Abstract
The potential benefits and risks of physical exercise on fetal development during pregnancy remain unclear. The aim was to analyze maternal oxidative stress status and the placental morphometry to relate to intrauterine growth restriction (IUGR) from diabetic female rats submitted to swimming program after embryonic implantation. Pregnant Wistar rats were distributed into 4 groups (11 animals/group): control—nondiabetic sedentary rats, control exercised—nondiabetic exercised rats, diabetic—diabetic sedentary rats, and diabetic exercised—diabetic exercised rats. A swimming program was used as an exercise model. At the end of pregnancy, the maternal oxidative stress status, placental morphology, and fetal weight were analyzed. The swimming program was not efficient to reduce the hyperglycemia-induced oxidative stress. This fact impaired placental development, resulting in altered blood flow and energy reserves, which contributed to a deficient exchange of nutrients and oxygen for the fetal development, leading to IUGR.
Keywords: physical exercise, diabetes, pregnancy, oxidative stress, placenta
Introduction
Diabetes mellitus is a group of metabolic diseases due to hyperglycemia, resulting from defects in insulin secretion and/or action.1 Diabetic pregnancy is complicated due to the metabolic demand of fetal, placental, and enclosure development. In this case, compensatory mechanisms are needed, and in cases where no compensatory mechanisms occur, it can result in maternal and fetal impairment.2,3
The balance between the fetal nutrient demand and the maternal–placental supply regulates the fetal growth.4 The altered fetal growth (macrosomia in human and intrauterine growth restriction [IUGR] in experimental animals) in pregnancies complicated by diabetes is the result of abnormal substrate availability in placental transfer capacity.4,5 Besides, diabetes causes an exacerbated oxidative stress in pregnancies, which is associated with an increase in embryonic oxygen-free radicals, because of its relatively weak antioxidant defense, especially at the early stages of organogenesis.2,6
Physical activity has long been known for its role in controlling glycemic levels by direct or indirect effects on insulin action.7,8 However, a major question remains regarding the correlation between the potential benefits and risks of physical exercise on fetal development during human pregnancy. A previous study demonstrated that swimming applied to diabetic rats from day 7 (after embryo implantation) to day 20 of pregnancy led to an improvement in maternal lipid metabolism, showing beneficial results.9 Besides, these rats presented reduced embryonic death rates (resorption) compared to diabetic nonexercised dams.10 Damasceno et al5 demonstrated that nondiabetic and diabetic rats exercised prior to and during whole pregnancy showed fetuses with IUGR.
Therefore, the objective of this study was to analyze maternal oxidative stress status and the placental morphometry to relate to IUGR in diabetic female rats submitted to swimming program after embryonic implantation, considering that exercise might lead to changes in the maternal oxidative stress status.
Materials and Methods
Experimental Animals
Wistar female rats, obtained from the São Paulo State University (UNESP) Vivarium (São Paulo, Brazil), were maintained in an experimental room under conditions with controlled temperature (22°C ± 2°C) and humidity (50% ± 10%) and a 12-hour light–dark cycle, with ad libitum access to commercial diet (Purina rat chow; Nestlé, St Louis, Missouri) and water. The procedures and animal handling were performed in accordance with the guidelines provided by the Brazilian College of Animal Experimentation in agreement with the International Guiding Principles for Biomedical Research Involving Animals promulgated by the Society for the Study of Reproduction and were authorized by the Ethical Committee for Animal Research of the UNESP, Brazil (Process number 353).
