Abstract
Phytosterols (PS) have long been recognized for their cholesterol-lowering action, however, recent work has highlighted triglyceride (TG)-lowering responses to PS that may have been overlooked in previous human interventions and mechanistic animal model studies. This review assesses the current state of knowledge regarding the effect of dietary PS supplementation on blood TG concentrations by examining the average therapeutic response, potential mechanisms, and metabolic and genetic factors that may contribute to inter-individual variability. Data from human intervention trials demonstrates that, compared to baseline concentrations, PS supplementation results in a variable TG-lowering response ranging from 0.8 to 28%. It is evident that hypertriglyceridemic individuals (>1.7 mmol/L) have a greater TG-lowering response to PS (11–28%) than subjects with normal plasma TG concentrations (0.8–7%). Although a genetic basis for the variable TG-lowering effects of PS is probable, there are only limited studies to draw on. The available data suggest that polymorphisms in the apolipoprotein E (apoE) gene may affect responsiveness, with PS-induced reductions in TG more readily evident in apoE2 than apoE3 or E4 subjects. Although only a minimal number of animal model studies have been conducted to specifically examine the mechanisms whereby PS may reduce blood TG concentrations, it appears that there may be multiple mechanisms involved including interruption of intestinal fatty acid absorption and modulation of hepatic lipogenesis and VLDL packaging and secretion.
In summary, the available data suggest that PS may be an effective therapy to lower blood TG, particularly in hypertriglyceridemic individuals. However, before PS can be widely recommended as a TG-lowering therapy, studies that are specifically powered and designed to fully access therapeutic responses and the mechanisms involved are required.
Introduction
Since discovery of the association between elevated blood cholesterol and increased cardiovascular disease (CVD) risk with early animal model [1] and epidemiological investigations [2], diet-based and pharmacological cholesterol-lowering therapies have become integral components of primary and secondary CVD prevention programs. Although these therapies have largely reduced the prevalence of high LDL-C amongst Americans, close to 33% of US adults still have elevated LDL-C and there is concern that high-risk individuals often fail to meet their LDL-target goals.
Phytosterols (PS), plant-based sterols that structurally resemble mammalian cholesterol, are arguably the best-defined nutraceutical approach to reduce blood cholesterol concentrations by interfering with intestinal cholesterol absorption. PS have a proven track record as ‘natural’ cholesterol-lowering agents with consistent LDL-cholesterol reductions in the range of 10–16% in numerous well-controlled clinical interventions [3] and pre-clinical studies that have defined the molecular mechanisms involved [4–6]. Although PS are highly regarded as effective for reducing circulating total- and LDL-C, they were traditionally believed to have no effect on triglyceride (TG) concentrations, an important independent CVD risk factor. However, recent animal and human studies have highlighted a potential role for PS in regulating blood TG concentrations (Tables 1 and 2). That the TG-lowering properties of PS are just now surfacing may seem unexpected given that their health benefits have been actively researched in controlled human studies since the 1950’s. However, a close assessment of previous clinical PS interventions reveals TG-lowering responses that may have been overlooked for a variety of reasons. First, the lipid hypothesis placed such a major emphasis on cholesterol as the major CVD risk factor that PS interventions were specifically designed and statistically powered to detect movement in the primary endpoint of LDL-C, not other lipid risk factors. Furthermore, recent work suggests that the TG lowering responses of PS are most clearly observed in hypertriglyceridemic individuals, however, the vast majority of PS interventions were designed with hypercholesterolemia as the main study inclusion criteria. Finally, the TG-lowering action of PS may have been difficult to discern as metabolic and genetic factors may contribute to a relatively variable response compared with the more consistent reductions observed in circulating cholesterol levels.
Table 1.
Selected clinical studies reporting changes in plasma triglyceride concentrations in response to phytosterol/phytostanol supplementation.
