Abstract
Objectives:
We sought to validate Cognitive Research Corporation’s Driving Simulator (CRCDS Mini-Sim) for studies of drug safety with respect to driving ability.
Methods:
A total of 30 healthy subjects were randomized to receive placebo or 7.5 mg zopiclone, a hypnotic known to impair driving, in random order during the 2 treatment periods of a 2 period crossover design.
Results:
Evening administration of 7.5 mg zopiclone increased next-day standard deviation of lateral lane position (SDLP) by 2.62 cm on average compared with evening administration of placebo, and caused significant effects on symmetry analysis. The magnitude of the change in SDLP is highly similar to changes previously observed using on-the-road driving methods.
Conclusions:
Further validation of the CRCDS Mini-Sim is warranted to develop this platform for drug safety studies.
Keywords: cognition, driving simulation, hypnotics, next-day residual drug effects, standard deviation of lane position
Introduction
In recent years, increasing attention has been focused on the safety of hypnotic drugs, in particular with respect to effects on driving ability [Verster et al. 2004, 2006, 2007; Verster and Mets, 2009; Leufkens and Vermeeren, 2009; Meskali et al. 2009]. Research has shown that many, but not all, currently marketed hypnotic drugs adversely affect driving ability as measured by standard deviation of lateral lane position (SDLP), a measure of weaving, the day after evening dosing [Verster et al. 2006]. The most direct method for assessing residual effects on SDLP involves testing drug effects on actual on-the-road driving (OTR). However, such studies are limited to a small number of highly specialized sites and alternatives to OTR may be useful with regard to cost, speed of execution, flexibility with regard to site, and ease of combination with other modalities.
Numerous driving simulator platforms have been developed to determine the effects of drugs, medical conditions, and other factors on driving performance [Lovsund et al. 1991; Lundqvist et al. 1997; Ranney et al. 1999; Lee et al. 2002; Barkley et al. 2005; Staner et al. 2005; Bulmash et al. 2006; Yamaguchi et al. 2006; Fildes et al. 2007; Bella, 2008; Brunnauer et al. 2008, 2009; Ting et al. 2008; Yan et al. 2008; De Winter et al. 2009; Stein and Dubinsky, 2011; Auberlet et al. 2012; Stough et al. 2012]. Cognitive Research Corporation’s Driving Simulator uses realistic depictions of driving scenarios based on actual roads that are visualized on a multiple monitor system, with actual auto seating and controls to maximize the realism of the drive. The platform has been found to have excellent sensitivity to alcohol and to compare favorably with another commonly used simulator platform [Kay et al. 2013].
The main goal of the present study was to use zopiclone, a commonly used nonbenzodiazepine hypnotic medication, as one step toward validating the CRCDS Mini-Sim for pharmacological studies. Zopiclone has been proposed as a ‘positive control’ drug for hypnotic safety studies because of its well-known residual effects on driving ability [Bocca et al. 1999, 2011; Vermeeren et al. 2002; Gustavsen et al. 2009; Verster et al. 2011]. We also tested the ability of zopiclone to impair performance on a computer-administered symbol-digit substitution test (CogScreen Symbol Digit Coding). A similar test has previously been shown to be sensitive to zopiclone [Leufkens et al. 2009], allowing us to verify the efficacy of the pharmacological manipulation using an independent test. Our primary hypothesis was that zopiclone increases SDLP the morning after administration. We also tested the secondary hypothesis that zopiclone decreases performance on a measure of attention and response speed, the CogScreen-PM Symbol Digit Coding (SDC) task.
Materials and methods
Measures
Simulated driving was assessed using the Cognitive Research Corporation’s Driving Simulator (CRCDS Mini-Sim). The CRCDS utilizes the University of Iowa National Advanced Driving Simulator (NADS) Mini-Sim simulator [Brown et al. 2010, 2013a, 2013b]. The Mini-Sim is a PC-based research driving simulator that provides a realistic automotive driving environment. The system consists of a single PC for simplicity and reliability, three front channel displays, an instrument panel display, a 2.1 audio system, a tactile transducer, and a separate display for the operator. The driving cockpit utilizes a full-size steering wheel and realistic pedals. The seat is an actual automotive seat which meets current US National Highway Traffic Safety Administration standards. The Mini-Sim uses a three-screen wide display to present a highly immersive and realistic driving environment.
The CRCDS was used to display the Country Vigilance Scenario (CVS) which has been demonstrated to be sensitive to detect the effects of alcohol and other factors on driving performance [Kay et al. 2013]. Components of the CVS include: mid-day daylight driving; two lane highway through remote rural countryside; uneventful drive with occasional long, wide curves and mild changes in grade; infrequent oncoming vehicles, 55 miles per hour (mph) posted throughout entire drive; and a secondary attention task of divided attention stimuli is presented throughout the entire drive. Periodically, targets are presented in boxes on the left side mirror and the right windshield column. When an arrow pointing to the left appears in the box on the inside of the left mirror, the participant’s task is to respond by hitting the center button on the left of the steering wheel as quickly as they can. When an arrow pointing to the right appears in the box on the column to the right of the windshield, the participant’s task is to respond by hitting the center button on the right of the steering wheel as quickly as they can. The participant is to ignore arrows pointing up which appear in the boxes on either side mirror. The arrow stimuli appear for 5 seconds, or until the participant makes a response. This task measures how quickly and accurately the participant responds to stimuli while driving. There are no verbal instructions during this drive. There are no verbal prompts for driving out of lane or driving too slowly. The driver is asked to drive the entire scenario at the posted speed limit of 55 mph. The driver is also graded on how steadily they can drive in their lane, with as little weaving as possible.
Endpoints from simulated driving included SDLP (in units of cm), speed deviation (m/sec), lane exceedances (count) and three measures of divided attention task performance (percent correct, standard deviation of reaction time, and median reaction time for correct items). Details regarding these endpoints are presented in the Statistical Analysis section below.
Cognition was assessed using subtests from the CogScreen computerized test battery [Crook et al. 2009]. CogScreen endpoints included SDC (number correct), previous number accuracy under multitasking conditions (dual task – single task performance; DIFPNACC), and tracking accuracy under multitasking conditions (dual task – single task performance; DIFTRERR, reported as absolute average error in pixels).
Other assessments included the Simulator Sickness Questionnaire [Kennedy et al. 1992], Karolinska Sleepiness Scale (KSS), Visual-Analog Scale Motivation (self-assessment of motivation to perform drive) and Visual-Analog Scale Performance (self-assessment of driving performance on the simulator).