Experimental Diabetes Induction
Diabetes was induced by streptozotocin (SIGMA Chemical Company, St Louis, Missouri). Streptozotocin was dissolved in citrate buffer (0.1 mol/L, pH 6.5) and administered (intravenously [IV]) at a dose of 40 mg/kg body weight. Nondiabetic rats received only (IV) citrate buffer. Blood glucose concentrations were measured by One Touch Ultra Johnson & Johnson glucometer (Johnson & Johnson, HDI Home Diagnostics Inc, Fort Lauderdale, Florida) 7 days after the induction of diabetes. For inclusion criteria, the diabetic state was confirmed by blood glucose levels ≥300 mg/dL. For nondiabetic adult rats that received only citrate buffer, the inclusion criteria used was blood glucose levels <120 mg/dL.11
Mating Procedure
All female rats were mated overnight with nondiabetic male rats. The day when sperms were found in the vaginal smear was designated as gestational day 0. The mating period consisted of 15 consecutive days, a period comprising approximately 3 estral cycles, until a replicate number of groups was obtained. However, during this period, nonmated female rats were considered to be infertile and were discarded from the study.12
Calculation of Sample and Experimental Groups
To calculate the sample size for this experiment, the blood glucose concentration was estimated in rats with severe diabetes, which were obtained from previous studies in the same laboratory. Considering a reduction of 10% and a power of 80%, the minimum number obtained was 11 participants per group. Then, after mating, the female rats were randomly distributed (by lot) into 4 experimental groups that constituted 11 animals/group: control (C)—sedentary nondiabetic, control exercised (CEx)—exercised nondiabetic, diabetic (D)—sedentary diabetic, and diabetic exercised (DEx)—exercised diabetic.
Exercise Program
For exercise, we used a swimming program according to the procedure by Volpato et al.10 To familiarize the rats to the swimming system (water), the rats were daily exposed to water for 15 minutes for 5 days in a cage (100 × 70 × 60 cm) containing water at a depth of 10 cm at 32°C. This period corresponded to the interval between diabetes induction and the mating period. Afterward, the female rats that were familiarized to the swimming system were placed in a cage containing water at a depth of 40 cm. Exercise on the first day under these conditions was about 20 minutes, with progressive increases of 10 minutes each day until they completed 60 minutes. Following, the rats were trained to swim for 1 hour daily until the end of pregnancy. Training for swimming was provided in water with a temperature of 32°C between 9 am and 10 am for 6 days a week. The pregnant rats that remained in water at a depth of 10 cm at 32°C were classified as sedentary.
Evaluation at Term of Pregnancy
Blood glucose levels and maternal weight were measured at approximately 9 am every 7 days until the end of pregnancy. At day 21 of pregnancy, after determination of maternal weight, the rats were anesthetized, and the uterine horns were exposed for weighing the fetuses and their respective placentas. The placental efficiency was calculated as the ratio of fetal weight and placental weight.13 One placenta from each uterine horn was sectioned medial sagitally and fixed in 10% buffered formalin before being processed for paraffin embedding.
Placental Morphometry
Formalin-fixed placentas were dehydrated in a graded ethanol series, embedded in paraffin according to a standard protocol, sectioned at 5 μm, and mounted on glass slides for hematoxylin–eosin staining. For histological analysis, 11 placental blocks (11 blocks/group—1 placenta/dam) were cut in the longitudinal direction. The placental morphometric analyses were performed in a computerized image system coupled to a photomicroscope through a digital camera. The slides were preselected to assure the presence of all placental layers in the sample. From each slide, 6 areas were randomly selected. The decidua and junctional zones (mm2) were evaluated at 100× magnification, while the labyrinthine region was evaluated at a magnification of 25×.
Determination of the Oxidative Stress Parameters
The samples of blood collected in heparinized tubes were processed and washed erythrocytes were collected for the determination of lipoperoxidation marker (malondialdehyde [MDA]) and antioxidant substances (superoxide dismutase [SOD] and glutathiones) according to the methodology of Damasceno et al.14
Statistical Analysis
Analysis of variance and Student-Newman-Keuls test were used for quantitative variables with normal distribution. Differences were considered statistically significant if P < .05.
Results
Maternal Glycemia
The blood glucose levels of the nondiabetic groups (C and CEx) were lower than 120 mg/dL during pregnancy. The swimming program did not alter the blood glucose levels of the nondiabetic (CEx vs C) and diabetic female rats (DEx vs D) throughout the pregnancy. The diabetic animals presented levels maintained above 300 mg/dL during pregnancy, regardless of the swimming program (Figure 1).
Figure 1.
