Abstract
The impaired synthesis of antigen-specific antibodies, which is indispensable for an adaptive immune response to infections, is a fundamental pathomechanism that leads to clinical manifestations in children with antibody production defects. The aim of this study was to evaluate the synthesis of antigen-specific antibodies following immunization in relation to peripheral blood B cell subsets in young children with hypogammaglobulinemia. Twenty-two children, aged from 8 to 61 months, with a deficiency in one or more major immunoglobulin classes participated in the study. Postvaccination antibodies against tetanus and diphtheria toxoids, the surface antigen of the hepatitis B virus, and the capsular Haemophilus influenzae type b polysaccharide antigen were assessed along with an immunophenotypic evaluation of peripheral blood B lymph cell maturation. A deficiency of antibodies against the tetanus toxoid was assessed in 73% of cases and that against the diphtheria toxoid was assessed in 68% of cases, whereas a deficiency of antibodies against the surface antigen of the hepatitis B virus was revealed in 59% of the children included in the study. A defective response to immunization with a conjugate vaccine with the Haemophilus influenzae type b polysaccharide antigen was demonstrated in 55% of hypogammaglobulinemic patients. Increased proportions of transitional B lymph cells and an accumulation of plasmablasts accompanied antibody deficiencies. The defective response to vaccine protein and polysaccharide antigens is a predominating disorder of humoral immunity in children with hypogammaglobulinemia and may result from a dysfunctional state of the cellular elements of the immune system.
INTRODUCTION
Antibody production defects are the most common category of pediatric primary immunodeficiencies (PIDs) (1, 2). The hallmark of these immunodeficiency conditions is the defective production of antigen-specific antibodies that are an indispensable element of the adaptive immune response to pathogens (3, 4). While the poor response to vaccines is another feature of humoral PIDs, the ability to synthesize postvaccination antibodies against toxoids and polysaccharides is the most specific expression of the immune response to antigens. In the evaluation of the immune response associated with antibody production, the response to vaccination against hepatitis B is not routinely recommended because of the large proportion of adults, up to between 1% and 3% of vaccinated individuals, who do not effectively synthesize antibodies against hepatitis B virus surface antigen (HBs). In infants and children, the efficiency of recombinant vaccines against hepatitis B, assessed on the basis of postvaccination anti-HBs antibody concentration over 10 mIU/ml, is estimated at 85% to 100% (5, 6). The minimal protective level of neutralizing antibodies against diphtheria and tetanus toxoids has been estimated at 0.01 to 0.1 IU/ml, whereas to achieve long-term immunity, a concentration of specific antibodies, up to 1.0 IU/ml, may be required. The synthesis of antibodies against the Haemophilus influenzae type b (Hib) polysaccharide capsular antigen (polyribosylribitol phosphate [PRP]) depends on the type of immunization, and the minimal protective level following the use of a conjugated vaccine has been estimated at 0.15 μg/ml, although long-term protection requires a concentration of 1.0 μg/ml (7, 8).
The purpose of the study was to evaluate the antigen-specific antibody response to vaccinations in young children with hypogammaglobulinemia. We also aimed to demonstrate the correlations between the production of antibodies against protein and polysaccharide antigens and the maturation of peripheral blood B lymph cell subsets.
MATERIALS AND METHODS
Study group.
Twenty-two children (17 boys and 5 girls), aged from 8 to 61 months (mean age, 26 months; median age, 23 months), who had been referred to the pediatric pneumonology, allergology, and immunology university clinic (Poznan University of Medical Sciences) because of recurrent respiratory tract infections and diagnosed with PIDs participated in the study. The project was accepted by the University Bioethical Committee. According to the Helsinki Declaration, written informed consent was obtained from the parents of all participating children.
The fundamental inclusion criteria were hypogammaglobulinemia regarding IgG or combined IgG and one or two major immunoglobulin class deficiencies. In accordance with this criteria, the study group was divided into 4 subgroups (Fig. 1). All children studied were infection free and had not been treated with antibiotics for at least 2 weeks before inclusion to the study. Immunoglobulin replacement therapy had not been administered prior to the study and was not carried out during the study in any of the participating children. Hence, the effect of passively transferred antigen-specific postvaccination antibodies on their levels measured in all children studied was excluded.
FIG 1.
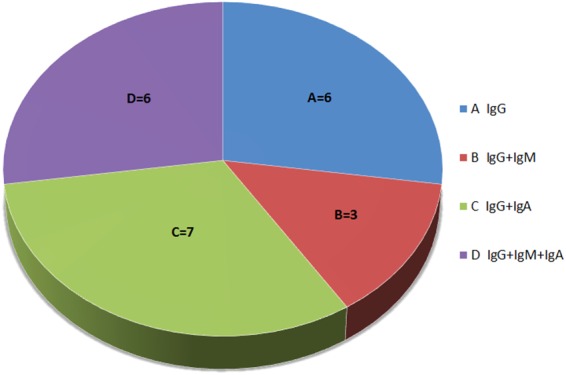
Subgroups of patients studied regarding the deficiency of major classes of immunoglobulins.