Subjects
The study was conducted at a single site, SGS in Antwerp, Belgium, as a randomized, double-blind, 2 period crossover study in healthy volunteers between the ages of 25 and 50 years (inclusive). Subjects were recruited through local advertising. Fully informed consent was obtained and all procedures were performed in accordance with the Declaration of Helsinki 1975, revised Hong Kong 1989. Eligible subjects included those who had: regular sleep patterns (bedtime between 21:00 and 24:00); were active drivers with a driver’s license who had driven an average minimum of 5000 km per year for the previous 3 years; did not demonstrate simulator sickness using a Simulator Sickness Questionnaire (SSQ); and did not have any visual or auditory impairment which would interfere with study-related procedures, with a body mass index of no greater than 30, and negative urine drug and breathalyzer screens.
In the initial enrollment period, 22 subjects were randomized in order to obtain at least 20 subjects with 2 evaluable periods to be included in an interim analysis. After the predefined interim analysis, results (see below) indicated the study did not meet the prespecified criteria to stop for efficacy or futility, an additional 8 subjects were randomized for a total of 30 subjects with 2 evaluable periods. A total of seven female subjects and a total of eight male subjects were randomized to placebo–zopiclone and the zopiclone–placebo conditions, respectively. The mean age (±standard deviation) for the placebo–zopiclone and zopiclone–placebo conditions was 40.7 ± 6.43 and 38.7 ± 7.10 years, respectively.
On dosing days, caffeine was not permitted from 10 hours prior to dosing until the time of discharge. At all other times, caffeine was limited to the equivalent of 480 mg/day. Alcohol was not allowed from 48 hours prior to study drug administration through 24 hours after study drug administration in each treatment period and for 24 hours prior to the pre study and post study assessments. At all other times alcohol was limited to approximately three drinks per day. Smoking was prohibited from 24 hours prior to dosing up to 12 hours after study drug administration. During the washout period, subjects were permitted to have no more than 2 cigarettes per day up to a maximum of 4 cigarettes per week. Study drug was administered with water and subjects were food restricted 1 hour prior to and 1 hour after study drug administration. Grapefruit products were not allowed beginning approximately 2 weeks prior to administration of the initial dose of study drug, throughout the study and until the post study assessment, and no fruit juices were allowed 12 hours prior to administration of study drug for each treatment period until time of discharge. The only concomitant medication that was permitted was acetaminophen.
Within 4 weeks of signing consent, subjects were admitted to the Clinical Research Unit (CRU) for Period 1. Prior to evening dosing on Day 1 of each treatment period, subjects completed driving simulator and cognitive battery practice. Plasma for zopiclone pharmacokinetics was drawn prior to dosing and subjects were then dosed with either zopiclone 7.5 mg or placebo at ~23:00 hours and remained in the clinic overnight.
On Day 2 of each treatment period, approximately 8 hours post dose, vital signs and plasma for zopiclone pharmacokinetics were collected and the Cog Screen-PM test battery and KSS were administered 30–60 minutes prior to the 60 minute (100 km) simulated drive approximately 10 hours post dose. Following the drive, subjects completed a Visual Analog Scale (VAS) for self-rating of driving performance and motivation.
Subjects were discharged after Period 1 procedures were completed. A minimum 5 day washout was required prior to the start of Period 2. Period 2 procedures also included having the subjects arrive at the CRU the day before the driving assessment to complete a practice drive and the practice cognitive testing session. Another plasma sample for zopiclone pharmacokinetics was collected, and subjects received either zopiclone 7.5 mg or placebo at ~23:00 hours while remaining in the clinic overnight. Procedures the following day were the same as for Period 1. On Day 2 of each treatment period, subjects completed the KSS prior to the 60 minute drive and a VAS to self-assess motivation and drive performance after the 60 minute drive.
Statistical methods
The SDLP measured over the entire length of the drive was the primary endpoint in this study. SDLP was calculated from the digitized driving data (digitized at 60 Hz) as follows. The first 1000 feet (~304 meters) of the drive were deleted as specified in the standard Mini-Sim data reduction protocol. For the remaining time points in the drive, lane deviation was defined by taking the driver’s current position and then building a Hermite spline using four points (the two points behind the driver’s position and the two points after the driver’s position) on the road. Lane deviation was set equal to the lateral distance between the estimate of the driver’s position and the lane center. SDLP was then calculated as the standard deviation of lane deviation. The total number of correct responses over two minutes on the SDC test (SDC correct) was the secondary endpoint in this study. Exploratory endpoints included: speed deviation (defined as deviation from the 55 mph speed that subjects were asked to drive at), lane exceedances (number of times that a tire passed a lane boundary) and dual tracking error (psychomotor; multitasking).
To address the primary hypothesis that zopiclone produces next-day residual impairment compared with placebo on the SDLP driving measure, the SDLP from the Mini-Sim driving simulator was examined using a mixed analysis of variance model appropriate for a two period crossover with fixed factors for treatment, period and sequence, and a random factor for subject within sequence. A paired t-test (α = 0.05, one-sided) using the mixed analysis of variance (ANOVA) model mean square error (MSE) was used to evaluate the SDLP treatment difference (zopiclone versus placebo). A 90% confidence interval (CI) for the difference in SDLP (zopiclone – placebo) using the mixed ANOVA MSE, the effect size [(mean zopiclone – mean placebo) / SQRT MSE)], and the corresponding two-sided 90% CI for the effect size were calculated.
There were no multiplicity adjustments amongst the exploratory endpoints. SDC and lane exceedances were analyzed using a similar model as described for SDLP; however, lane exceedances required a transformation. Because previous experience with the driving simulator had indicated that a natural log transformation [y = ln(x + 1)] would be most appropriate for the analysis of lane exceedances, lane exceedances were analyzed on the natural log (x + 1) scale and reported as x = exp(y). Treatment differences for lane exceedance are reported as percent change.
SDLP was also analyzed to examine balance in ‘extreme’ values, that is, treatment differences above a range of thresholds between 1 and 6 cm (commonly referred to as ‘symmetry analysis’). For each threshold, the number of impaired and improved subjects was calculated and the hypothesis that the two frequencies are equal was tested using McNemar’s test. The corresponding p values were calculated using an exact binomial test (see Center for Drug Evaluation and Research Application 022328Orig1s000).
A predefined interim analysis was conducted after the data from the initial 22 subjects became available to allow for stopping for futility or continuing with additional enrollment. After failing to meet the prespecified criteria for stopping for efficacy (i.e. p values for SDLP and SDC both had to be <0.0034), an additional 8 subjects were enrolled. At the end of the study, the SDLP and SDC treatment contrasts were tested at α = 0.0498 to maintain an overall type I error rate of 0.05.
A total of 30 subjects completing the study was estimated to yield 99% power to detect a true SDLP difference (zopiclone – placebo) of 4.2 cm given a standard deviation (SD) of 5.73, and 86% power to detect a true SDC difference (zopiclone – placebo) of 3.24 given a standard deviation of 6.45. The overall power to detect the above named changes in both SDLP and SDC with 30 subjects completing the study was 85%. This estimate assumes independence between SDLP and SDC, and is therefore slightly conservative.
