Abstract
Trastuzumab (TZ), a monoclonal antibody against human epidermal growth factor receptor type 2 (HER2), is an important biological agent used for the treatment of positive breast cancer. This paper presents a 46-year-old female patient who developed right heart failure and right ventricular dysfunction while on TZ due to breast cancer, and returned to normal following the discontinuation of the drug. As far as we know, this is the first case report related to a patient presenting with right ventricular dysfunction and induced cardiotoxicity while on TZ.
Keywords: cardiotoxicity, trastuzumab, right ventricle failure
Introduction
Human epidermal growth factor receptor type 2 (HER2) is overexpressed in 25% of breast cancer cases. This is a more aggressive disease with a higher recurrence rate [Pegram et al. 2004] and associated with increased mortality [Slamon et al. 1987]. Trastuzumab (TZ), a monoclonal antibody against HER2, is an important biological agent used for the treatment of positive breast cancer. However, the risk of developing cardiotoxicity increases during the treatment. This paper presents a 46-year-old female patient who developed right heart failure and right ventricular dysfunction while on TZ treatment and returned to normal following the discontinuation of the drug. As far as we know, this is the first case report related to a patient presenting with right ventricular dysfunction and induced cardiotoxicity while on TZ.
Case report
A 46-year-old female patient was referred to the cardiology department from the medical oncology clinic due to the complaints of weakness, shortness of breath and swelling in the legs. We understood from medical history and the reports filed 4 years ago that she had undergone surgery and radiotherapy due to invasive ductal breast cancer, then had taken cyclophosphamide, adriamycin and 5-fluorouracil protocols for 3 months, and then had received docetaxel through chemotherapy for 3 months. TZ and paclitaxel were initiated 1.5 years after the diagnosis due to bone metastasis and recurrence of the disease in the other breast. Then, paclitaxel was stopped upon the finding of HER2 positivity.
At the 12th month of the treatment with TZ alone, weakness, shortness of breath and swelling in the legs developed. Her physical examination revealed normal breathing sounds and bilateral (2 +) pretibial edema. Laboratory tests (complete blood count, fasting blood glucose, liver and renal function tests, D-dimer levels) revealed no pathology. The electrocardiogram was normal. A transthoracic echocardiographic examination (TTE) performed 4 months ago revealed left ventricular ejection fraction (LVEF) was 60%, and the patient had a normal size of heart chambers. The control TTE showed that the left ventricular systolic function (LVEF 56%) decreased, interventricular septum and right ventricular free wall was hypokinetic, and there was moderate tricuspid regurgitation (TR).
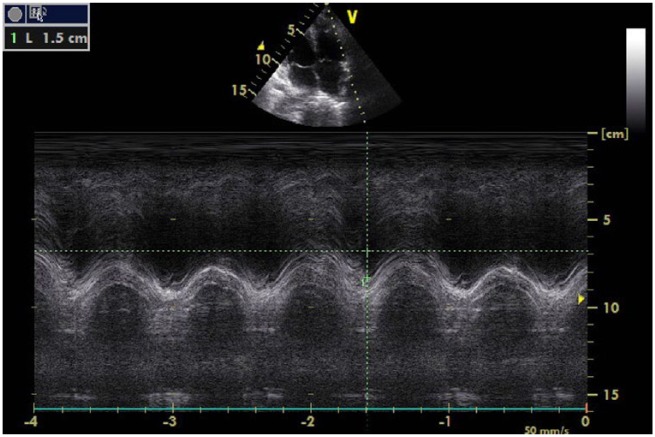
The systolic pulmonary artery pressure was measured as 38 mmHg via TR jet. Left ventricular systolic and diastolic functions were within normal range. In apical four-chamber views, the right atrium diameter was measured to be 58 mm × 42mm and the right ventricle (RV) diameter was found to be 41 mm annulus level, which were interpreted to be dilated. The tricuspid annular plane systolic excursion (TAPSE) value was determined as 15 mm (Figure 1). Furthermore, the diameter of the proximal right ventricular outflow tract (RVOT) was calculated to be 38 mm on the parasternal long axis and the diameter of the distal RVOT increased and was calculated to be 28 mm on the parasternal short axis. Right ventricle fractional area change was found to be 27% and tissue Doppler echocardiography revealed that the right ventricle myocardial performance index was 0.64 and the S’ velocity was 0.12 m/s. Conventional echocardiographic parameters of the patient are listed in Table 1.
Figure 1.
The tricuspid annular plane systolic excursion (TAPSE) value was 15 mm during the admission.
Table 1.