Vaccinations against hepatitis B, tetanus, diphtheria, and Hib were carried out in all of the children studied according to the current immunization program. A full implementation of the above-mentioned immunizations enabled an evaluation of the specific postvaccination immune response. Immunization against hepatitis B, using the viral surface antigen, is carried out according to the time frame of 0, 1, and 6 months of life. The vaccination against tetanus with the use of the toxoid consists of a primary vaccination comprising 3 doses of a vaccine administered from the 2nd month of life at 6-week intervals and of 1 dose of the booster vaccination at 16 to 18 months of life. Immunization with diphtheria toxoid is given together with the tetanus vaccine. Immunization against Hib with the capsular polysaccharide PRP antigen conjugated with protein, preferably carried out along with tetanus and diphtheria toxoids, is composed of 3 doses of primary vaccination administered from the 2nd month of life at 6-week intervals and of 1 dose of the booster vaccination at 16 to 18 months of life. The examinations of serum-specific antibody levels were carried out 6 to 10 weeks after the administrations of last doses of vaccines.
Assessment of antibody levels.
Serum samples obtained from clotted peripheral blood were used for further analysis of the major classes of immunoglobulins and postvaccination antigen-specific antibody levels.
Immunoglobulins G, A, and M were measured with the use of an immunoturbidimetric assay (Beckman Coulter, USA). Specific IgG antibodies against tetanus toxoid were determined with the use of an anti-tetanus enzyme-linked immunosorbent assay (ELISA) (Euroimmun, Germany). Specific IgG antibodies against diphtheria toxoid were measured with the use of the EC129.00 anti-diphtheria ELISA IgG (Sekisui Virotech, Germany). Specific IgG antibodies against Hib PRP were determined with the use of the ELISA Immunozym HiB-IgG test (Progen Biotechnik, Germany). For the purpose of determining postvaccination antibodies against the HBs antigen, an immunoenzymatic test with microparticles (microparticle enzyme immunoassay [MEIA]) AxSYM Ausab (Abbott Diagnostics Division, Germany) was applied. The detection limits of ELISAs for postvaccination antibodies were: 0.005 IU/ml, 0.05 IU/ml, 0.1 μg/ml, and 2.0 mIU/ml, respectively.
Peripheral blood lymph cell immunophenotyping.
Peripheral venous blood samples anticoagulated with EDTA-K2 were stored at temperatures of between 4°C and 8°C and processed within 24 h. Cells were labeled with the following murine fluorochrome-stained monoclonal antibodies: anti-CD45 fluorescein isothiocyanate (FITC), anti-CD14 phycoerythrin (PE), anti-CD19 PE, anti-CD19 peridinin chlorophyll protein (PerCP), anti-IgM FITC, anti-IgD FITC, anti-CD38 allophycocyanin (APC), anti-CD27 PE, or anti-CD21 FITC (Becton-Dickinson, USA). Blood samples were mixed with antibodies, incubated in a lysing solution (FACS lysing solution, Becton-Dickinson), centrifuged twice, and suspended in a phosphate-buffered saline (PBS) (Roche, Germany). Acquisition of cells and analysis were carried out with the use of the flow cytometer FACSCanto and FACSDiva software (Becton-Dickinson). With sequential gating on biparametric scattering, the following B lymph cell subpopulations were identified: CD45hi CD14− lymphocytes, memory B cells CD19+ CD27+, naive B cells CD19+ CD27−, nonswitched memory B cells/marginal zone B cells CD19+ CD27+ IgD+, transitional B cells CD19+ CD38hi IgMhi, plasmablasts CD19+ CD38+ IgM−, and immature B cells CD19+ CD21lo. The relative values of peripheral blood lymphocytes, B cells of the total lymphocyte population, and B cell subsets were calculated. Absolute counts of all cell subsets were calculated from the leukocyte count obtained from the analyzer XT 2000i (Sysmex, Japan). A comparative analysis was done with a group of 111 healthy children, aged 5 months to 5 years, whose peripheral blood B lymph cell immunophenotyping had been carried out by Piątosa et al. (9) and served to elaborate the reference values for pediatric populations at different age groups.
Statistical analysis.
Spearman's rank correlation coefficient (rho) was used to test the direction and strength of the relationship between the two variables. Its level of significance (P < 0.05) was statistically significant. The nonparametric comparison Kruskal-Wallis test was used to analyze quantitative traits that did not assume normal distribution. The Mann-Whitney test was used to compare the average of the two groups, and the Fisher exact test was used to determine nonrandom associations between categorical variables. All statistical analyses were performed with the use of Statistica v9 software.
RESULTS
Antibody production.