Statistical analyses were conducted with SAS version 9.2 and R version 2.15.2.
Results
A total of 30 subjects were randomly assigned to study treatment. All 30 subjects received the intended treatment and completed the trial. The interim data analysis is described in the Statistical Methods section above. All 30 subjects were analyzed in the primary analysis. Subject characteristics are shown in Table 1. There were no serious adverse events (SAEs) noted during the study and all recorded nonserious adverse experiences were of mild intensity. All subjects noting adverse events recovered, except for one subject with cytomegalovirus infection which was considered definitely not related to study drug treatment.
Table 1.
Summary of demographic variables and subject allocation.
| Age range | Sex | Treatment sequence |
|
|---|---|---|---|
| Placebo–Zopiclone | Zopiclone–Placebo | ||
| 25–35 | Female | 2 | 2 |
| Male | 3 | 2 | |
| 36–45 | Female | 3 | 3 |
| Male | 4 | 3 | |
| 46–50 | Female | 2 | 2 |
| Male | 1 | 3 | |
Primary hypothesis: SDLP
As illustrated in Figure 1, Table 2 and Supplementary Figure 1, evening administration of 7.5 mg zopiclone in healthy young subjects increased the next-day SDLP on the CRCDS Mini-Sim driving simulator by 2.62 cm (p < 0.001, one-sided) compared with evening administration of placebo. The effect size (treatment difference / SDwithin) and 90% CI for the effect size were 1.07 and (0.58–1.55), respectively.
Figure 1.
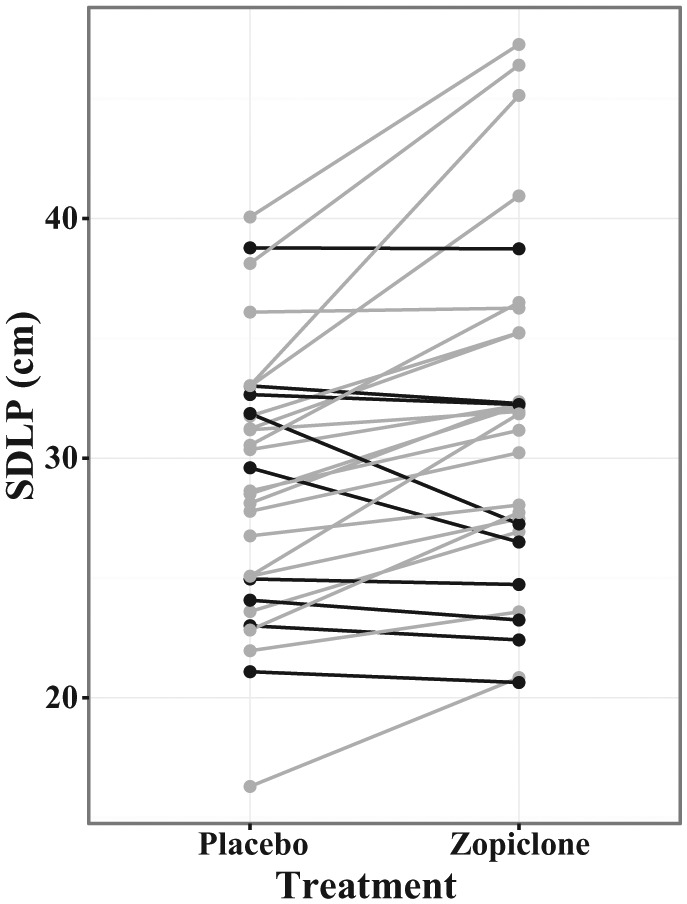
SDLP versus treatment.
SDLP is shown plotted as a function of treatment condition, with lines connecting data values from individual subjects. Subjects with higher SDLP in the zopiclone condition are plotted in grey and subjects with lower SDLP in the zopiclone condition are plotted in black. SDLP was higher in the zopiclone condition than in the placebo condition. See Table 2 for statistical results.
Table 2.
Least squares means, confidence intervals, effects sizes, effects size confidence intervals and p values for the driving variables.
| Variable | Analysis scale | Treatment | LSMean | CI for mean* | ES | ES 90% CI | p value (one-sided) |
|---|---|---|---|---|---|---|---|
| SDLP | Raw | Placebo | 28.97 | (26.56, 31.38) | |||
| Zopiclone 7.5 mg | 31.59 | (29.18, 33.99) | |||||
| Zopiclone versus placebo | 2.62 | (1.54, 3.69) | 1.07 | (0.58, 1.55) | 0.000 | ||
| Lane exceedances | Natural log | Placebo | 41.90 | (27.03, 64.94) | |||
| Zopiclone 7.5 mg | 51.46 | (33.20, 79.76) | |||||
| Zopiclone versus placebo | 22.80 | (−2.4, 54.6) | 0.39 | (−0.04, 0.82) | 0.070 | ||
| Speed deviation | Raw | Placebo | 0.86 | (0.74, 0.98) | |||
| Zopiclone 7.5 mg | 0.85 | (0.73, 0.97) | |||||
| Zopiclone versus placebo | −0.01 | (−0.07, 0.05) | −0.07 | (−0.49, 0.36) | 0.397 |
Estimates are from a linear mixed model appropriate for a two-period crossover study with fixed factors for sequence, period and treatment with a random factor for subject within sequence. All analyses were performed on the raw (nontransformed) scale; treatment differences are reported as differences. All 30 subjects were included in the analysis. Analysis for lane exceedances is shown on the back-transformed natural log scale because a ln(y + 1) transformation was performed on this endpoint prior to statistical analysis. The treatment difference is reported as the percent change; marginal treatment means are reported as geometric means.
90% CI for treatment LSMean differences, 95% CI for marginal LSMeans.
CI, confidence interval; ES, effects size; LSMean, least squares mean; SDLP, standard deviation of lateral lane position.
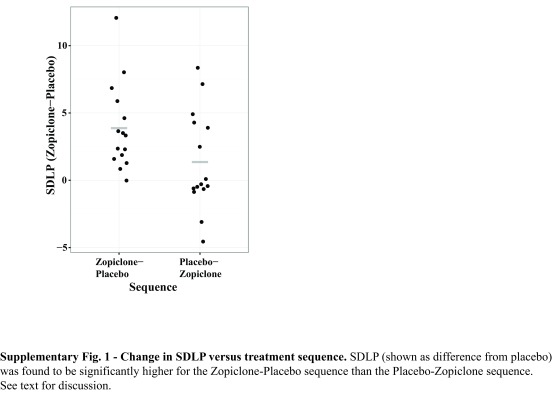
Upon graphical examination (see Supplementary Figure 1), SDLP change appeared to vary with treatment sequence. Examination of the zopiclone effect on SDLP in each treatment sequence separately revealed a larger effect in the zopiclone–placebo treatment sequence (3.88 cm, p = 0.0002) than in the placebo–zopiclone treatment sequence (1.36 cm, p = 0.09).