Patient’s conventional echocardiographic parameters.
| Parameters | Before anthracycline | Before TZ | While using TZ | After TZ |
|---|---|---|---|---|
| LA diameter, PSLA, mm | 38 | 38 | 39 | 38 |
| LV EDD, PSLA, mm | 44 | 45 | 46 | 46 |
| LV ESD, PSLA, mm | 26 | 28 | 28 | 27 |
| LV EF, % | 60 | 56 | 56 | 57 |
| IVS thickness, PSLA, mm | 11 | 11 | 11 | 11 |
| PW thickness, PSLA, mm | 10 | 10 | 10 | 10 |
| RA diameter, AP4C, mm | 51 × 32 | 52 × 33 | 58 × 42 | 54 × 38 |
| RV diameter, AP4C, mm | 32 | 33 | 41 | 36 |
| TR degree | 1 | 1 | 2 | 1 |
| Systolic PAP | 35 | 36 | 38 | 36 |
| MR degree | minimal | minimal | minimal | minimal |
| LAVI, ml/m2 | 29 | 29 | 30 | 29 |
AP4C, apical four-chamber view; EDD, end diastolic diameter; ESD, end systolic diameter; EF, ejection fraction; IVS, interventricular septum; LA, left atrium; LV, left ventricle; LAVI, left atrial volume index; MR, mitral regurgitation; PAP, pulmonary artery pressure; PSLA, parasternal long axis; PW, posterior wall; RA, right atrium; RV, right ventricle; TR, tricuspid regurgitation; TZ, trastuzumab.
The etiological assessment of the patient showed that she had right ventricular systolic dysfunction. Thoracic computed tomography scans were taken for the diagnosis of pulmonary embolism, which was within the normal range, while ventilation perfusion scintigraphy revealed a low probability for pulmonary embolism. A spirometry test showed no findings associated with pulmonary obstructive lung disease or bronchial asthma. Coronary angiography and right heart catheterization were not performed due to the absence of risk factors related to coronary artery disease and pulmonary artery pressure that was within the normal ranges.
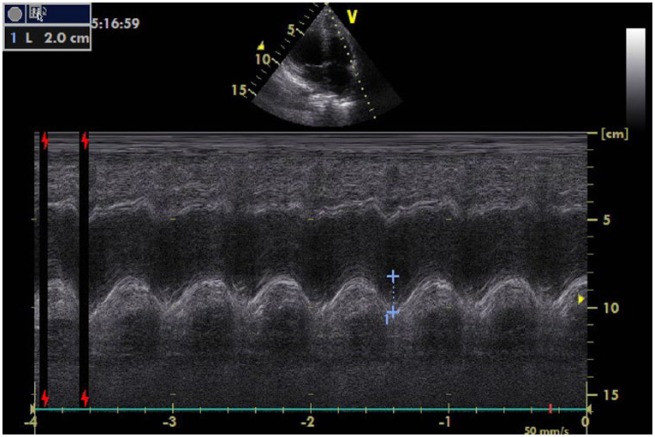
The right heart failure was thought to be associated with the use of TZ in the patient, who did not use any medication other than TZ. Following consultation with the oncology clinic, TZ treatment was discontinued; beta-blockers, angiotensin-converting enzyme inhibitor (ACEI) and diuretic therapy was initiated. The follow-up TTE made 6 weeks later revealed that left and right ventricular function had improved. Examination showed that: the TAPSE value was 20 mm; the diameter of the proximal RVOT was 34 mm on the parasternal long axis; the diameter of the distal RVOT had increased and was 24 mm on the parasternal short axis; the right ventricle fractional area change was 38%; and tissue Doppler echocardiography revealed that the value of the right ventricle myocardial performance index was 0.50 and S′ velocity was 0.13 m/s (Figure 2).
Figure 2.
Six weeks after the examination, the right ventricular systolic function returned to normal, the tricuspid annular plane systolic excursion (TAPSE) value was found to be 20 mm.
In our patient, the right ventricular dysfunction increased while on TZ, whereas it improved when the drug was withdrawn; this was thought to be due to the TZ-related cardiotoxicity.
Discussion
The incidence of TZ-induced cardiomyopathy ranged from 15 to 27% in different trials and was higher when TZ and anthracycline (AS) were used concurrently. When AS and TZ were administered simultaneously, the incidence of New York Heart Association class III or IV heart failure was found to be 16% [Slamon et al. 2001].
TZ often leads to a reduction in the asymptomatic LVEF and the main symptoms in patients with symptomatic palpitations, shortness of breath and chest pain. Patients having left bundle branch block and developing mimicking acute coronary syndrome and left ventricular dysfunction have been reported in the literature [Ribeiro et al. 2012].