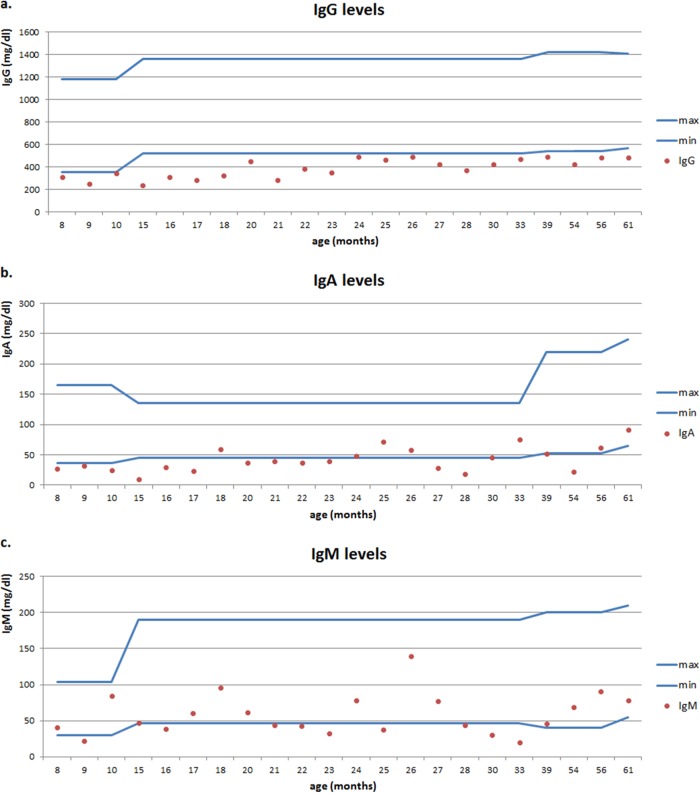
Individual levels of major classes of immunoglobulin in each child studied are presented in Fig. 2.
FIG 2.
Individual serum immunoglobulin G (a), A (b), and M (c) levels in children studied. Min, minimum; max, maximum.
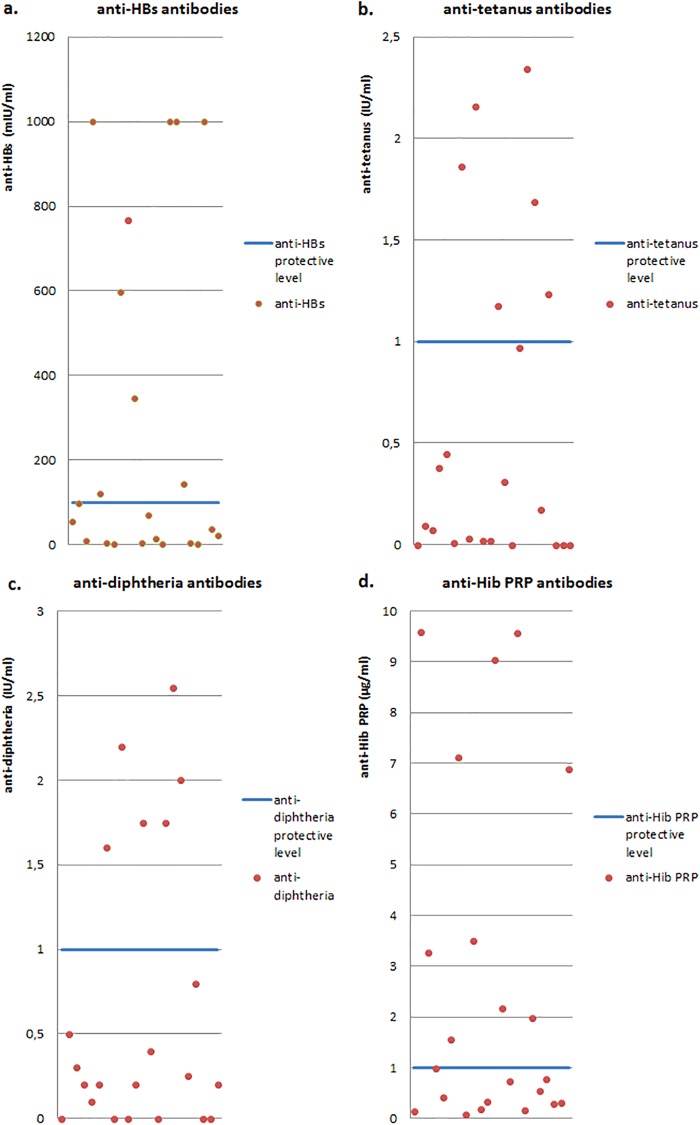
Specific anti-HBs antibody levels below 100 mIU/ml were observed in 13 of the children studied (59%), whereas in 7 children (32%), levels of anti-HBs antibodies were below the threshold of 10 mIU/ml. It is worth noting that an impaired response to vaccination against hepatitis B was revealed in as many as 6 children, that is, in all children demonstrating IgG deficiency (subgroup A).
