Abstract
Elimination programs for Wuchereria bancrofti and Onchocerca volvulus are in critical need of sensitive, specific, and point-of-contact (POC) tools that can be used for surveillance years beyond cessation of mass drug administration when infection intensities are low. Previously, Wb123 and Ov16 were identified individually as potential filarial antigens for an antibody-based POC test. The present study compares single-antigen Wb123- and Ov16-based POC tests with an integrated configuration to detect antibodies to Wb123 and Ov16 simultaneously. Wb123 and Ov16 isolates were striped onto lateral flow strips containing anti-IgG4. Sera from W. bancrofti-, O. volvulus-, and other helminth-infected or -uninfected individuals were added to the strips with buffer. Strips were read for the appearance of a positive or negative test line for both antigens at 20 min and following drying. Sensitivity, specificity, and predictive values were calculated for the single-antigen and biplex strips. Single and biplex lateral flow strips showed nearly identical results, with >90% sensitivity for Ov16 and >92% sensitivity for Wb123. Overall specificities for the single and biplex tests were 98% and 96% for Ov16 and Wb123, respectively. Biplex tests performed as well as the single-antigen tests regardless of the intensity of patient IgG4 response. The high sensitivity and specificity make these new biplex tests extremely useful for POC long-term surveillance following mass drug administration in Africa that should reduce time and cost in areas where bancroftian filariasis and onchocerciasis are coendemic.
INTRODUCTION
Initiatives to eliminate seven of the world's most neglected tropical diseases (NTDs) have been a focus of the World Health Organization in collaboration with many government and programmatic organizations since 2000. A major stimulus for these elimination programs has been the generous donations from pharmaceutical manufacturers of the medicines needed to treat these diseases. More recently, it has been recognized that the integration of programs for individual NTDs would save considerable time, money, and human resources, especially since many of these infections have overlapping geographic endemicity and share once yearly single-dose treatment regimens. Such integration would be of particular value for surveillance activities and especially for postelimination surveillance when resources for these diseases will be even more limited.
Two of these coendemic NTDs targeted for elimination in Africa are infections caused by Wuchereria bancrofti, the principal cause of lymphatic filariasis (LF), and Onchocerca volvulus, the etiologic agent of onchocerciasis (river blindness). The coincidence of these 2 filarial infections in Africa has stimulated the need for improved (ideally, rapid point-of-contact [POC]) diagnostic tools, especially in two specific programmatic settings (surveillance and mapping). First, since mass drug administration (MDA) programs based on ivermectin and/or albendazole for treating O. volvulus and W. bancrofti in areas where these infections are coendemic have proven so successful, many countries now have an urgent need for new tools to conduct post-MDA surveillance. Second, in other countries (particularly in central Africa where Loa loa infection is also endemic), MDA has been delayed, largely because it is uncertain whether the levels of prevalence of W. bancrofti and O. volvulus are above the thresholds to warrant MDA given the risk of serious adverse effects following treatment with ivermectin in individuals heavily infected with Loa loa (1). In addition, further mapping for O. volvulus infection will be necessary because a change in the global target for onchocerciasis (from control to elimination) means that the full extent of endemic onchocerciasis (including areas where the infection is hypoendemic) will now have to be mapped in detail.
Antibody-based assays are well suited for post-MDA surveillance and for mapping because of their ability to identify both past and current infection without dependency on the timing of MDA. While current WHO guidelines specify using the antigen-based immunochromatographic test (ICT) for transmission assessment surveys (TAS) for bancroftian filariasis (2), it is now recognized that this test is less sensitive for detection of early infection (i.e., prior to the appearance of adult worms) than are antibody-based assays. The inability to identify early infections as well as ongoing exposure to filariae following MDA makes the ICT problematic for long-term monitoring of children 6 to 7 years of age, who have been selected as the sentinel population for post-MDA surveillance (2–4). Having been born during or after MDA, these children are likely to be uninfected, with little to no exposure to the parasite, or have very low parasite burdens, making antibody assays even more useful. In addition, there is currently no antigen-based assay for the detection of onchocerciasis, making an antibody assay currently the only available tool.
Two highly specific and sensitive filarial antigens, Ov16 for O. volvulus infection (5) and Wb123 for W. bancrofti infection (6), have been used as the basis of immunoassays in a variety of formats (e.g., enzyme-linked immunosorbent assay [ELISA], luciferase immunoprecipitation system [LIPS], and Luminex) for post-MDA surveillance (5–8) with particular emphasis on the detection of infection prior to patency. All of these assays require relatively sophisticated instrumentation and have typically been performed in well-equipped centralized laboratories. Because Ov16- and Wb123-based IgG4 immunoassays can be configured to allow for near 100% specificity, they hold great promise for mapping and post-MDA surveillance in Africa where highly prevalent coincident filarial infections (Loa loa and Mansonella spp.) confound less specific immunoassays (7, 9). Most useful, however, for programmatic needs would be rapid diagnostic tests (RDT) where central laboratory processing is not needed.
Fortunately, Ov16 and Wb123 antigens have recently been developed as individual, standalone, rapid (10- to 20-min) lateral flow tests for point-of-contact detection of O. volvulus-specific or W. bancrofti-specific IgG4 antibodies (10–12). These tests can be used with serum, whole blood, or eluates of dried blood spots and have been shown to detect specific antibodies in individuals with infections that span many geographically diverse regions of the world where filarial infection is endemic. Because of the often overlapping endemicity of W. bancrofti and O. volvulus infections in Africa and because of the potential financial savings of having a single 2-antigen test from the overall cost-of-goods and the programmatic operational costs, the present study focused on demonstrating the feasibility of a lateral flow biplex strip test configured to simultaneously detect IgG4 antibodies to Ov16 and Wb123.
MATERIALS AND METHODS
Ethics statement.
Several protocols approved by the Institutional Review Board of the NIAID were used to collect patient serum samples, with most collected under NCT00001230, NCT00342576, or 92-I-0155 (inactive). Some samples were collected as part of a large international field project approved by the respective governments. Written informed consent was obtained from all subjects.
Construction and testing of biplex strips.
The protocol for the production of the lateral flow strips and for testing the strips with serum (and whole blood) has been described previously (10, 11), and the present study utilized a second-generation design to allow for the striping of both antigens with similar results in sensitivity and specificity (Fig. 1A). A BioDot XYZ reagent dispenser was used to apply Wb123 antigen (0.8 mg/ml), Ov16 antigen (1 mg/ml), and control antibody (goat anti-mouse IgG; Jackson ImmunoResearch, West Grove, PA) stripes onto the nitrocellulose membrane and to spray anti-human IgG4 antibody (Hybridoma Reagent Laboratory, Baltimore, MD) conjugated to gold colloid onto the conjugate pad material. Lateral flow strip materials were assembled on Lohmann diagnostic strip backing (300 by 75 mm) in a card format, using a 25-mm-wide nitrocellulose membrane, 22-mm-wide conjugate pad material, and 32-mm-wide absorbent wick material. Cards were cut into 4-mm-wide test strips and stored sealed with desiccant at room temperature until use.
FIG 1.
Structures of the IgG4 biplex lateral flow tests. (A) Identification of the components of the biplex strips and the appearance of the Wb123 (0.8 mg/ml), Ov16 (1 mg/ml), and IgG control lines following the movement of sample and anti-human IgG4 in buffer toward the absorbent pad. (B) Schematic of the biplex test in the prototype cassette with positive results shown in the results window. O, W, and C indicate the locations of the Ov16, Wb123, and control lines, respectively.
The protocol for testing the strips was identical to that described previously (11). Briefly, 2.5 μl of patient serum or plasma was added to the nitrocellulose membrane on the test strip approximately 4 mm from the conjugate pad, and the strip was then placed vertically in a well of a flat-bottom plate with 100 μl of lateral flow test running buffer. Strips were read at 20 min, removed from the well, and then read again when dry (usually overnight). A sensitivity/specificity calculator was used to determine both of these parameters as well as the positive and negative predictive values for the single and biplex assays.
Construction and testing of Ov16-Wb123 biplex cassettes.
Following the analysis of the biplex strips, a small subset of sera with variable reactivity to Ov16 or Wb123 was used in an initial test of a prototype POC biplex cassette device (Fig. 1B). The biplex strips first underwent a period of optimization in which the position of the antigens on the strips was reversed and the concentrations of Ov16 and Wb123 were adjusted to 0.4 mg/ml and 0.7 mg/ml, respectively. This alteration in antigen concentration resulted in a measureable increase in specificity with little loss in sensitivity (data not shown).