Secondary hypothesis: SDC
Evening administration of 7.5 mg zopiclone in healthy young subjects decreased the next-day SDC number correct by 2.67 (p = 0.007, one-sided) compared with evening administration of placebo (Figure 2). The effect size and 90% CI for the effect size were -0.67 and (-1.12 to -0.22), respectively (see Table 3). A sequence effect, examined both graphically and in the linear mixed model, was not observed for this endpoint.
Figure 2.
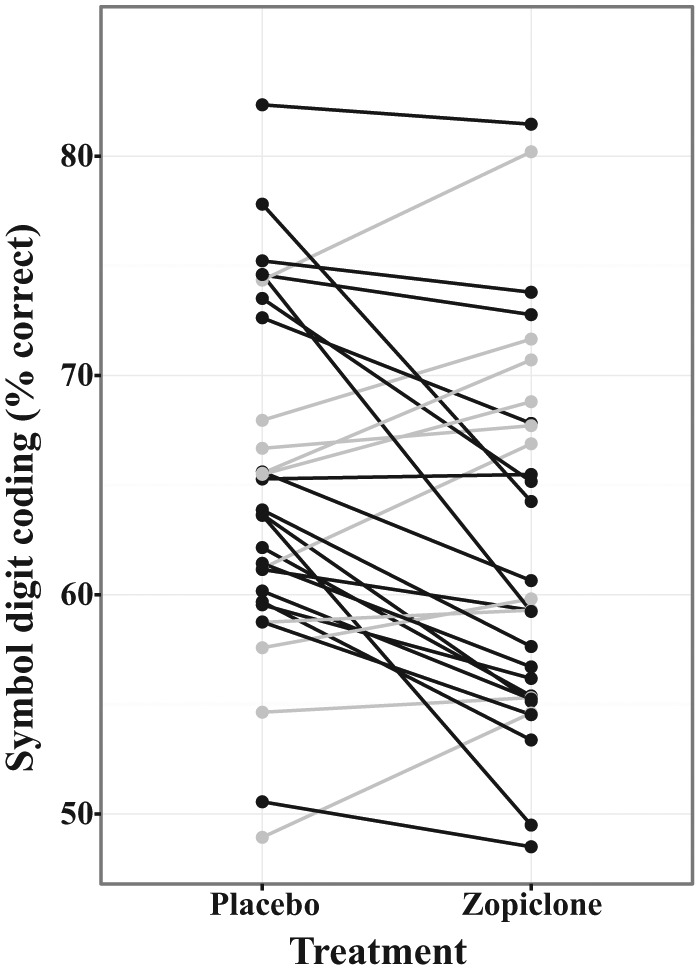
Symbol digit coding (SDC) versus treatment.
SDC is shown plotted as a function of treatment condition, with lines connecting data values from individual subjects. Subjects with higher SDC performance in the zopiclone condition are plotted in grey, and subjects with lower SDC performance in the zopiclone condition are plotted in black. On average, zopiclone cause a worsening of performance on SDC. See Table 3 for statistical results.
Table 3.
Least squares means, standard errors, confidence intervals, effect sizes and p values for the cognition variables.
| CogScreen test (variable) | Definition (Domain) | Treatment | LSMean | CI for Mean* | ES | ES 90% CI | p value (one-sided) |
|---|---|---|---|---|---|---|---|
| Symbol digit coding (SDC) | Percent correct | Placebo | 64.93 | (61.83, 68.03) | |||
| SDCCOR | (Processing speed; attention) | Zopiclone 7.5 mg | 62.27 | (59.17, 65.37) | |||
| Zopiclone versus placebo | −2.67 | (−4.40, −0.93) | −0.67 | (−1.12, −0.22) | 0.007 | ||
| Dual-Alone task (DIF) | Difference in previous number accuracy | Placebo | −9.00 | (−12.6, −5.36) | |||
| DIFPNACC | (Divided attention) | Zopiclone 7.5 mg | −9.28 | (−12.9, −5.64) | |||
| Zopiclone versus placebo | −0.28 | (−3.93, 3.37) | −0.03 | (−0.46, 0.39) | 0.449 | ||
| DIF | Difference in tracking error | Placebo | 22.36 | (16.71, 28.01) | |||
| DIFTRERR | (Divided attention) | Zopiclone 7.5 mg | 24.48 | (18.83, 30.13) | |||
| Zopiclone versus placebo | 2.12 | (−1.18, 5.42) | 0.28 | (−0.15, 0.71) | 0.142 | ||
| Dual Task (DTT) | Absolute tracking error, single task condition | Placebo | 9.24 | (7.51, 10.98) | |||
| DTTAABS | (Divided attention) | Zopiclone 7.5 mg | 9.64 | (7.91, 11.38) | |||
| Zopiclone versus placebo | 0.40 | (−1.10, 1.90) | 0.12 | (−0.31, 0.54) | 0.326 | ||
| DTT | Absolute tracking error, dual task condition | Placebo | 31.60 | (25.43, 37.78) | |||
| DTTDABS | (Divided attention) | Zopiclone 7.5 mg | 34.12 | (27.95, 40.30) | |||
| Zopiclone versus placebo | 2.52 | (−0.47, 5.51) | 0.37 | (−0.06, 0.80) | 0.081 | ||
| DTT | Previous number dual accuracy | Placebo | 89.55 | (85.91, 93.18) | |||
| DTTPDACC | (Divided attention) | Zopiclone 7.5 mg | 89.11 | (85.48, 92.75) | |||
| Zopiclone versus placebo | −0.44 | (−3.89, 3.02) | −0.06 | (−0.48, 0.37) | 0.416 |
Estimates are from a linear mixed model appropriate for a two-period crossover study with fixed factors for sequence, period and treatment with a random factor for subject within sequence. All analyses were performed on the raw (nontransformed) scale; treatment differences are reported as differences. All 30 subjects were included in the analysis. The treatment difference is reported as the percent change; marginal treatment means are reported as geometric means.
90% CI for treatment LSMean differences, 95% CI for marginal LSMeans.
CI, confidence interval; ES, effects size; LSMean, least squares mean; SDLP, standard deviation of lateral lane position.
SDCCOR, symbol digit coding correct responses; DIFPNACC, dual task test previous number accuracy; DIFTRERR, dual task test tracking error; DTTAABS, tracking alone; DTTPDACC, previous number dual.
Exploratory analyses
Symmetry analysis
The SDLP symmetry analysis for the evening administered zopiclone 7.5 mg versus placebo treatment differences for a range of impairment thresholds is shown in Table 4. Zopiclone led to a significant asymmetry (number of subjects with impairment versus improvement, at each threshold) relative to placebo at most of the impairment thresholds tested.