The level of anti-tetanus toxoid antibodies below 1.0 IU/ml was noted in 16 children (73%), and in 11 patients (50%), the minimal protective level of 0.1 IU/ml was not achieved. Impaired production of specific anti-tetanus antibodies was demonstrated in as many as 7 children, that is, in all children exhibiting IgG and IgA deficiencies (subgroup C).
Antibodies against diphtheria toxoid decreased below the minimal protective level of 0.1 IU/ml in 6 children (27%) and below the level of 1.0 IU/ml in 16 children (73%), and among them, 6 children were classified to group C. A combined defect of the immune response to tetanus and diphtheria toxoids was demonstrated in as many as 15 of the children.
The level of specific antibodies against Hib PRP below 1.0 μg/ml was revealed in 12 of 22 children studied (55%), while the level below the minimal protective concentration of 0.15 μg/ml was demonstrated in 2 children. Among 12 children in whom the defective response to the Hib vaccine with PRP was shown, in as many as 10 children, it was accompanied by impaired production of antibodies against tetanus and diphtheria toxoids (Fig. 3).
FIG 3.
Levels of antigen-specific postvaccination antibodies: anti-HBs (a), anti-tetanus toxoid (b), anti-diphtheria toxoid (c), and anti-PRP Hib (d).
The Spearman's rank correlation coefficient and its level of significance for the relationship between serum IgG levels and levels of antibodies against HBs antigen, tetanus, and diphtheria toxoids, and Hib PRP antigen were as follows: rho values of −0.381, −0.295, −0.145, and −0.017, respectively (P > 0.05).
Peripheral blood B cell subsets.
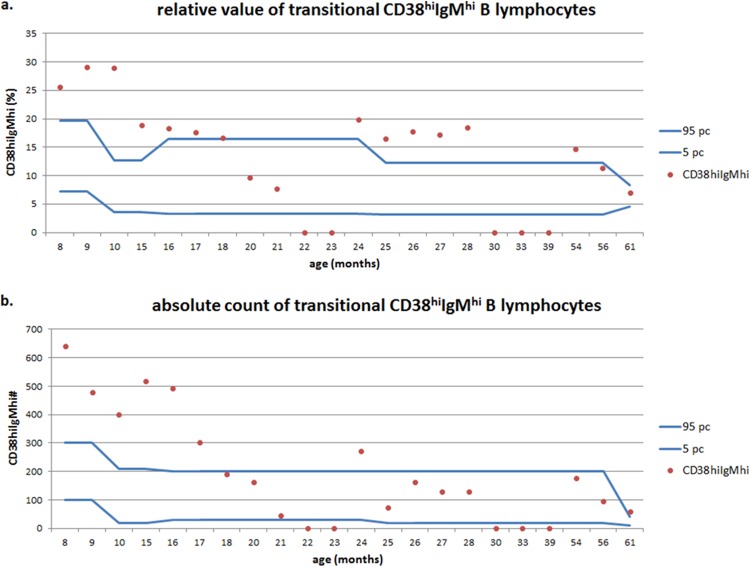
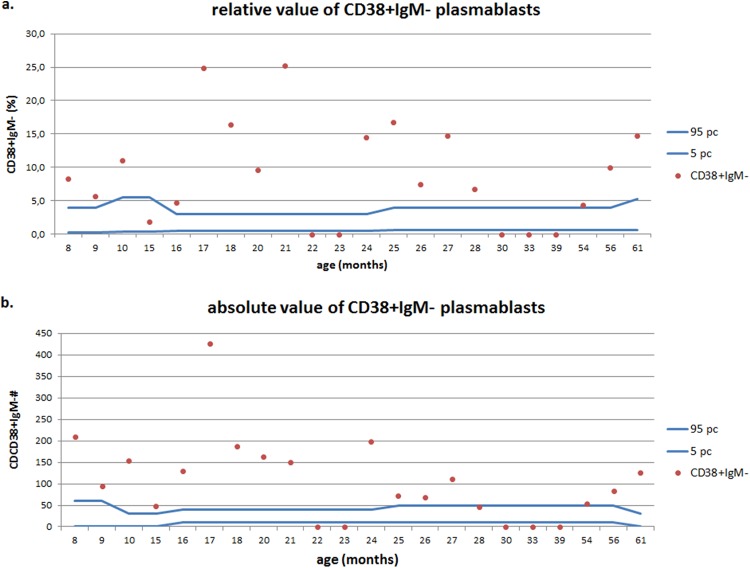
In 18 of the children studied, the relative value and absolute count of immature B cells (CD19+ CD21lo) were within the age-matched normal range. The absolute count was increased in two children and decreased in another two children, although these changes did not correlate with serum immunoglobulin levels (rho, −0.198 and 0.010, respectively; P > 0.05). Of 22 children from the study group, in 13 of them (59%) the relative value of transitional B cells (CD19+ CD38hi IgMhi) increased above the 95th percentile of the age-matched range, whereas in 6 children this increase in the relative value of this B cell subset was accompanied by the normal value of its absolute count (Fig. 4). The level of significance (P = 0.039) was revealed for the relationship between the relative value of transitional B cells and subgroup C (IgG and IgA deficiency) of hypogammaglobulinemia. In six of the children studied (27%), a decreased relative value and, in two of the children, a decreased absolute count of mature naive B cells (CD19+ CD27− IgD+) was revealed that did not correlate with serum immunoglobulin levels (rho, −0.029 and 0.021, respectively; P > 0.05). The relative value of the total pool of memory B cells (CD19+ CD27+) was increased above the 95th percentile of the age-matched range in six of the children studied (27%), and their absolute count increased in 3 children. In 4 children who were under the age of 2 years, a decrease in the relative value of nonswitched IgD+ memory B cells was observed. The percentage of plasmablasts (CD19+ CD38+ IgM−) in peripheral blood in children usually does not exceed 5 and does not show substantial variations in different age groups. In the study group, in as many as 16 children (73%), the relative value and the absolute count of this B cell subset increased above the upper limit of the age-matched range (Fig. 5). The deviations of total memory B cell, marginal zone-like B cell subsets, or plasmablasts did not correlate with serum immunoglobulin levels.