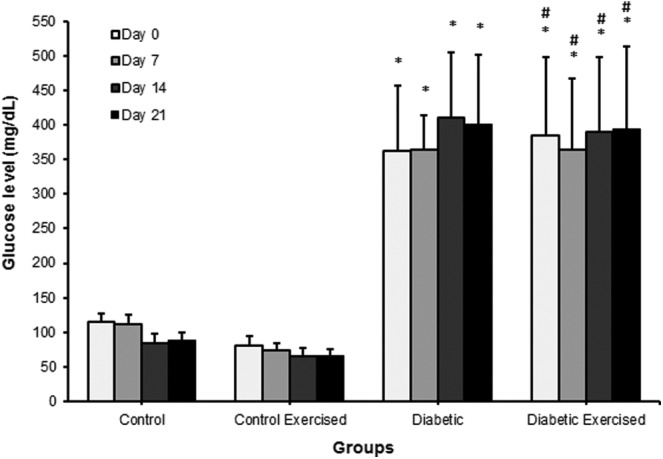
Blood glucose levels from nondiabetic or diabetic rats, not exercised or exercised, after the embryonic implantation period. Data shown as the mean ± standard deviation (ANOVA—Student-Newman-Keuls posttest) *P < .05—Statistically significant difference compared with control group. #P < .05—Statistically significant difference compared with control exercised group. ANOVA indicates analysis of variance.
Maternal Oxidative Stress Parameters
Superoxide dismutase activity increased in the exercised nondiabetic animals compared to the sedentary group (C). The sedentary diabetic female rats (D) had increased level of MDA compared to C group. The DEx rats had decreased levels of glutathione peroxidase (GSH-Px) and elevated SOD activity compared to the C group. This same group presented increased MDA levels and decreased SOD levels when compared to the D group (Table 1).
Table 1.
Oxidative Stress Status of Nondiabetic or Diabetic Rats, Not Exercised or Exercised, After the Embryonic Implantation Period.a
| Groups | ||||
|---|---|---|---|---|
| Control | Control Exercised | Diabetic | Diabetic Exercised | |
| MDA, nmol/L/gHb | 54.60 ± 40.15 | 65.80 ± 21.84 | 319.7 ± 191.78b | 544.2 ± 148.21b,c |
| SOD, UI/mgHb | 7.17 ± 3.74 | 19.63 ±5.41b | 8.55 ± 4.99 | 16.34 ± 6.80b,c |
| Thiol group, µmol/L/gHb | 0.76 ± 0.66 | 1.23 ± 0.37 | 0.64 ± 0.56 | 1.09 ± 0.29 |
| GSH-Px, UI/gHb | 0.48 ± 0.36 | 0.23 ± 0.08 | 0.22 ± 0.15 | 0.10 ± 0.04b |
Abbreviations: MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; ANOVA, analysis of variance.
aData shown as the mean ± standard deviation (ANOVA—Student-Newman-Keuls posttest).
b P < .05—statistically significant difference compared with control group.
c P < .05—statistically significant difference compared with diabetic group.
Maternal Reproductive Outcomes and Placental Morphometry
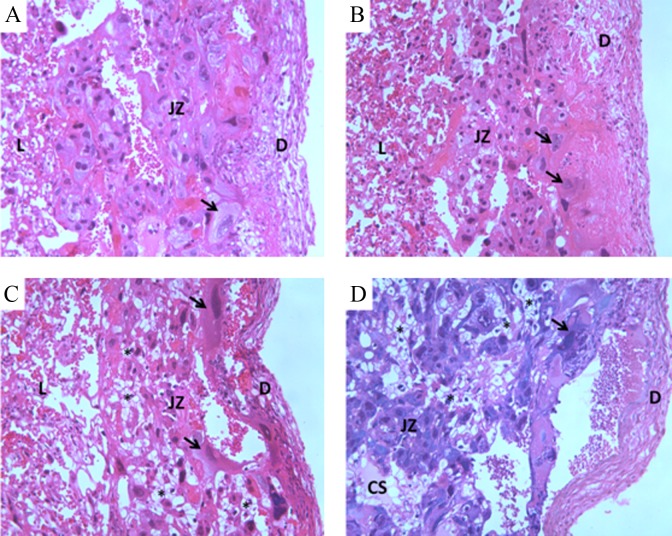
The maternal weight gain, with and without uterine content, was lower in all experimental groups compared to C group (Figure 2). The fetal weight was also lower in all experimental groups than the C group. The CEx rats presented placentas with the lowest weights and the placental efficiency indexes in the diabetic rats (D and DEx) were decreased in relation to C group. The mean area of the placental decidua from dams of the D and DEx groups was significantly lower compared to that of the C group. The mean area of the placental labyrinthine was lower in all groups compared to the C group (Table 2). Figure 3 shows placental morphology and structural changes in the placenta of diabetic groups. The disarrangement observed in diabetic placentas (D and DEx) was characterized by aberrant cell size in placental layers, ectopic and spread giant cells, and presence of cystic spaces.