Table 4.
SDLP symmetry analysis.
| Threshold | Number of subjects |
Proportion |
McNemar statistic | p value | |||
|---|---|---|---|---|---|---|---|
| Impaired | Improved | Neutral | Impaired | Improved | |||
| 1.00 | 19 | 2 | 9 | 0.63 | 0.07 | 13.76 | 0.0002 |
| 1.25 | 18 | 2 | 10 | 0.60 | 0.07 | 12.80 | 0.0004 |
| 1.50 | 18 | 2 | 10 | 0.60 | 0.07 | 12.80 | 0.0004 |
| 1.75 | 17 | 2 | 11 | 0.57 | 0.07 | 11.84 | 0.0007 |
| 2.00 | 16 | 2 | 12 | 0.53 | 0.07 | 10.89 | 0.0013 |
| 2.25 | 16 | 2 | 12 | 0.53 | 0.07 | 10.89 | 0.0013 |
| 2.50 | 13 | 2 | 15 | 0.43 | 0.07 | 8.07 | 0.0074 |
| 2.75 | 13 | 2 | 15 | 0.43 | 0.07 | 8.07 | 0.0074 |
| 3.00 | 13 | 2 | 15 | 0.43 | 0.07 | 8.07 | 0.0074 |
| 3.25 | 13 | 1 | 16 | 0.43 | 0.03 | 10.29 | 0.0018 |
| 3.50 | 11 | 1 | 18 | 0.37 | 0.03 | 8.33 | 0.0063 |
| 3.75 | 10 | 1 | 19 | 0.33 | 0.03 | 7.36 | 0.0117 |
| 4.00 | 9 | 1 | 20 | 0.30 | 0.03 | 6.40 | 0.0215 |
| 4.25 | 9 | 1 | 20 | 0.30 | 0.03 | 6.40 | 0.0215 |
| 4.50 | 7 | 1 | 22 | 0.23 | 0.03 | 4.50 | 0.0703 |
| 4.75 | 7 | 0 | 23 | 0.23 | 0.00 | 7.00 | 0.0156 |
| 5.00 | 6 | 0 | 24 | 0.20 | 0.00 | 6.00 | 0.0313 |
| 5.25 | 6 | 0 | 24 | 0.20 | 0.00 | 6.00 | 0.0313 |
| 5.50 | 6 | 0 | 24 | 0.20 | 0.00 | 6.00 | 0.0313 |
| 5.75 | 6 | 0 | 24 | 0.20 | 0.00 | 6.00 | 0.0313 |
| 6.00 | 5 | 0 | 25 | 0.17 | 0.00 | 5.00 | 0.0625 |
Symmetry analysis was conducted on the SDLP data using a range of impairment thresholds for SDLP. For each threshold, the number of subjects who were impaired, improved, or neither is shown, as is the proportion impaired and improved. Equivalence of the number of impaired and unimpaired subjects was tested using McNemar’s test, with p values calculated using an exact binomial test.
SDLP, standard deviation of lateral lane position.
Lane exceedance and speed deviation
Lane exceedances seemed to trend toward statistical significance (p = 0.07), although no adjustments for multiplicity were performed (Table 2). No residual zopiclone effects were observed for speed deviation or on the divided attention tasks embedded in the driving scenarios (Table 2).
Psychomotor testing
Zopiclone effects on exploratory cognitive endpoints are shown in Table 3. No statistically significant zopiclone effects were observed for CogScreen endpoints other than SDC.
Correlations between driving and cognition
Spearman correlations between the driving variables and CogScreen tests were calculated as an exploratory analysis and are shown in Supplementary Table 1. There were no significant (uncorrected for multiple comparisons) correlations between changes in cognition and changes in driving variables. SDC performance was negatively correlated with the standard deviation of reaction time on the divided attention task (-0.37, p < 0.05) under the zopiclone condition.
Motivation and drive performance VAS
Results for zopiclone effects on VAS measuring self-reported driving performance and self-reported motivation to drive are shown in Supplementary Table 2. No significant zopiclone effects were observed on these measures, indicating that subjects lacked awareness of the impact of zopiclone on their driving performance and that zopiclone impairment did not affect their willingness to drive.
Discussion
Results show that the CRCDS Mini-Sim platform is sensitive to the next-day effects of evening zopiclone on driving performance. The observed effect size using this platform is similar to that observed in previous studies of zopiclone effects on driving ability using OTR driving methods. It should be noted that the absolute magnitude of SDLP obtained from this platform is significantly higher than that obtained using OTR methods. In this study we observed a mean SDLP of 28.97 cm after placebo administration compared with 17.6 cm from a previous OTR study under placebo conditions. However, the average change in SDLP due to drug was found to be very similar for the Mini-Sim and previously published studies testing the effects of zopiclone using OTR driving. For example, in this study we observed a mean change in SDLP (zopiclone minus placebo) of 2.62 (90% CI 1.54–3.69) and an effect size and 90% CI of 1.07 and (0.58, 1.55), respectively. Previous work using OTR driving for the zopiclone condition found a mean change in SDLP of 2.76 (1.39, 4.12) using a similar statistical approach [Leufkens et al. 2009] and similar to a more recent study [Leufkens and Vermeeren, 2014]. In a recent meta-analysis, the effects of zopiclone were found to cause an average increment of 3.0 cm in SDLP (range was 1.6–4.5 cm for studies examining driving 8.5–11 hours after dosing) [Verster et al. 2011]. Therefore, the results obtained with the Mini-Sim are similar to results obtained with OTR methods.
It is important to note that the absolute but not relative SDLP change due to zopiclone was similar using the simulator and a previous value obtained OTR. That is, the absolute changes of 2.62 and 2.76 using OTR and simulator methods, respectively, were similar, but the percent change from baseline (approximately 16% and 9%, respectively) were less similar. Theoretically, such a relationship is consistent with a model where OTR and simulated driving relate to the underlying (latent) construct of driving ability with a similar slope but different intercepts. The fact that SDLP values are higher for the simulator than OTR suggests that the intercept is higher for the simulator than actual driving. It is possible that the simulator is sensitive to certain types of lane position variation, such as high frequency components, that are not detectable OTR. The higher absolute SDLP values that are seen from simulators when comparing with OTR may be related to the impact that the visual display under simulation conditions has on perceived speed (and to a lesser extent perceived lane position). Another important difference is that the consequences of risky driving are very real in the case of OTR driving, which may increase the motivation to drive well in OTR studies. Further research will be required to determine the origin of these differences. In any case, the effect size observed was very similar using the two approaches, which is a function of a change from baseline SDLP, rather than percent change in SDLP.