FIG 4.
Relative values (a) and absolute counts (b) of transitional B lymphocytes in children with hypogammaglobulinemia.
FIG 5.
Relative values (a) and absolute counts (b) of plasmablasts in children with hypogammaglobulinemia.
DISCUSSION
The antibody response to the antigenic challenge with vaccines provides an essential insight into the immune status of children suspected of having primary antibody deficiencies. As prophylactic vaccines provide a source of standardized antigenic exposure, the measurements of antibody titers can reflect the antigen-specific immune response to microorganisms and supply a reasonable correlate for protection against infection (10). Vaccines against tetanus and diphtheria with toxoids, being protein thymus-dependent antigens, induce the activation of T helper cells and the synthesis of IgG antibodies by B cells already in young infants. Primary vaccination carried out within the first 6 months and a booster vaccination given in the 18th month of life is aimed at developing a long-term seroprotection against infections. However, data regarding the immunogenicity of vaccines with toxoids are not entirely consistent. A high seroprotection rate exceeding 1.0 IU/ml against diphtheria and tetanus after 3 doses of primary immunization reaching 98% and 100%, respectively, was reported by Zarei et al. in Iran (11). Likewise, maintenance of the specific antibody response was shown in 81% and 96% of Austrian children ages 4 to 8 years who had received 4 doses of anti-diphtheria and anti-tetanus vaccine (12). However, in a study by Postfay-Barbe et al. (13), the administration of 1 booster dose of tetanus toxoid in 10 to 11-year-old children who had received 3 doses of primary vaccination during early infancy induced the minimal antibody level of 0.1 IU/ml in only 55% of the patients studied, suggesting that the immaturity of the immune response to vaccination was applicable within the first months of life along with a failure to generate a long-term immune memory. Dorsey and Orange (14) demonstrated anti-tetanus antibodies present in 30% of children with transient hypogammagammaglobulinemia of infancy (THI) and considered this impaired immune response to immunization along with increased B cell number as the principal pathomechanisms of THI. In our study, 50% of patients with hypogammaglobulinemia did not achieve 0.1 IU/ml, that is, the minimal protective level of anti-tetanus IgG antibodies, and as many as 73% of patients did not achieve 1.0 IU/ml, the antibody level associated with long-term seroprotection. Likewise, a defective response to vaccination with diphtheria toxoid related to a specific antibody level below 0.1 IU/ml was demonstrated in 27% of cases and a level below 1.0 IU/ml in 68% of the children studied. While in THI and in other frequent antibody production defects in children, such as IgG subclass deficiency, IgA deficiency, and specific anti-polysaccharide, an antibody deficiency response to protein and protein-conjugated vaccines is typically preserved, not least in a subset of pediatric patients with hypogammaglobulinemia, in whom the response to vaccines is impaired, and a lack of evidence for specific diagnosis of PID may indicate a delayed maturation of the immune response with antibodies that can also be consistent with THI (10). In the case of a severe deficiency in congenital antibody production, such as common variable immunodeficiency (CVID), an insufficient response to vaccines is a typical finding, displaying however wide intersubject variability of antibody responsiveness. A positive response to polypeptide vaccines was assessed in 23% of CVID patients, with 18% of them demonstrating a similar response to polysaccharide vaccines (15); therefore, it may be assumed that hyporesponsiveness to vaccines and the lack of postvaccination specific antibodies is not an indispensable condition for a diagnosis of CVID.