Figure 2.
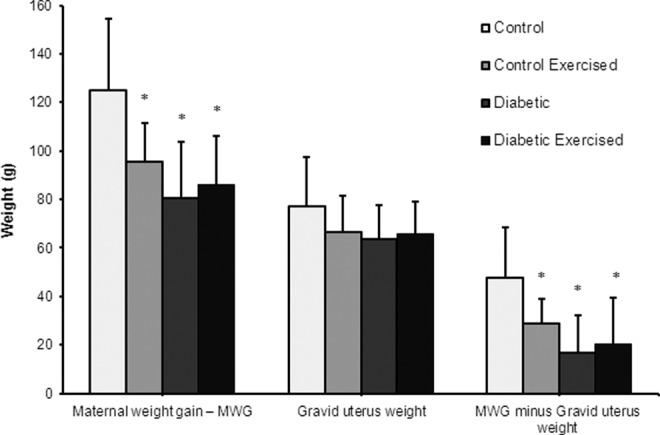
Maternal and uterine weights from nondiabetic or diabetic rats, not exercised or exercised, after the embryonic implantation period. Data shown as the mean ± standard deviation (ANOVA—Student-Newman-Keuls posttest) *P < .05—Statistically significant difference compared with control group. ANOVA indicates analysis of variance.
Table 2.
Fetal and Placental Analysis From Nondiabetic or Diabetic Rats, not Exercised or Exercised, After the Embryonic Implantation Period.a
| Groups | ||||
|---|---|---|---|---|
| Control | Control Exercised | Diabetic | Diabetic Exercised | |
| Fetuses | ||||
| Fetal weight, g | 5.32 ± 0.48 | 4.53 ± 0.40b | 4.22 ± 0.59b | 3.84 ± 0.45b,c |
| Placenta | ||||
| Placental weight, g | 0.47 ± 0.08 | 0.39 ± 0.08b | 0.66 ± 0.16b | 0.55 ± 0.12b,c |
| Placental efficiency | 11.60 ± 1.63 | 11.85 ± 1.86 | 6.70 ± 1.65b | 7.24 ± 1.57b,c |
| Decidual area, mm2 | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.05 ± 0.01b | 0.05 ± 0.02b |
| Junctional area, mm2 | 0.18 ± 0.04 | 0.16 ± 0.03 | 0.17 ± 0.03 | 0.16 ± 0.03 |
| Labyrinthine area, mm2 | 4.14 ± 0.41 | 3.93 ± 0.44b | 3.85 ± 0.47b | 3.86 ± 0.32b |
aData shown as the mean ± standard deviation (ANOVA—Student-Newman-Keuls posttest) and proportions (%; Fisher exact test).
b P < .05—statistically significant difference compared with control group.
c P < .05—statistically significant difference compared with diabetic group.
Figure 3.
Microscopic images of the placentas (hematoxylin and eosin) at day 21 of pregnancy from nondiabetic or diabetic rats, not exercised or exercised, after the embryonic implantation period. A indicates control group; B indicates control exercised group; C indicates diabetic group; D indicates diabetic exercised group. D, decidua area; JZ, junctional zone; L, labyrinthine area; CS, cystic spaces presented transudate; arrow, Giant cells; asterisk, glycogen cells (magnification ×40). ANOVA indicates analysis of variance.