In the literature, there are limited data relating to the effect of TZ treatment on right ventricular function. In a recent study including 42 patients who used TZ due to breast cancer, no echocardiographic change was reported on the right ventricular sizes and functions at 6-month follow up [Lange et al. 2012]. However, in a study conducted by Grover and colleagues, AS and TZ treatment effect on left ventricle (LV) and RV function was assessed by cardiac magnetic resonance imaging (MRI) and the patients were reported to have right ventricular systolic dysfunction when left ventricular function was found to be slightly affected. This can be explained by the fact that the right ventricle more sensitive to chemotherapeutics due to its thinner structure containing fewer myofibrils [Grover et al. 2013].
In patients treated with TZ, left ventricular function must be assessed prior to the treatment and at 3 month intervals. LVEF is the most common parameter used for the evaluation of cardiac function. Cardiac MRI is the gold standard for the accurate assessment of LV volumes and LVEF; however, it cannot be used as a screening test due to its high cost and limited availability. TTE is the method recommended for the evaluation of left ventricular function. TTE has multiple advantages as it is easily accessible, without any radiation exposure, and can assess LV and RV systolic and diastolic dysfunction, heart valve disease, pericarditis and pericardial effusion [Bovelli et al. 2010]. Moreover, it has been reported that other echocardiographic techniques such as tissue velocity imaging can identify cardiotoxicity without LVEF declines [Fallah-Rad et al. 2011].
When TZ induced cardiotoxicity occurs, cardiologists and oncologists should consider the risk of stopping treatment. Once treatment with TZ is discontinued, cardiac function improves in majority of these patients. In patients with heart failure, treatment with beta-blockers and ACEI has been shown to reduce signs of heart failure [Jones et al. 2009]. Furthermore, it has also been shown that those patients treated with beta-blockers and ACEI are at lower risk of developing heart failure during TZ treatment. In normotensive patients, however, prophylactic treatment provided to conceal the symptoms of heart failure is not recommended [Cardinale et al. 2006].
Conclusion
TZ plays an important role in the treatment of HER2+ breast cancer. Guidelines recommend that LVEF should be monitored regularly throughout TZ treatment. However, cardiotoxicity in these patients, as in our case, can accompany right ventricular failure; thus, right ventricular function in these patients should also be considered through echocardiographic examination.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Nermin Bayar, Cardiology Department, Antalya Education and Research Hospital, Varlık mahallesi, Kazım Karabekir caddesi, Soğuksu-Antalya, Turkey.
Selçuk Küçükseymen, Cardiology Department, Antalya Education and Research Hospital, Antalya, Turkey.
Sevil Göktaş, Medical Oncology Department, Antalya Education and Research Hospital, Antalya, Turkey.
Şakir Arslan, Cardiology Department, Antalya Education and Research Hospital, Antalya, Turkey.
References
- Bovelli D., Plataniotis G., Roila F. and ESMO Guidelines Working Group (2010) Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol 21(Suppl. 5): v277–v282. [DOI] [PubMed] [Google Scholar]
- Cardinale D., Colombo A., Sandri M., Lamantia G., Colombo N., Civelli M., et al. (2006) Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensinconverting enzyme inhibition. Circulation 114: 2474–2481. [DOI] [PubMed] [Google Scholar]
- Fallah-Rad N., Walker J., Wassef A., Lytwyn M., Bohonis S., Fang T., et al. (2011) The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol 57: 2263–2270. [DOI] [PubMed] [Google Scholar]
- Grover S., Leong D., Chakrabarty A., Joerg L., Kotasek D., Cheong K., et al. (2013) Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospective study using novel cardiac imaging and biochemical markers. Int J Cardiol 168: 5465–5467. [DOI] [PubMed] [Google Scholar]
- Jones A., Barlow M., Barrett-Lee P., Canney P., Gilmour I., Robb S., et al. (2009) Management of cardiac health in trastuzumab treated patients with breast cancer: updated United Kingdom National Cancer Research Instute recommendations for moniting. Br J Cancer 100: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Ebner B., Wess A., Kögel M., Gajda M., Hitschold T., et al. (2012) Echocardiography signs of early cardiac impairment in patients with breast cancer and trastuzumab therapy. Clin Res Cardiol 101: 415–426. [DOI] [PubMed] [Google Scholar]
- Pegram M., Pienkowski T., Northfelt D., Eiermann W., Patel R., Fumoleau P., et al. (2004) Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst 96: 759–769. [DOI] [PubMed] [Google Scholar]
- Ribeiro K., Miranda C., Andrade J., Galli L., Tiezzi D., Oliveira H., et al. (2012) Trastuzumab-induced myocardiotoxicity mimicking acute coronary syndrome. Case Rep Oncol 5: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D., Clark G., Wong S., Levin W., Ullrich A., McGuire W. (1987) Human breast cancer: correlation of relapse and survival with amplicification of the HER- 2/neu oncogene. Science 235: 177–182. [DOI] [PubMed] [Google Scholar]
- Slamon D., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792. [DOI] [PubMed] [Google Scholar]