The investigation of anti-HBs antibodies is admittedly not recommended routinely as a diagnostic test for defects of antigen-specific immunity (16) because impaired response to vaccination against hepatitis B may be an isolated abnormality and not accompanied by any exponents of the immunodeficiency. However, the practical aspect of the examination must be stressed, owing to the defined anti-HBs antibody level of 100.0 mIU/ml that is considered protective from infection in patients with immunodeficiencies. In our study, the anti-HBs antibody level below 10 mIU/ml, the minimal protective level, was demonstrated in 32% of patients and that below 100 mIU/ml, associated with long-term seroprotection, was revealed in 59% of the children.
Polysaccharides of encapsulated bacteria are thymus-independent type 2 antigens and are characterized by a lack of ability to stimulate T helper cells, weak induction of the immune response in the neonatal period, impaired stimulation of class switch recombination, and antibody production in children younger than 2 years of age. The response to polysaccharide antigens is associated with marginal zone B cells, and their number is reduced in neonates and infants. The synthesis of IgG2, along with IgG4, the most effective antibody isotypes against polysaccharides is at that time defective (17, 18). Vaccines containing protein-polysaccharide conjugates stimulate B cells and, in contrast to native Hib PRP polysaccharides, induce the response to thymus-dependent protein antigens and provide immunogenicity in infants and children younger than 2 years of age (19). In as many as 12 children (55%), the level of anti-PRP antibodies was below 1.0 μg/ml.
It is worth noting that 5 of the children studied manifested defective production of all four types of postvaccination antibodies; the impaired production of three types of antibodies was demonstrated in 11 children and the impaired production of 1 type of antibody was observable in 4 children; that is, all together, the deficiency of antigen-specific antibody response to immunization was revealed in as many as 20 of the children (91%) studied. This phenomenon supports the hypothesis by Dorsey and Orange (14) regarding the relationship between hypogammaglobulinemia and impaired response to vaccines; however, the proportion of children with this immune defect noted in our study was even higher than in the aforementioned report. No correlation between the doses of vaccines that had been administered in children studied and the levels of antigen-specific postvaccination antibodies was observed.
It must be emphasized that active immunization in the neonatal and early infantile period, accompanied by the immaturity of the adaptive immune response leading to long-term immune memory, may be considered the major cause of the impairment of the antibody synthesis following vaccination (20, 21). The immaturity of and limitations of T cell functions in neonates and young children can be important contributors. Delayed maturation of dendritic cells in early life and impaired secretion of interleukin-12 (IL-12), type I IF (interferon), and IL-18 can result in defective T cell ability to produce cytokines, the CD4+ T cell response biased toward a Th2 phenotype and promotion of regulatory T cells. Furthermore, the hypothesis of active suppression by immune regulatory cells was put forward recently (22), which involves immune suppressive pathways of mesenchymal stromal cells, myeloid-derived stromal cells, CD5+ B cells, and regulatory T cells. Important factors that may influence vaccine efficacy in infants and children is the occurrence of genetic variability within the major histocompatibility complex (MHC) region. A number of single nucleotide polymorphisms (SNPs) within MHC class I and II genes are associated with a specific antibody response, immunoglobulin, and IgG subclasses production (23, 24). Additionally, immunogenetic studies have also shown associations between polymorphisms in gene-encoding immune response proteins and variation in response to vaccines. Among others, associations between an SNP in the forkhead box protein 1 (FOXP1) gene, a transcription factor regulating B cell development (25) and in various cytokine genes, such as gamma interferon (IFN-γ), IL-4, IL-4R, IL-10, IL-10RA, IL-12B, and tumor necrosis factor (TNF), and the vaccine efficacy have also been shown (26, 27). Another proposed inhibitory mechanism that may contribute to inadequate infant response to vaccine is related to high titers of maternal antibodies that persist over a period of 6 to 12 months. The reduced antibody generation by maternal antibodies to protein tetanus and hepatitis B vaccines as well as conjugated Hib vaccine have been reported (28, 29), and this phenomenon may be explained by a hypothesis of the downregulation of B cell responses mediated through a cross-link between a B cell receptor and the inhibitory Fcγ-receptor IIB by a vaccine-antibody complex (29).
Interestingly, in our study antibody production defects were accompanied by an increased proportion of transitional B cells, a population that does not proliferate but instead differentiates into naive mature B cells; therefore, this expansion of recent bone marrow emigrants may be a mechanism related to the small number of mature B cells due to a peripheral maturation defect. These findings are consistent with observations concerning the underlying immunologic disease-causing mechanisms in common variable immunodeficiency (CVID), in which patients fail to produce sufficient amounts of antigen-specific antibodies due to defects in B cell differentiation and maturation. As mutations in genes encoding T and B cell activation molecules and receptors, such as inducible T cell costimulator (ICOS), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), B cell activating factor-receptor (BAFF-R), B cell receptor complex (CD19, CD81, CD21), and CD20 have been detected in less than 10% of patients affected by CVID (30), the identification of genetic defects is limited by disease immunologic heterogeneity. A classification of CVID dependent of immune parameters of the B cell compartment with analysis of replication history and somatic hypermutation status has been proposed by Driessen et al. (31). The pathophysiologic B cell pattern associated with peripheral maturation or survival defects was associated with a normal transitional B cell number and a reduction of naive mature and memory B cells in CVID patients and also in the hypogammaglobulinemic children studied. On the other hand, the generation of high-affinity B cell receptors in memory B cells and production of antigen-specific antibodies, depending on the somatic hypermutation (SHM) process in the variable region of immunoglobulin genes in children of various ages, differs considerably and increases rapidly during the first 2 years of life (32). Therefore, in young children a delayed maturation of SHM levels might consequently suggest an impaired maturation of the immune system.
The concurrent increase in the proportion of plasmablasts, precursors of plasma cells that are the major source of high-affinity antibodies, may thus lead to the hypothesis of a maturational block at the terminal stage of B cell development. This phenomenon, not reported in previous studies, may be explained as the expression of immunological immaturity leading eventually to an impaired antigen-specific antibody response. Further studies of the antigen-specific B cell repertoire are required to define the correlates of protection and vaccine efficacy in the particular population of immunodeficient children (33, 34).
ACKNOWLEDGMENTS
We acknowledge the assistance of Renata Jenek in the assessment of postvaccination antibodies and Stanislaw Nowak for help with statistical analysis.
REFERENCES
- 1.de Vries E, Driessen G. 2011. Primary immunodeficiencies in children: a diagnostic challenge. Eur J Pediatr 170:169–177. doi: 10.1007/s00431-010-1358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiehm RE. 2007. The four most common pediatric immunodeficiencies. Adv Exp Med Biol 601:15–26. [DOI] [PubMed] [Google Scholar]
- 3.Fried AJ, Bonilla FA. 2009. Pathogenesis, diagnosis, and management of primary antibody deficiencies and infections. Clin Microbiol Rev 22:396–414. doi: 10.1128/CMR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kainulainen L, Vuorinen T, Rantakokko-Jalava K, Osterback R, Ruuskanen O. 2010. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol 126:120–126. doi: 10.1016/j.jaci.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madaliński K. 2002. Persistence of immunity following vaccination against hepatitis B. Przegl Epidemiol 56:605–613. (In Polish.) [PubMed] [Google Scholar]
- 6.Keating GM, Noble S. 2003. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 63:1021–1051. doi: 10.2165/00003495-200363100-00006. [DOI] [PubMed] [Google Scholar]
- 7.Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin Vaccine Immunol 17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piątosa B, Wolska-Kusnierz B, Pac M, Siewiera K, Gałkowska E, Bernatowska E. 2010. B cell subsets in healthy children: reference values for evaluation of B cell maturation process in peripheral blood. Cytometry B Clin Cytom 78:372–381. [DOI] [PubMed] [Google Scholar]
- 10.Orange JS, Ballow M, Stiehm ER, Ballas Z, Chinen J, De La Morena M, Kumararatne D, Harville TO, Hesterberg P, Koleilat M, McGhee S, Perez EE, Raasch J, Scherzer R, Schroeder H, Seroogy C, Huissoon A, Sorensen RU, Katial R. 2012. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 130(Suppl):1–24. doi: 10.1016/j.jaci.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Zarei S, Jeddi-Tehrani M, Akhondi MM, Zeraati H, Pourheidari F, Ostadkarampour M, Tavangar B, Shokri F. 2009. Primary immunization with a triple diphtheria-tetanus-whole cell pertussis vaccine in Iranian infants: an analysis of antibody response. Iran J Allergy Asthma Immunol 8:85–93. [PubMed] [Google Scholar]