OTR driving has been the most commonly used platform for assessing residual effects of hypnotics. An important safety benchmark is based on data regarding impairment due to 0.05% blood alcohol levels, based on epidemiological data showing an increase in accident risk at this level [Borkenstein et al. 1974; Krüger et al. 1995]. A randomized, 4 period, 4 treatment crossover study using OTR methods showed that a 0.05% blood alcohol level increases SDLP values by about 2.4 cm on average, thereby connecting the 2.4 cm threshold with accident risk [Louwerens et al. 1987]. Although the driving conditions used in this study were significantly different from subsequent OTR studies (i.e. they were conducted on a different highway that was closed to traffic and in a different vehicle), the results are similar to those determined in more recent studies [Ramaekers et al. 2000; Kuypers et al. 2006].
At present, the precise scaling relationship between the Mini-Sim and OTR is not known and essentially only 2 points of correspondence are known, namely at the practice drive and the morning after treatment administration (placebo and 7.5 mg zopiclone). The relationship of the two driving platforms could be linear or curvilinear when examined across a broad range of driving impairment. Furthermore, only averages are available at present, and OTR versus driving simulator within-subject analyses have not been conducted. However, as long as the scaling relationship is monotonic, which is highly likely, the Mini-Sim will yield the same relative ordering of drug effects as OTR, making it highly useful for safety studies.
Although the magnitude of the SDLP change was similar for the simulator and OTR based on a previous study, such a precise correspondence is not required for simulator approaches to be useful. Rather than a precise equivalence between simulator approaches and OTR, what is needed is construct validity for the simulator. That is, it is important that the simulator tests the same underlying construct of ‘driving ability’ that is tested using OTR methods, such that a change in SDLP on a simulator is predictive of a change in SDLP using OTR methods. Taken together with previous simulator studies, there is growing evidence that OTR and simulator approaches are sensitive to the same pharmacological manipulations. The utility of simulator approaches is that studies can be implemented at any study site, including specialized settings such as sleep and epilepsy centers, at relatively low cost. It is likely that precise predictions of crash risk will, however, require OTR approaches for the foreseeable future, since only OTR approaches are related to alcohol impairment and crash risk based on currently available data that are accepted by regulators. Therefore, a drug signal on a simulator platform could be followed by an OTR study to more precisely allow crash risk to be quantified. Precise inferences regarding crash risk from a driving simulator study are not yet possible and will require direct comparisons between simulated and OTR driving.
The mechanism of hypnotic effects on driving performance is not entirely clear, but some information is available. Flunitrazepam but not zopiclone, triazolam or lormetazepam impair reaction time on an information processing task [Harrison et al. 1985]. When zolpidem, zopiclone and flunitrazepam were compared on a collision anticipation task, only flunitrazepam impaired performance [Berthelon et al. 2003]. Bocca and Denise studied the effects of zolpidem, zopiclone and flunitrazepam on two ocular saccade tasks, and demonstrated effects of all three drugs on visuospatial performance and marked effects of flunitrazepam on alertness studied [Bocca and Denise, 2000] Although it was initially believed that zopiclone has few if any effects on cognition, more recent studies have indicated otherwise [O‘Hanlon, 1995]. For example, one study [Leufkens et al. 2009] compared gaboxadol, zolpidem, zopiclone and placebo, given before bed or in the middle of the night. With regard to World List Learning, evening administration of zopiclone and middle-of-the-night administration of zolpidem resulted in lower delayed recall scores, fewer words recognized correctly and slower responses. Critical tracking performance was impaired by middle-of-the-night gaboxadol and zolpidem. Divided attention tracking error and target detection reaction times were impaired by middle-of-the-night gaboxadol and zolpidem as well as evening zopiclone, and evening gaboxadol impaired tracking performance. Symbol digit substitution test scores were impaired by middle-of-the-night zopiclone and gaboxadol as well as evening zopiclone. In the present study, we observed impairment of SDC by zopiclone, consistent with previous results [Leufkens et al. 2009].
Relationships between zopiclone-induced driving impairment and zopiclone-induced cognition impairment were difficult to detect. We did observe a modest association between previous number accuracy under multitasking conditions and speed deviation (ρ = 0.36, p = 0.051). The previous number task is a variant of the n-back test used to assess working memory. We also noted correlations between changes in SDC and SDLP under placebo conditions and zopiclone conditions, but not with changes in SDLP. Our results, taken together with previous research, suggest that zopiclone impairs declarative memory recall, attention and processing speed/executive functioning. However, it is difficult to account for the driving impairment that we observed on the basis of changes in the cognitive domains that were measured in the present study. Further research is warranted in this area.
The results of the post hoc analysis of the effects of treatment sequence highlight the fact that the Mini-Sim has certain important limitations. In particular, we observed that subjects who underwent testing in the order placebo–zopiclone (P-Z) showed only a trend, but subjects in the zopiclone–placebo (Z-P) sequence showed a significant treatment effect. This pattern was specific to the SDLP measure and was not seen in the case of the cognition endpoints. The simplest interpretation of these results involves a simple ‘symmetric’ practice effect. Subjects randomized to the Z-P sequence may have experienced improved performance on the Mini-Sim in Period 2 because of their initial experience the day after receiving zopiclone, leading to an exaggerated apparent effect of zopiclone for that treatment sequence. Conversely, subjects randomized to the P-Z sequence may have shown a diminished apparent drug effect in Period 2 while on zopiclone because of the benefits of practice gained in Period 1 while on placebo. Under this model, the magnitude of this practice effect can be estimated by subtracting the mean increase in SDLP due to zopiclone for the two sequences from the overall mean increase in SDLP due to zopiclone, which yields a value of 1.243 cm for the practice effect. Because our design is balanced, the practice effect would cancel out when averaging across sequence under this model, and the mean increase in SDLP of 2.62 cm reported here would be the best estimate of the true effect of zopiclone on SDLP. Future studies using the Mini-Sim should include additional ‘practice’ drives that would likely reduce or eliminate the practice effect.
A number of additional limitations of this work should be acknowledged. First, weaving, as measured by SDLP, is only one measure of driving performance, although it appears to be the most sensitive measure for hypnotics and other sedating drugs. It is important to note that we included the entire drive in the calculation of SDLP, whereas the standard procedure for OTR driving involves editing out portions of the drive, such as while passing or during traffic jams [Verster and Roth, 2011]. The need for such editing is, however, reduced in the case of the Mini-Sim, where events do not preclude maintaining speed and position. The utility of driving simulators is somewhat reduced by the presence in some subjects of simulator sickness. Risk for simulator sickness appears to be due to subject factors as well as simulator design factors [Classen et al. 2011] that may be amenable to optimization to reduce the incidence of this difficulty. Finally, future studies should include the addition of a habituation night that would reduce any effects of sleep disruption during the first night on the unit.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors wish to thank Amy Harman for her logistical support for this study.