Discussion
In the present study, the effect of exercise on blood glucose level was not observed in the diabetic rats during pregnancy. The lack of exercise effect on maternal hyperglycemia in diabetic pregnant female rats was previously observed in other studies.5,9,10 Similarly, clinical investigations with diabetic pregnant women confirmed this fact.15
Among the complications of diabetes, oxidative stress has been widely studied. Oxidative stress is a condition in which the production of reactive oxygen species (ROS) is alarmingly high and the available antioxidant defenses is limited, resulting in damage to DNA, proteins, sugars, and lipids caused by the excessive free radicals.16 Reactive oxygen species include free radicals such as superoxide (•O2 −), hydroxyl (•OH), peroxyl (•RO2), and hydroperoxyl (•HRO2 −) as well as nonradical species such as hydrogen peroxide (H2O2).17,18 Production of 1 ROS may lead to the production of others through radical chain reactions. Exposure to free radicals from a variety of sources has led organisms to develop a series of defense mechanisms,19 such as preventative and repair mechanisms, physical, and antioxidant defenses. Enzymatic antioxidant defenses include SOD, GSH-Px, and catalase (CAT). Under normal conditions, •O2 − is quickly eliminated by antioxidant defense mechanisms. •O2 − is dismutated to H2O2 by manganese SOD in the mitochondria and by copper SOD in the cytosol.20 H2O2 is converted into H2O and O2 by GSH-Px or CAT in the mitochondria and lysosomes, respectively. H2O2 can also be converted into the highly reactive •OH radical in the presence of transition elements like iron and copper. Glutathione is highly abundant in the cytosol (1-11 mmol/L), nuclei (3-15 mmol/L), and mitochondria (5-11 mmol/L) and is the major soluble antioxidant in these cell compartments. The following are the main protective roles of glutathione against oxidative stress21: (1) glutathione is a cofactor of several detoxifying enzymes against oxidative stress, for example, GSH-Px, detoxifying H2O2, and lipid peroxides by the catalytic action of GSH-Px. The capacity of glutathione to regenerate the most important antioxidants is linked with the redox state of the glutathione disulfide–glutathione couple (GSSG/2GSH).22 Considering the antioxidant defense of the endocrine pancreas, it was verified that βcells are particularly sensitive to ROS because they are low in free-radical quenching (antioxidant) enzymes such as CAT, GSH-Px, and SOD.23 Therefore, the ability of oxidative stress to damage mitochondria and markedly blunt insulin secretion is not surprising.24 Evidence in both, experimental and clinical studies, suggests that free radical-mediated oxidative stress plays a major role in the pathogenesis of both type 1 and type 2 diabetes.25,26
Maritim et al27 reviewed in detail that diabetes has multiple effects on the protein levels and activity of the antioxidant enzymes, which further augment oxidative stress by causing a suppressed defense response.
The oxidative stress may be analyzed in the red blood cells (RBCs). This might be explained because RBCs are vulnerable to oxidative damage because of their continuous exposure to oxygen and their high concentrations of polyunsaturated fatty acids and heme iron.28 In the present study, the diabetic groups presented increased MDA levels, a marker of lipid peroxidation, in analysis of washed RBCs, confirming oxidative stress.
Several research about oxidative damage indicate that exercise exacerbates the generation of ROS, some of which are free radicals.29,30 Numerous studies have shown that muscle cells also release superoxide into the extracellular space,31 so free radicals readily reach the blood and act on other cells.32 Several potential alternative sources of free radicals, such as oxidase systems associated with membranes, nitric oxide production, and phagocytic processes,33 as well as an increase in lactate formation, as happens in exhaustive exercise,34 have been proposed to contribute significantly to the overproduction of free radicals.35
In relation to the influence of swimming on pregnant rats, this study showed that the exercise contributed to an increased SOD enzymatic activity in nondiabetic and diabetic rats. However, the swimming decreased GSH-Px activity in diabetic status. These results show that increased SOD activity was not sufficient to reduce the elevated lipid peroxidation in the diabetic dams as a function of the uncontrolled metabolism due to severe hyperglycemia. Witt et al36 verified that the exercise resulted in an increased free radical concentration in muscle and other tissues and membrane damage, as evidenced by lipid peroxidation, which depends on the state of training, duration, intensity of exercise, and the tissue examined.