Cassettes containing the Ov16-Wb123 biplex test strips were prepared by manually encasing the 4-mm-wide bare test strips into hard plastic cassettes made from 3 press-together parts of custom design. An adhesive-edged blood filter is applied to the sample well part of the cassette during the assembly process. The fully assembled test cassettes were individually sealed into vapor-resistant pouches containing 0.5 mg clay desiccant to ensure humidity control.
For testing of samples, 6 μl of serum or plasma was added to the cassettes. The sample filter portion of the cassette was then lifted up by pulling the tab on the top of the cassette followed by the addition of 75 μl of lateral flow test running buffer to the buffer well (Fig. 1B). Cassettes were examined for results at 30 min from the addition of buffer and after drying (overnight).
RESULTS AND DISCUSSION
We recently reported on the performance of the Wb123 lateral flow strips using a panel of sera from patients with W. bancrofti, O. volvulus, L. loa, and Strongyloides stercoralis, as well as sera from non-helminth-infected individuals (11). A subset of these sera was also used to analyze the performance of Ov16 in a single antigen format strip (10). To determine whether the reactivity of individual patient sera would be the same when 2 antigens are bound to the assay strips, these same sera (plus some additional sera) (Table 1) were used to compare the Ov16-Wb123 biplex format with the original single-format studies. Only patients with a diagnosis for a single filarial infection were used in the analyses; therefore, a subset of patients from Ghana with known O. volvulus infection were removed from the analysis for Wb123 because it was later determined that these patients were from a region where W. bancrofti may have been endemic, but their LF status was not determined at the time the sera were collected.
TABLE 1.
Study population
| Infection | Region | Ov16 (no. of patients tested) |
Wb123 (no. of patients tested) |
||
|---|---|---|---|---|---|
| Single | Biplex | Single | Biplex | ||
| Onchocerca volvulus | Ecuador | 69 | 69 | 46 | 69 |
| Guatemala | 41 | 41 | 41 | 41 | |
| Ghana | 64 | 64 | 3 | 4 | |
| Cameroon | 4 | 4 | 4 | 4 | |
| Expatriatesa | 18 | 18 | 18 | 18 | |
| Total | 196 | 196 | 112 | 136 | |
| Wuchereria bancrofti | Cook Islands | 19 | 59 | 59 | 59 |
| India | 18 | 18 | 18 | 18 | |
| Haiti | 0 | 16 | 16 | 16 | |
| Guyana | 2 | 2 | 2 | 2 | |
| Total | 39 | 95 | 95 | 95 | |
| Loa loa | Nigeria | 1 | 1 | 1 | 1 |
| Expatriates | 40 | 40 | 40 | 40 | |
| Total | 41 | 41 | 41 | 41 | |
| Strongyloides stercoralis | Jamaica | 1 | 1 | 1 | 1 |
| Central America | 12 | 12 | 12 | 12 | |
| West Africa | 1 | 1 | 1 | 1 | |
| Southeast Asia | 18 | 18 | 18 | 18 | |
| Middle East | 1 | 1 | 1 | 1 | |
| Expatriates | 8 | 8 | 8 | 8 | |
| Total | 41 | 41 | 41 | 41 | |
| None (helminth-uninfected) | Ecuador (Quito) | 20 | 20 | 20 | 20 |
| Guatemala (Guatemala City) | 17 | 17 | 17 | 17 | |
| Haiti (Port au Prince) | 0 | 6 | 6 | 6 | |
| Mali (Bamako) | 18 | 18 | 18 | 18 | |
| North America | 22 | 22 | 22 | 22 | |
| Total | 77 | 83 | 83 | 83 | |
| Total strips for all regions | 394 | 456 | 372 | 396 | |
Expatriates were infected patients from regions where various infections were endemic.
Photographs of sample biplex strips are illustrated in Fig. 2A. The results of the biplex tests showed stability over time; only 3/456 (0.66%) strips for Ov16 and 3/396 (0.76%) strips for Wb123 changed from negative to a very weak positive (barely detectable with the naked eye) between the 20 min read and when completely dry 24 h later. For this reason and also since it had been previously demonstrated with the Ov16 monoplex strips that a stable reading was maintained for at least 70 days (10), only the results from the dry (overnight) strips are reported for the sake of consistency and simplicity.
FIG 2.
Illustration of the lateral flow biplex test strips. (A) Photographs of individual patient sera showing a negative, Ov16 single positive, Wb123 single positive, and double positive result using the biplex format of the lateral flow strips. The position of the antigen lines is shown, and strips have been aligned by the control lines. (B) Comparison of Ov16 results demonstrating the similarity between the single format and biplex format lateral flow strips. The reactivity of 3 O. volvulus-infected patients with strong, average, and weaker IgG4 reactivity to Ov16 is shown in the single format and biplex format strips; in each set of strips, the Ov16 lines are aligned. The patients showed no reactivity to the Wb123 test line, the position of which is indicated by the labeled arrow in the top biplex strips. Photographs in panels A and B have had equivalent adjustments to approximate the color tone of the original strips.
Despite the different number of patients tested in the biplex format, the overall sensitivity of the single and biplex strips was nearly identical for both antigens, 90.8% and 90.3%, respectively, for Ov16 and 92.6% in each format for Wb123 (Table 2). Similarly, the specificities were essentially identical, with 98% and 96% overall specificities for Ov16 and Wb123, respectively. The specificities to the individual comparator groups were likewise equivalent in the biplex compared to the single-antigen format, ranging from 97.6% to 100% for Ov16 and 91.9% to 100% for Wb123. The primary cross-reactors to Wb123 in both formats were sera from 9/87 and 13/110 Ov-infected patients from Ecuador and Guatemala, regions where W. bancrofti is not endemic. All but 4 of these sera had faint test line results, and many became negative when slightly lower concentrations of Wb123 were used for striping (data not shown).
TABLE 2.
Comparison of sensitivity and specificity rates for Ov16 and Wb123 in the original (single) and biplex format lateral flow assay strips
| Antigen result | Sensitivity (%) |
Specificity (%) |
||
|---|---|---|---|---|
| Single | Biplex | Single | Biplex | |
| Ov16 results for O. volvulus-infected vs: | ||||
| All non-O. volvulus | 90.8 | 90.3 | 98.0 | 98.1 |
| W. bancrofti | 100 | 97.9 | ||
| Loa loa | 100 | 100 | ||
| Strongyloides stercoralis | 95.1 | 97.6 | ||
| Helminth uninfected | 97.4 | 97.6 | ||
| Wb123 results for W. bancrofti-infected vs: | ||||
| All non-W. bancrofti | 92.6 | 92.6 | 95.7 | 96.0 |
| O. volvulus | 90.2 | 91.9 | ||
| Loa loa | 100 | 100 | ||
| Strongyloides stercoralis | 100 | 100 | ||
| Helminth uninfected | 98.8 | 98.8 | ||
While this study was not population based, the positive and negative predictive values were nevertheless calculated and were found to also be highly similar between the single-antigen and biplex strips (data not shown). The positive predictive value in the biplex strips for Ov16 (97.3%) was higher than that for Wb123 (88.0%), owing to the larger number of patients with onchocerciasis for Ov16 testing and the higher degree of cross-reactivity to Wb123. The negative predictive values were high overall for both Ov16 and Wb123 (93.1% and 97.6%, respectively).
The above results show that the Ov16 and Wb123 test lines perform as well in the new biplex lateral flow assay strips as they do in the single antigen format. In further illustration of this, Fig. 2B shows the response to Ov16 of 3 O. volvulus-infected patients with variable reactivity (from strong to weak) to the antigen. As can be seen, regardless of how strong the antibody response, the single and biplex strips have similar intensities. In addition, the similar performance between the strips was further demonstrated by the fact that the only change in patient status for either Ov16 or Wb123 was a change from a weak positive to a negative response in 3 O. volvulus patients (of 196 tested) to Ov16 between the single and biplex strips.