Footnotes
Conflict of interest statement: T.H. and G.G.K. have a financial interest in Cognitive Research Corporation, the company that developed and markets the Mini-Sim. The remaining authors have no conflicts of interest to declare in preparing this article.
Funding: This work was funded by Merck.
Contributor Information
Arthur A. Simen, Merck Research Laboratories, Merck Sharp & Dohme, North Wales, PA 19454, USA. Present address: Pfizer Worldwide Research & Development, Cambridge, MA 02139, USA
Cynthia Gargano, Johnson & Johnson, Springhouse, PA 19477.
Jang-Ho Cha, Merck Research Laboratories, Merck Sharp & Dohme, North Wales, PA, USA.
Melissa Drexel, Merck Research Laboratories, Merck Sharp & Dohme, North Wales, PA, USA.
An Bautmans, Merck ESD EU, MSD Europe Inc., Belgium.
Ingeborg Heirman, Merck ESD EU, MSD Europe Inc., Belgium.
Tine Laethem, Merck ESD EU, MSD Europe Inc., Belgium.
Thomas Hochadel, Cognitive Research Corporation, Saint Petersburg, FL, USA.
Lien Gheyle, SGS Life Science Services, Antwerp, Belgium.
Kim Bleys, SGS Life Science Services, Antwerp, Belgium.
Chan Beals, Merck Research Laboratories, Merck Sharp & Dohme, North Wales, PA, USA.
Aubrey Stoch, Merck Research Laboratories, Merck Sharp & Dohme, North Wales, PA, USA.
Gary G. Kay, Cognitive Research Corporation, Saint Petersburg, FL, USA
Arie Struyk, Merck Research Laboratories, Merck Sharp & Dohme, North Wales, PA, USA.
References
- Auberlet J., Rosey F., Anceaux F., Aubin S., Briand P., Pacaux M., et al. (2012) The impact of perceptual treatments on driver’s behavior: from driving simulator studies to field tests – first results. Accid Anal Prev 45: 91–98. [DOI] [PubMed] [Google Scholar]
- Barkley R., Murphy K., O’Connell T., Connor D. (2005) Effects of two doses of methylphenidate on simulator driving performance in adults with attention deficit hyperactivity disorder. J Safety Res 36: 121–131. [DOI] [PubMed] [Google Scholar]
- Bella F. (2008) Driving simulator for speed research on two-lane rural roads. Accid Anal Prev 40: 1078–1087. [DOI] [PubMed] [Google Scholar]
- Berthelon C., Bocca M., Denise P., Pottier A. (2003) Do zopiclone, zolpidem and flunitrazepam have residual effects on simulated task of collision anticipation? J Psychopharmacol 17: 324–331. [DOI] [PubMed] [Google Scholar]
- Bocca M., Denise P. (2000) Residual effects of hypnotics on disengagement of spatial attention.J Psychopharmacol 14: 401–405. [DOI] [PubMed] [Google Scholar]
- Bocca M., Le Doze F., Etard O., Pottier M., L’Hoste J., Denise P. (1999) Residual effect of zolpidem 10 mg and zopiclone 7.5 mg versus flunitrazepam 1 mg and placebo on driving performance and ocular saccades. Psychopharmacology 143: 373–379. [DOI] [PubMed] [Google Scholar]
- Bocca M., Marie S., Lelong-Boulouard V., Bertran F., Couque C., Desfemmes T., et al. (2011) Zolpidem and zopiclone impair similarly monotonous driving performance after a single nighttime intake in aged subjects. Psychopharmacology 214: 699–706. [DOI] [PubMed] [Google Scholar]
- Borkenstein R., Crowther R., Shumate R., Ziel W., Zylman R. (1974) The role of the drinking driver in traffic accidents (the Grand Rapids Study). Blutalkohol 11 (Suppl. 1): 7–13. [Google Scholar]
- Brown T., He Y., Roe C., Schnell T. (2010) Is more better? Night vision enhancement system’s pedestrian warning modes and older drivers. Ann Adv Automot Med 54: 343–350. [PMC free article] [PubMed] [Google Scholar]
- Brown T., Johnson R., Milavetz G. (2013a) Identifying periods of drowsy driving using EEG. Ann Adv Automot Med 57: 99–108. [PMC free article] [PubMed] [Google Scholar]
- Brown T., Milavetz G., Murry D. (2013b) Alcohol, drugs and driving: implications for evaluating driver impairment. Ann Adv Automot Med 57: 23–32. [PMC free article] [PubMed] [Google Scholar]
- Brunnauer A., Laux G., David I., Fric M., Hermisson I., Moller H. (2008) The impact of reboxetine and mirtazapine on driving simulator performance and psychomotor function in depressed patients. J Clin Psychiatry 69: 1880–1886. [DOI] [PubMed] [Google Scholar]
- Brunnauer A., Laux G., Zwick S. (2009) Driving simulator performance and psychomotor functions of schizophrenic patients treated with antipsychotics. Eur Arch Psychiatry Clin Neurosci 259: 483–489. [DOI] [PubMed] [Google Scholar]
- Bulmash E., Moller H., Kayumov L., Shen J., Wang X., Shapiro C. (2006) Psychomotor disturbance in depression: assessment using a driving simulator paradigm. J Affect Disord 93: 213–218. [DOI] [PubMed] [Google Scholar]
- Classen S., Bewernitz M., Shechtman O. (2011) Driving simulator sickness: an evidence-based review of the literature. Am J Occupat Ther 65: 179–188. [DOI] [PubMed] [Google Scholar]
- Crook T., Kay G., Larrabee G. (2009) Computer based cognitive testing. In: Grant I., Adams K. (eds), Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders, New York: Oxford University Press, 3: 84–100. [Google Scholar]
- De Winter J., De Groot S., Mulder M., Wieringa P., Dankelman J., Mulder J. (2009) Relationships between driving simulator performance and driving test results. Ergonomics 52: 137–153. [DOI] [PubMed] [Google Scholar]
- Fildes B., Charlton J., Muir C., Koppel S. (2007) Driving responses of older and younger drivers in a driving simulator. Annu Proc Assoc Adv Automot Med 51: 559–572. [PMC free article] [PubMed] [Google Scholar]
- Gustavsen I., Al-Sammurraie M., Morland J., Bramness J. (2009) Impairment related to blood drug concentrations of zopiclone and zolpidem compared to alcohol in apprehended drivers. Accid Anal Prev 41: 462–466. [DOI] [PubMed] [Google Scholar]
- Harrison C., Subhan Z., Hindmarch I. (1985) Residual effects of zopiclone and benzodiazepine hypnotics on psychomotor performance related to car driving. Drugs Exp Clin Res 11: 823–829. [PubMed] [Google Scholar]
- Kay G., Ahmad O., Brown T., Veit A. (2013) Comparison of the MiniSim and STISIM driving simulators for the detection of impairment: an alcohol validation study, In: Proceedings of the 7th International Driving Symposium on Human Factors in Driver Assessment, Training, and Vehicle Design, Bolton Landing New York. [Google Scholar]