The exercised dams of the different experimental groups showed a lower gain of maternal weight associated with the lower corporal weight of the fetuses. This decrease is characteristic of IUGR, which is related to fetal hypoxia caused by the practice of exercise, and this was exacerbated by the uncontrolled hyperglycemia, corroborating the previous results of this research group.10 The effects of physical exercise during fetal development are controversial, mainly regarding the intensity level of the exercise that is undertaken (light, moderate, or intense), and there are several conflicting reports regarding the effects of intense exercise on the risk of IUGR.37–39 One possible explanation for confounder factors in the interpretation of results is the physiological stress caused by exercise in experimental animals.40 Swimming during pregnancy increases the plasmatic corticosterone levels in rats,41,42 and the effects of swimming-induced stress during pregnancy persist on birth weight through 2 subsequent generations.43 Vaughan et al44 showed that fetuses of corticosterone-treated dams always weighed less than normal. The corticosterone induced fetal growth restriction, which was associated with morphological and functional changes in placental phenotype that depends on gestational age, particularly related to placental amino acid transport.
The exercised nondiabetic rats presented reduced placental weight and labyrinthine area, leading to reduced fetal weight. In the diabetic female rats, it showed higher placental weights, showing reduced placental efficiency, which confirms the inability of the larger placenta to transfer nutrients to the developing fetus, causing IUGR. The reduction in decidual area from diabetic dams confirms that the decidua is highly influenced by the hyperglycemic status.12 Besides, these results show that swimming did not protect this area, whereas lipid peroxidation process is exacerbated, which might alter decidual zone, interfering with placental development. The exercised nondiabetic and diabetic rats presented a significant decrease in labyrinthine area, the site of maternal–fetal exchange. Hewitt et al45 showed that administration of glucocorticoids causes a permanent deficit in labyrinthine blood vessels in the rat placenta. As discussed previously, the stress caused by exercise contributed to a deficit in nutrient transport confirmed by decreased exchange area in the placenta. In addition, the hyperglycemia associated with exercise caused structural disarrangement in placenta, which demonstrated ectopic and spread giant cells and presence of cystic spaces.
The placental alterations verified in this study could justify that IUGR was caused by exercise program, which is related to a reduction in placental blood flow in the rats submitted to acute strenuous exercise. Although the literature showed that swimming program used in this experiment is moderate,46 the impaired maternal and fetal outcomes suggest an exercise of strong/severe intensity.
Thus, the swimming program was not efficient to reduce the hyperglycemia-induced oxidative stress. This fact impaired placental development, resulting in altered blood flow and energy reserves, which contributed to a deficient exchange of nutrients and oxygen for the fetal development, leading to IUGR. These findings reinforce the necessity to reach a good glycemic control combined with interdisciplinary and professional discussion about exercise intensity and time of exposure for women during pregnancy.
Acknowledgments
The authors thank students Isabela Lovizutto Iessi and Aline Bueno for the care of the animals.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank the FAPESP for financial support (Process Number: 05/02386-7 - Coordinator: DC Damasceno and 08/00358-7 - Coordinator: IMP Calderon) and the fellowship for student Viviane Maria Ribeiro (Process Number: 2008/03881-0 - supervisor: GT Volpato).