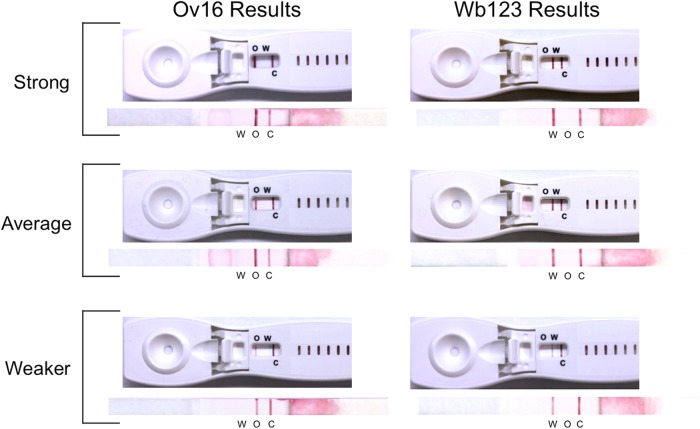
Following the comparison of the single and biplex formats, a small subset of sera (3 O. volvulus and 3 W. bancrofti patients) were selected for their variable reactivity to Ov16 and Wb123, respectively, and were retested in an initial look at the new prototype cassette devices (Fig. 3). As illustrated, regardless of the change in antigen position and concentration (as described in Materials and Methods), results using the biplex cassettes compared well with the performance seen with the bare biplex strips, regardless of patient reactivity. In addition to plasma or serum, whole blood and eluted blood spots have been shown to work equally well in these cassettes (data not shown). The ability to use whole blood will be essential in resource-limited countries.
FIG 3.
Comparison of patient serum reactivity in the biplex prototype cassettes and the biplex strips. The same serum sample was tested against each cassette-strip pair for Ov16 (left) and Wb123 (right), and the antigen test lines are aligned for each pair. Sera with strong (top samples), average (middle samples), and weaker (bottom samples) IgG4 antibody reactivity are shown. O, W, and C indicate the locations of the Ov16, Wb123, and control lines, respectively. An equivalent adjustment was made to each photograph to approximate the original color tone of the cassettes and strips.
One limitation of antibody assays is that they cannot distinguish current active infection from past infection in individuals from regions where these infections are endemic, as previously infected or exposed persons may still be antibody positive. This limitation may be of particular importance in mapping new regions. A POC antibody-based test does not replace the antigen-based ICT, which is still the best means for detection of active W. bancrofti infection and will more than likely continue to be used for TAS, at least for the foreseeable future. Nevertheless, antibody prevalence in a treated population still shows a steep decline, particularly in young children, following MDA (8). Moreover, as more communities are evaluated with these antibody assays, further knowledge will be gleaned in how these assays behave across all age groups prior to and following MDA.
An additional limitation of this biplex antibody assay is that even a small decrease in specificity from 100% could mean that validation of an interruption of transmission might be problematic in large population-based assessments. This potential problem could be eliminated by using confirmatory tests (e.g., other antibody assays or the ICT [for lymphatic filariasis]). However, Ov16 has already been used successfully for post-MDA surveillance in the Americas, and with regard to Wb123 the specificities are well within the range of the ICT currently used for TAS. With prior knowledge of the deficiencies in any given assay, the problem of false positives or negatives can be overcome by increasing the sample size.
Even with these limitations, antibody assays do provide certain distinct advantages. Their ability to measure ongoing exposure to the infective stages of filariae and to detect infection at an earlier time point, particularly with low infection intensities prior to patency (5, 7) as may be seen in children, makes an antibody-based assay highly useful for post-MDA surveillance and monitoring of a population for years following cessation of MDA. In addition, recent findings have determined that the ICT is cross-reactive in loiasis patients with high microfilarial burdens, which is problematic in regions where Loa loa is coendemic (13). In contrast, measurements of Wb123 antibodies using the prototype biplex cassettes and eluted blood spots from 44 patients with false-positive ICTs and with Loa loa microfilarial levels of >30,000/ml were uniformly negative (data not shown). Lastly, the lack of an antigen-based POC assay for onchocerciasis makes an assay for detection of Ov16 antibodies currently the only viable alternative for post-MDA surveillance.
The integration of two filarial antigens in one POC device will ultimately save time, money, and human resources for both post-MDA surveillance and any additional mapping that is required for both bancroftian filariasis and onchocerciasis in regions where coendemicity is suspected. In addition, the ability to use a single finger-prick to measure antibodies for 2 often coendemic diseases should be more acceptable for testing in communities, particularly for young children. Moreover, the fact that these devices give a stable long-term reading and can be used with either serum or whole blood (10) makes them highly attractive for long-term field surveillance. These biplex devices are currently undergoing additional optimization and elevated temperature stability testing in advance of licensing a commercially manufactured integrated rapid POC biplex test. Multiple independent assessments of this biplex device are under way to ensure an unbiased examination of its usefulness in meeting the needs of the global elimination programs and in reaching the elimination goals for both O. volvulus and W. bancrofti infection.