- Kennedy R., Fowlkes J., Berbaum K., Lilienthal M. (1992) Use of a motion sickness history questionnaire for prediction of simulator sickness. Aviat Space Environ Med 63: 588–593. [PubMed] [Google Scholar]
- Krüger H., Kazenwadel J., Vollrath M. (1995) Grand Rapids effects revisited: accidents, alcohol and risk, In: Proceedings of the 13th International Conference on Alcohol, Drugs and Traffic Safety (T’95), Adelaide, Australia. [Google Scholar]
- Kuypers K., Samyn N., Ramaekers J. (2006) MDMA and alcohol effects, combined and alone, on objective and subjective measures of actual driving performance and psychomotor function. Psychopharmacology 187: 467–475. [DOI] [PubMed] [Google Scholar]
- Lee J., Mcgehee D., Brown T., Reyes M. (2002) Collision warning timing, driver distraction, and driver response to imminent rear-end collisions in a high-fidelity driving simulator. Hum Factors 44: 314–334. [DOI] [PubMed] [Google Scholar]
- Leufkens T., Lund J., Vermeeren A. (2009) Highway driving performance and cognitive functioning the morning after bedtime and middle-of-the-night use of gaboxadol, zopiclone and zolpidem. J Sleep Res 18: 387–396. [DOI] [PubMed] [Google Scholar]
- Leufkens T., Vermeeren A. (2009) Highway driving in the elderly the morning after bedtime use of hypnotics: a comparison between temazepam 20 mg, zopiclone 7.5 mg, and placebo. J Clin Psychopharmacol 29: 432–438. [DOI] [PubMed] [Google Scholar]
- Leufkens T., Vermeeren A. (2014) Zopiclone’s residual effects on actual driving performance in a standardized test: a pooled analysis of age and sex effects in 4 placebo-controlled studies. Clin Ther 36: 141–150. [DOI] [PubMed] [Google Scholar]
- Louwerens J., Gloerich A., De Vries G., Brookhuis K., O’hanlon J. (1987) The relationship between drivers’ blood alcohol concentration (BAC) and actual driving performance during high speed travel. In: Noordzij P., Roszbach R. (eds), Alcohol, Drugs and Traffic Safety. Amsterdam: Excerpta Medica, 183-192. [Google Scholar]
- Lovsund P., Hedin A., Tornros J. (1991) Effects on driving performance of visual field defects: a driving simulator study. Accid Anal Prev 23: 331–342. [DOI] [PubMed] [Google Scholar]
- Lundqvist A., Alinder J., Alm H., Gerdle B., Levander S., Ronnberg J. (1997) Neuropsychological aspects of driving after brain lesion: simulator study and on-road driving. Appl Neuropsychol 4: 220–230. [DOI] [PubMed] [Google Scholar]
- Meskali M., Berthelon C., Marie S., Denise P., Bocca M. (2009) Residual effects of hypnotic drugs in aging drivers submitted to simulated accident scenarios: an exploratory study. Psychopharmacology) 207: 461–467. [DOI] [PubMed] [Google Scholar]
- O’Hanlon J. (1995) Zopiclone’s residual effects on psychomotor and information processing skills involved in complex tasks such as car driving: a critical review. Eur Psychiatry 10(Suppl. 3): 137s–143s. [DOI] [PubMed] [Google Scholar]
- Ramaekers J., Robbe H., O’Hanlon J. (2000) Marijuana, alcohol and actual driving performance. Hum Psychopharmacol 15: 551–558. [DOI] [PubMed] [Google Scholar]
- Ranney T., Simmons L.-, Masalonis A. (1999) Prolonged exposure to glare and driving time: effects on performance in a driving simulator. Accid Anal Prev 31: 601–610. [DOI] [PubMed] [Google Scholar]
- Staner L., Ertle S., Boeijinga P., Rinaudo G., Arnal M., Muzet A., et al. (2005) Next-day residual effects of hypnotics in dsm-iv primary insomnia: a driving simulator study with simultaneous electroencephalogram monitoring. Psychopharmacology 181: 790–798. [DOI] [PubMed] [Google Scholar]
- Stein A., Dubinsky R. (2011) Driving simulator performance in patients with possible and probable Alzheimer’s disease. Ann Adv Automot Med 55: 325–334. [PMC free article] [PubMed] [Google Scholar]
- Stough C., Downey L., King R., Papafotiou K., Swann P., Ogden E. (2012) The acute effects of 3,4-methylenedioxymethamphetamine and methamphetamine on driving: a simulator study. Accid Anal Prev 45: 493–497. [DOI] [PubMed] [Google Scholar]
- Ting P., Hwang J., Doong J., Jeng M. (2008) Driver fatigue and highway driving: a simulator study. Physiol Behav 94: 448–453. [DOI] [PubMed] [Google Scholar]
- Vermeeren A., Riedel W., Van Boxtel M., Darwish M., Paty I., Patat A. (2002) Differential residual effects of zaleplon and zopiclone on actual driving: a comparison with a low dose of alcohol. Sleep 25: 224–231. [PubMed] [Google Scholar]
- Verster J., Mets M. (2009) Psychoactive medication and traffic safety. Int J Environ Res Public Health 6: 1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster J., Roth T. (2011) Standard operation procedures for conducting the on-the-road driving test, and measurement of the standard deviation of lateral position (SDLP). Int J Gen Med 4: 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster J., Spence D., Shahid A., Pandi-Perumal S., Roth T. (2011) Zopiclone as positive control in studies examining the residual effects of hypnotic drugs on driving ability. Curr Drug Saf 6: 209–218. [DOI] [PubMed] [Google Scholar]
- Verster J., Veldhuijzen D., Patat A., Olivier B., Volkerts E. (2006) Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf 1: 63–71. [DOI] [PubMed] [Google Scholar]
- Verster J., Veldhuijzen D., Volkerts E. (2004) Residual effects of sleep medication on driving ability. Sleep Med Rev 8: 309–325. [DOI] [PubMed] [Google Scholar]
- Verster J., Volkerts E., Olivier B., Johnson W., Liddicoat L. (2007) Zolpidem and traffic safety – the importance of treatment compliance. Curr Drug Saf 2: 220–226. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Wakasugi J., Sakakima J. (2006) Evaluation of driver stress using biomarker in motor-vehicle driving simulator. Conf Proc IEEE Eng Med Biol Soc 1: 1834–1837. [DOI] [PubMed] [Google Scholar]
- Yan X., Abdel-Aty M., Radwan E., Wang X., Chilakapati P. (2008) Validating a driving simulator using surrogate safety measures. Accid Anal Prev 40: 274–288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials