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes millitus. Diabet Care. 2014;37 (suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 2. Eriksson UJ. Congenital anomalies in diabetic pregnancy. Semin Fetal Neonatal Med. 2009;14 (2):85–93. [DOI] [PubMed] [Google Scholar]
- 3. Volpato GT, Calderon IM, Sinzato S, et al. Effect of Morus nigra aqueous extract treatment on the maternal-fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2011;138 (3):691–696. [DOI] [PubMed] [Google Scholar]
- 4. Jansson T, Cetin I, Powell TL, et al. Placental transport and metabolism in fetal overgrowth – A workshop report. Placenta. 2006;27 (suppl A):S109–S113. [DOI] [PubMed] [Google Scholar]
- 5. Damasceno DC, Silva HP, Vaz GF, et al. Diabetic rats exercised prior to and during pregnancy: maternal reproductive outcome, biochemical profile, and frequency of fetal anomalies. Reprod Sci. 2013;20 (7):730–738. [DOI] [PubMed] [Google Scholar]
- 6. Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24 (1):31–41. [DOI] [PubMed] [Google Scholar]
- 7. Devlin JT. Effects of exercise on insulin sensitivity in humans. Diabetes Care. 1992;15 (11):1690–1693. [DOI] [PubMed] [Google Scholar]
- 8. Kim C. Gestational diabetes: risks, management, and treatment options. Int J Womens Health. 2010;2:339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volpato GT, Damasceno DC, Campos KE, Rocha R, Rudge MV, Calderon IM. Avaliação do efeito do exercício físico no metabolismo de ratas diabéticas prenhes. Rev Bras Med Esp. 2006;12 (5):229–233. [Google Scholar]
- 10. Volpato GT, Damasceno DC, Kempinas WG, Rudge MV, Calderon IM. Effect of exercise on the reproductive outcome and fetal development of diabetic rats. Reprod Biomed Online. 2009;19 (6):852–858. [DOI] [PubMed] [Google Scholar]
- 11. Damasceno DC, Kiss AC, Sinzato YK, et al. Maternal-fetal outcome, lipid profile and oxidative stress of diabetic rats neonatally exposed to streptozotocin. Exp Clin Endocrinol Diabetes. 2011;119 (7):408–413. [DOI] [PubMed] [Google Scholar]
- 12. Sinzato YK, Volpato GT, Iessi IL, et al. Neonatally induced mild diabetes in rats and its effect on maternal, placental, and fetal parameters. Exp Diabetes Res. 2012;2012:108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572 (pt 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Damasceno DC, Sinzato YK, Lima PH, et al. Effects of exposure to cigarette smoke prior to pregnancy in diabetic rats. Diabetol Metab Syndr. 2011;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avery MD, Leon AS, Kopher RA. Effects of a partially home-based exercise program for women with gestacional diabetes. Obstet Gynecol. 1997;89 (1):10–15. [DOI] [PubMed] [Google Scholar]
- 16. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39 (1):44–84. [DOI] [PubMed] [Google Scholar]
- 17. Turko IV, Marcondes S, Murad F. Diabetes-associated nitration of tyrosine and inactivation of succinyl-CoA:3-oxoacid CoA-transferase. Am J Physiol Heart Circ Physiol. 2001;281 (6):H2289–H294. [DOI] [PubMed] [Google Scholar]
- 18. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622. [DOI] [PubMed] [Google Scholar]
- 19. Cadenas E. Basic mechanisms of antioxidant activity. Biofactors. 1997;6 (4):391–397. [DOI] [PubMed] [Google Scholar]
- 20. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and {beta}-cell dysfunction? Diabetes. 2003;52(1):1–8. [DOI] [PubMed] [Google Scholar]
- 21. Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16 (10):577–586. [DOI] [PubMed] [Google Scholar]
- 22. Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: Implication in redox and detoxification. Clin Chim Acta. 2003;333 (1):19–39. [DOI] [PubMed] [Google Scholar]
- 23. Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46 (11):1733–1742. [DOI] [PubMed] [Google Scholar]
- 24. Gisela D, Peter KD, Martina D. Oxidative stress and beta-cell dysfunction. Eur J Physiol. 2010;460 (4):703–718. [DOI] [PubMed] [Google Scholar]