ACKNOWLEDGMENTS
We thank Eric Ottesen for his helpful suggestions in editing the manuscript.
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and, in part, by the Bill and Melinda Gates Foundation.
One of the authors (T.B.N.) has a patent application pending related to methods of detection of Wb123 (U.S. application 13/882,850) and has, through the National Institutes of Health, provided Wb123 antigen through nonexclusive licensing agreements. All other authors report no conflicts of interest for this study.
REFERENCES
- 1.Basanez MG, Pion SD, Churcher TS, Breitling LP, Little MP, Boussinesq M. 2006. River blindness: a success story under threat? PLoS Med 3:e371. doi: 10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2011. Global programme to eliminate lymphatic filariasis—monitoring and epidemiological assessment of mass drug administration: a manual for national elimination programmes. WHO, Geneva, Switzerland. [Google Scholar]
- 3.Moss DM, Priest JW, Boyd A, Weinkopff T, Kucerova Z, Beach MJ, Lammie PJ. 2011. Multiplex bead assay for serum samples from children in Haiti enrolled in a drug study for the treatment of lymphatic filariasis. Am J Trop Med Hyg 85:229–237. doi: 10.4269/ajtmh.2011.11-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ. 2006. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet 367:992–999. doi: 10.1016/S0140-6736(06)68426-2. [DOI] [PubMed] [Google Scholar]
- 5.Lobos E, Weiss N, Karam M, Taylor HR, Ottesen EA, Nutman TB. 1991. An immunogenic Onchocerca volvulus antigen: a specific and early marker of infection. Science 251:1603–1605. doi: 10.1126/science.2011741. [DOI] [PubMed] [Google Scholar]
- 6.Kubofcik J, Fink DL, Nutman TB. 2012. Identification of Wb123 as an early and specific marker of Wuchereria bancrofti infection. PLoS Negl Trop Dis 6:e1930. doi: 10.1371/journal.pntd.0001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamlin KL, Moss DM, Priest JW, Roberts J, Kubofcik J, Gass K, Streit TG, Nutman TB, Eberhard ML, Lammie PJ. 2012. Longitudinal monitoring of the development of antifilarial antibodies and acquisition of Wuchereria bancrofti in a highly endemic area of Haiti. PLoS Negl Trop Dis 6:e1941. doi: 10.1371/journal.pntd.0001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steel C, Kubofcik J, Ottesen EA, Nutman TB. 2012. Antibody to the filarial antigen Wb123 reflects reduced transmission and decreased exposure in children born following single mass drug administration (MDA). PLoS Negl Trop Dis 6:e1940. doi: 10.1371/journal.pntd.0001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lammie PJ, Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E. 2004. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis—a multicenter trial. Filaria J 3:9. doi: 10.1186/1475-2883-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden A, Steel C, Yokobe L, Jackson E, Barney R, Kubofcik J, Peck R, Unnasch TR, Nutman TB, de los Santos T, Domingo GJ. 2013. Extended result reading window in lateral flow tests detecting exposure to Onchocerca volvulus: a new technology to improve epidemiological surveillance tools. PLoS One 8:e69231. doi: 10.1371/journal.pone.0069231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steel C, Golden A, Kubofcik J, LaRue N, de Los Santos T, Domingo GJ, Nutman TB. 2013. Rapid Wuchereria bancrofti-specific antigen Wb123-based IgG4 immunoassays as tools for surveillance following mass drug administration programs on lymphatic filariasis. Clin Vaccine Immunol 20:1155–1161. doi: 10.1128/CVI.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weil GJ, Steel C, Liftis F, Li BW, Mearns G, Lobos E, Nutman TB. 2000. A rapid format antibody card test for diagnosis of onchocerciasis. J Infect Dis 182:1796–1799. doi: 10.1086/317629. [DOI] [PubMed] [Google Scholar]
- 13.Bakajika DK, Nigo MM, Lotsima JP, Masikini GA, Fischer K, Lloyd MM, Weil GJ, Fischer PU. 2014. Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the Democratic Republic of Congo. Am J Trop Med Hyg 91:1142–1148. doi: 10.4269/ajtmh.14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]