of the relatively small number of subjects and the variability of response.
Increased circulating levels of TNF-α and IL-6 without specific antigen stimulation have been hypothesized to be markers for chronic inflammation in aging (19). We noted that those with nonmeningeal disseminated disease had a pattern of increased release of TNF-α and IL-6 compared to that in those with pulmonary coccidioidomycosis, and this declined over time. We devised a score to reflect this cytokine relationship and found that it confirmed that there was a diminished Th1 response and increased inflammatory cytokine response pattern among those with disseminated disease during the first year of diagnosis compared to the responses in those with pulmonary disease. These results might suggest an early inflammatory pattern in those with nonmeningeal disseminated coccidioidomycosis.
The weaknesses of this study include its descriptive nature, the relatively small number of subjects, including healthy controls, and the fact that the subjects were studied at various times after their initial diagnosis. In some of the disseminated-disease cases, the studies were undertaken many years after diagnosis and likely do not reflect their initial immune response. In addition, the subject groups differed markedly in their time of diagnosis, age, and racial makeup, and these factors may have affected the cytokine response. Finally, the number of cytokines surveyed was relatively limited.
A strength of the study is the use of a whole-blood assay with a coccidioidal antigen preparation that is relatively simple to perform and capable of detecting cytokine profiles (20). With this assay, cytokine patterns emerged among the different subject groups, and the results serve as preliminary data to further explore whether a cytokine biosignature predicts protective and nonprotective immune responses in human coccidioidomycosis.
ACKNOWLEDGMENTS
This proposal was supported by funding from the Pulmonary Division of the Department of Medicine, College of Medicine of the University of Arizona. Clinical and laboratory space were generously provided by the Southern Arizona Veterans Affairs Health Care Center (SAVAHCS).
REFERENCES
- 1.Pappagianis D. 1988. Epidemiology of coccidioidomycosis. Curr Top Med Mycol 2:199–238. [DOI] [PubMed] [Google Scholar]
- 2.Ampel NM. 2007. The complex immunology of human coccidioidomycosis. Ann N Y Acad Sci 1111:245–258. doi: 10.1196/annals.1406.032. [DOI] [PubMed] [Google Scholar]
- 3.Smith CE, Beard RR, Whiting EG, Rosenberger HG. 1946. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health 36:1394–1402. doi: 10.2105/AJPH.36.12.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drutz DJ, Catanzaro A. 1978. Coccidioidomycosis. Part I. Am Rev Respir Dis 117:559–585. [DOI] [PubMed] [Google Scholar]
- 5.Ampel NM, Christian L. 2000. Flow cytometric assessment of human peripheral blood mononuclear cells in response to a coccidioidal antigen. Med Mycol 38:127–132. doi: 10.1080/mmy.38.2.127.132. [DOI] [PubMed] [Google Scholar]
- 6.Nesbit L, Johnson SM, Pappagianis D, Ampel NM. 2010. Polyfunctional T lymphocytes are in the peripheral blood of donors naturally immune to coccidioidomycosis and are not induced by dendritic cells. Infect Immun 78:309–315. doi: 10.1128/IAI.00953-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ampel NM, Kramer LA, Kerekes KM, Johnson SM, Pappagianis D. 2001. Assessment of the human cellular immune response to T27K, a coccidioidal antigen preparation, by flow cytometry of whole blood. Med Mycol 39:315–320. doi: 10.1080/mmy.39.4.315.320. [DOI] [PubMed] [Google Scholar]
- 8.Ampel NM, Hector RF, Lindan CP, Rutherford GW. 2006. An archived lot of coccidioidin induces specific coccidioidal delayed-type hypersensitivity and correlates with in vitro assays of coccidioidal cellular immune response. Mycopathologia 161:67–72. doi: 10.1007/s11046-005-0218-8. [DOI] [PubMed] [Google Scholar]
- 9.Wüthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest 121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesbit LA, Knox KS, Nguyen CT, Roesch J, Wheat LJ, Johnson SM, Pappagianis D, Chavez S, Ampel NM. 2013. Immunological characterization of bronchoalveolar lavage fluid in patients with acute pulmonary coccidioidomycosis. J Infect Dis 208:857–863. doi: 10.1093/infdis/jit246. [DOI] [PubMed] [Google Scholar]
- 11.Wieden MA, Galgiani JN, Pappagianis D. 1983. Comparison of immunodiffusion techniques with standard complement fixation assay for quantitation of coccidioidal antibodies. J Clin Microbiol 18:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ampel NM, Kramer LA, Li L, Carroll DS, Kerekes KM, Johnson SM, Pappagianis D. 2002. In vitro whole-blood analysis of cellular immunity in patients with active coccidioidomycosis by using the antigen preparation T27K. Clin Diagn Lab Immunol 9:1039–1043. doi: 10.1128/CDLI.9.5.1039-1043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ampel NM, Nelson DK, Chavez S, Naus KA, Herman AB, Li L, Simmons KA, Pappagianis D. 2005. Preliminary evaluation of whole-blood gamma interferon release for clinical assessment of cellular immunity in patients with active coccidioidomycosis. Clin Diagn Lab Immunol 12:700–704. doi: 10.1128/CDLI.12.6.700-704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pappagianis D. 2001. Serologic studies in coccidioidomycosis. Semin Respir Infect 16:242–250. doi: 10.1053/srin.2001.29315. [DOI] [PubMed] [Google Scholar]
- 15.Jurado JO, Pasquinelli V, Alvarez IB, Pena D, Rovetta AI, Tateosian NL, Romeo HE, Musella RM, Palmero D, Chuluyan HE, Garcia VE. 2012. IL-17 and IFN-gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol 91:991–1002. doi: 10.1189/jlb.1211619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marín ND, Paris SC, Rojas M, Garcia LF. 2012. Reduced frequency of memory T cells and increased Th17 responses in patients with active tuberculosis. Clin Vaccine Immunol 19:1667–1676. doi: 10.1128/CVI.00390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunnari G, Pinzone MR, Vancheri C, Palermo F, Cacopardo B. 2013. Interferon-gamma and interleukin-17 production from PPD-stimulated PBMCs of patients with pulmonary tuberculosis. Clin Invest Med 36:E64–E71. [DOI] [PubMed] [Google Scholar]
- 18.Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, Lambrecht BN, Hendriks RW, Kleinjan A. 2012. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 51:37–46. doi: 10.1093/rheumatology/ker316. [DOI] [PubMed] [Google Scholar]
- 19.Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. 2003. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol 132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva D, Ponte CG, Hacker MA, Antas PR. 2013. A whole blood assay as a simple, broad assessment of cytokines and chemokines to evaluate human immune responses to Mycobacterium tuberculosis antigens. Acta Trop 127:75–81. doi: 10.1016/j.actatropica.2013.04.002. [DOI] [PubMed] [Google Scholar]