- 12.Paulke-Korinek M, Fischmeister G, Grac A, Rendi-Wagner P, Kundi M, Mohzadeh-Rabbani A, Moritz K, Fenninger B, Jarisch R, Jasinska J, Holzmann H, Wiedermann U, Kollaritsch H. 2011. Peristence of antibodies in 4-8 year old Austrian children after vaccination with hexavalent DTaP-HBV-IPV/Hib and MMR vaccines. Vaccine 29:5130–5136. doi: 10.1016/j.vaccine.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Postfay-Barbe KM, Kobela M, Sottas C, Grillet S, Taguebue J, Ekoe T, Lambert PH, Lecoultre C, Siegrist CA. 2010. Frequent failure of adolescent booster response to tetanus toxoid despite infant immunization: waning of infancy-induced immune memory? Vaccine 28:4356–4361. doi: 10.1016/j.vaccine.2010.04.060. [DOI] [PubMed] [Google Scholar]
- 14.Dorsey MJ, Orange JS. 2006. Impaired specific antibody response and increased B-cell population in transient hypogammaglobulinemia of infancy. Ann Allergy Asthma Immunol 97:590–595. doi: 10.1016/S1081-1206(10)61085-X. [DOI] [PubMed] [Google Scholar]
- 15.Goldacker S, Draeger R, Warnatz K, Huzly D, Salzer U, Thiel J, Eibel H, Schlesier M, Peter HH. 2007. Active vaccination in patients with common variable immunodeficiency (CVID). Clin Immunol 124:294–303. doi: 10.1016/j.clim.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Abraham RS. 2011. Relevance of laboratory testing for the diagnosis of primary immunodeficiencies: a review of case-based examples of selected immunodeficiencies. Clin Mol Allergy 9:6. doi: 10.1186/1476-7961-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson AR, Roifman CM. 2007. Low serum immunoglobulin G2 levels in infancy can be transient. Pediatrics 120:e543–e547. doi: 10.1542/peds.2006-3613. [DOI] [PubMed] [Google Scholar]
- 18.Klein Klouwenberg P, Bont L. 2008. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin Dev Immunol 2008:628963. doi: 10.1155/2008/628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly DF, Moxon ER, Pollard AJ. 2004. Haemophilus influenzae type b conjugate vaccines. Immunology 113:163–174. doi: 10.1111/j.1365-2567.2004.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breukels MA, Spanjaard L, Sanders L, Rijkers GT. 2001. Immunological characterization of conjugated Haemophilus influenzae type b vaccine failure in infants. Clin Infect Dis 32:1700–1705. doi: 10.1086/320755. [DOI] [PubMed] [Google Scholar]
- 21.Siegrist CA. 2007. The challenges of vaccine responses in early life: selected examples. J Comp Pathol 137(Suppl):4–9. [DOI] [PubMed] [Google Scholar]
- 22.Gervassi AL, Horton H. 2014. Is infant immunity actively suppressed or immature? Virology (Auckl) 2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yucesoy B, Talzhanov Y, Johnson VJ, Wilson NW, Biagini RE, Wang W, Frye B, Weissman DN, Germolec DR, Luster MI, Barmada MM. 2013. Genetic variants within the MHC region are associated with immune responsiveness to childhood vaccinations. Vaccine 4:5381–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinetti M, De Sivestri A, Belloni C, Pasi A, Tinelli C, Pistorio C, Salvaneschi L, Rondini G, Avanzini MA, Cuccia M. 2000. Humoral response to recombinant hepatitis B virus vaccine at birth: role of HLA and beyond. Clin Immunol 97:234–240. doi: 10.1006/clim.2000.4933. [DOI] [PubMed] [Google Scholar]
- 25.Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M. 2010. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun 11:232–238. doi: 10.1038/gene.2010.1. [DOI] [PubMed] [Google Scholar]
- 26.Yucesoy B, Johnson VJ, Fluharty K, Kashon ML, Slaven JE, Wilson NW, Weissman DN, Biagini RE, Germolec DR, Luster MI. 2009. Influence of cytokine gene variations on immunization to childhood vaccines. Vaccine 27:6991–6997. doi: 10.1016/j.vaccine.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 27.Newport MJ. 2015. The genetic regulation of infant immune responses to vaccination. Front Immunol 6:18. doi: 10.3389/fimmu.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niewiesk S. 2014. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Gui XE, Teter C, Zhong H, Pang Z, Ding L, Li F, Zhou Y, Zhang L. 2014. Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine 32:6091–6097. doi: 10.1016/j.vaccine.2014.08.078. [DOI] [PubMed] [Google Scholar]
- 30.Yazdani R, Hakemi MG, Sherkat R, Homayouni V, Farahani R. 2014. Genetic defects and the role of helper T-cells in the pathogenesis of common variable immunodeficiency. Adv Biomed Res 3:2. doi: 10.4103/2277-9175.124627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Driessen GJ, van Zelm MC, van Hagen PM, Hartwig NG, Trip M, Warris A, de Vries E, Barendregt BH, Pico I, Hop W, van Dongen JJ, van der Burg M. 2011. B-cell replication history and somatic hypermutation status identify distinct pathophysiologic backgrounds in common variable immunodeficiency. Blood 118:6814–6823. doi: 10.1182/blood-2011-06-361881. [DOI] [PubMed] [Google Scholar]
- 32.Schatorje EJ, Driessen GJ, van Hout RW, van der Burg M, de Vries E. 2014. Levels of somatic hypermutations in B cell receptors increase during childhood. Clin Exp Immunol 178:394–398. doi: 10.1111/cei.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centlivre M, Combadiere B. 2015. New challenges in modern vaccinology. BMC Immunol 16:18. doi: 10.1186/s12865-015-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson CW. 2015. Applying contemporary immunology to elucidate heterologous effects of infant vaccines and to better inform maternal-infant immunization practices. Front Immunol 6:64. doi: 10.3389/fimmu.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]