- 25. West IC. Radicals and oxidative stress in diabetes. Diabetes Med. 2000;17 (3):171–180. [DOI] [PubMed] [Google Scholar]
- 26. Agnieszka P, Dorota R, Iren A, Maciej J, Stefan A. High glucose concentration affects the oxidant/antioxidant balance in cultured mouse podocytes. J Cell Biochem. 2011;112 (6):1661–1672. [DOI] [PubMed] [Google Scholar]
- 27. Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17 (1):24–38. [DOI] [PubMed] [Google Scholar]
- 28. Smith JA. Exercise, Training and Red Blood Cell Turnover. Sports Med. 1995;19 (1):9–31. [DOI] [PubMed] [Google Scholar]
- 29. Bloomer RJ, Fisher-Wellman KH. Blood oxidative stress biomarkers: influence of sex, exercise training status, and dietary intake. Gender Med. 2008;5 (3):218–218. [DOI] [PubMed] [Google Scholar]
- 30. Jackson MJ. Free radicals generated by contracting muscle: by-products of metabolism or key regulators of muscle function? Free Radic Biol Med. 2008;44 (2):132–141. [DOI] [PubMed] [Google Scholar]
- 31. McArdle F, Pattwell DM, Vasilaki A, McArdle A, Jackson MJ. Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med. 2005;39 (5):651–657. [DOI] [PubMed] [Google Scholar]
- 32. Ashton T, Rowlands CC, Jones E, et al. Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J Appl Physiol Occup Physiol. 1998;77 (6):498–502. [DOI] [PubMed] [Google Scholar]
- 33. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88 (4):1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ali MA, Yasui F, Matsugo S, Konishi T. The lactate-dependent enhancement of hydroxyl radical generation by the Fenton reaction. Free Radic Res. 2000;32 (5):429–438. [DOI] [PubMed] [Google Scholar]
- 35. Berzosa C, Cebrián I, Fuentes-Broto L, et al. Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. J Biomed Biotechnol. 2011;2011:540458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Witt EH, Rezhick AZ, Viguie CA, Starke-Reed P, Packer L. Exercise, oxidative damage and effects of antioxidant manipulation. J Nutr. 1992;122 (3 suppl):766–773. [DOI] [PubMed] [Google Scholar]
- 37. Brown W. The benefits of physical activity during pregnancy. J Sci Med Sport. 2002;5 (1):37–45. [DOI] [PubMed] [Google Scholar]
- 38. Kardel KR, Kase T. Tranining during pregnancy: effects on fetal development and birth. Am J Obstet Gynecol. 1998;178 (2):280–286. [DOI] [PubMed] [Google Scholar]
- 39. Sternfeld B, Quesenberry CP, Eskenazi B, Newman LA. Exercise during pregnancy and pregnancy outcome. Med Sci Sports Exerc. 1995;27 (5):634–640. [PubMed] [Google Scholar]
- 40. Rosa BV, Firth EC, Blair HT, Vickers MH, Morel PC. Voluntary exercise in pregnant rats positively influences fetal growth without initiating a maternal physiological stress response. Am J Physiol Regul Integr Comp Physiol. 2011;300 (5):R1134–1141. [DOI] [PubMed] [Google Scholar]
- 41. Akhavan MM, Emami-Abarghoie M, Safari M, et al. Serotonergic and noradrenergic lesions suppress the enhancing effect of maternal exercise during pregnancy on learning and memory in rat pups. Neurosci. 2008;151 (4):1173–1183. [DOI] [PubMed] [Google Scholar]
- 42. Contarteze RV, Manchado Fde B, Gobatto CA, De Mello MA. Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp Biochem Physiol A Mol Integr Physiol. 2008;151 (4):415–422. [DOI] [PubMed] [Google Scholar]
- 43. Pinto ML, Shetty PS. Influence of exercise-induced maternal stress on fetal outcome in Wistar rats: inter-generational effects. Br J Nutr. 1995;73 (5):645–653. [DOI] [PubMed] [Google Scholar]
- 44. Vaughan OR, Sferruzzi-Perri AN, Fowden AL. Maternal corticosterone regulates nutrient allocation to fetal growth in mice. J Physiol. 2012;590 (pt 21):5529–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147 (12):5568–5574. [DOI] [PubMed] [Google Scholar]
- 46. Lancha AH, Jr, Recco MB, Abdalla DS, Curi R. Effect of aspartate, asparagine, and carnitine supplementation in the diet on metabolism of skeletal muscle during a moderate exercise. Physiol Behav. 1995;57 (2):367–371. [DOI] [PubMed] [Google Scholar]