| Study | n | Duration | Design | Dose | Vehicle | Baseline TG (mmol/L) |
Effect on TG Level (% change from baseline) |
|
|---|---|---|---|---|---|---|---|---|
| Maki et al. [7] | 28 7M 21F |
Primary hypercholesterolaemia | 6 wks | Randomized, single-blinded, placebo-controlled, crossover NCEP TLC weight maintenance diet |
1.8 g/d(81% PS & 19% PSA) | Capsule | 1.41 | vs. Base: ↓4% vs. Ctl:↓ 9.1% |
| Sialvera et al. [9] | 101 60M 48F |
Metabolic syndrome | 2 mo | Randomized, single-blinded, placebo-controlled, parallel-arm Western-type diet |
4 g/d PS | Yogurt beverage | 2.20 | vs. Base: ↓24% vs. Ctl: ↓12.5% |
| Sanchez-Muniz et al. [8] | 217 | Hypercholesterolaemic, stratified by carrier of the Apo E2 (E2E2), E3 (E3E3) and E4 (E3E4, E4E4) alleles | 5 wks | Randomized, double-blinded, placebo-controlled parallel-arm NCEP Step 1 diet |
1.1–2.2 g/d PS | Margarine | E2: 1.66 E3: 1.33 E4: 1.69 |
E2 vs. Ctl: ↓15.5% E3 vs. Ctl: ns E4 vs. Ctl: ns |
| Theuwissen et al. [10] | 28 16M 12F |
Elevated triglyceride levels | 3 wks | Randomized, double-blinded, placebo-controlled parallel-arm Habitual diet |
2.5 g/d PSA | Margarine | 2.63 | vs. Base: ↓11% (subjects with baseline TG > 2.3 mmol/L) |
| Plat et al. [11] | 18 | Dyslipidemic metabolic syndrome | 8 wks | Randomized, double-blinded, Placebo-controlled, parallel-arm | 2.0 g/d PSA | Yogurt beverage | 2.21 | vs. Base: ↓28% |
| 75 | Normolipidemic | 8 wks | Randomized, double-blinded, Placebo-controlled, parallel-arm | 3.8–4.1 g/d PSA | Margarine and shortenings | 1.02 | ns | |
| Rideout et al. [12] | 33 15M 18F |
Normolipidemic | 28 d | Randomized, placebo-controlled, crossover Controlled feeding trial (50% CHO, 35% fat, 15% protein) |
1.95 g/d PS | Low fat soy beverage | 1.65 | ns |
| 23 10M 13F |
Hypercholesterolaemic | 28 d | Randomized, placebo-controlled, crossover Controlled feeding trial (50% CHO, 35% fat, 15% protein) |
1.95 g/d PS | Moderate fat soy beverage | 2.06 | vs. Ctl: ↓9.4% | |
| Plat et al. [13] | 36 23M 13F |
Metabolic syndrome | 8 wks | Randomized, placebo-controlled parallel-arm | 2.0 g/d PSA | Yogurt beverage | 2.21 | vs. Base: ↓15.9% vs. Ctl: ↓44.5% |
| Clifton et al. [14] | 151 77M 74F |
Hypercholesterolemic | 6 wks | Randomized, double-blinded, placebo-controlled parallel-arm Habitual diet |
Wk 0–3: 1.6 g/d PS Wk 3–6: 3.0 g/d PS |
Margarine | 1.92 |
Wk 0–3 vs. Base: ↓12.5% vs. Ctl: ↓17.4% Wk: 3–6 vs. Base: ↓12.5% vs. Ctl: ↓21.5% |
| Plana et al. [15] | 83 | Hypercholesterolaemic | 6 wks | Multi-centre, randomized, double-blind, placebo-controlled, parallel-arm | 1.6 g/d PS | Yogurt beverage | 1.55 | vs. Base: ↓7% vs. Ctl: ↓14% |
| Judd et al. [16] | 53 26M 27F |
Normocholesterolaemic and mildly hypercholesterolaemic | 3 wks | Randomized, placebo-controlled, double-blind, crossover | 3.6 g/d PS | Salad dressing | 1.46 | vs. Ctl: ↓7.3% |
| Davidson et al. [17] | 84 46M 38F |
Healthy | 8 wks | Randomized, double-blind, placebo-controlled, parallel-arm Habitual diet |
3.0 g/d PS 6.0 g/d PS 9.0 g/d PS |
Reduced fat spread and/or salad dressing | 1.20 1.18 1.47 |
3.0 g/d, vs. Base: ↓13.3% 6.0 g/d, vs. Base: ns 9.0 g/d, vs. Base: ns |
| Jones et al. [18] | 15M | Hypercholesterolaemic | 21 d | Randomized, double-blind, placebo-controlled, crossover Controlled feeding trial (50% CHO, 35% fat, 15% protein) |
1.84 g/d PS 1.84 g/d PSA |
Margarine | 2.52 2.39 |
PS vs. Base: ↓18.9% vs. Ctl: ↓1.0% PSA vs. Base: ↓17.4% vs. Ctl: ↓0.9% |
| Shaghaghi et al. [19] | 47 25M 22 F |
Mild to moderately hypercholesterolaemic | 29 d | Randomized, placebo-controlled, crossover Habitual diet |
2.0 g/d water dispersible PS | Yogurt | 1.68 | vs. Base: ↓0.8% vs. Ctl: ↓13.9% |
Table 2.
Selected pre-clinical studies reporting plasma and or tissue triglyceride responses following phytosterol/phytostanol supplementation.
| Author | Model | Diet & Design | Result | Notes |
|---|---|---|---|---|
| Hamster model | ||||
| Rideout et al. (2013)[29] | Male Syrian Golden | Semi-synthetic ‘Western’ diet2% PS supplementation (Reducol) 6 wks feeding |
↓ blood TG (49%) Shift in hepatic FA (↑ 16:0; ↓16:1 and 18:1 ↓ de novo lipogenesis (44%) ↓ intestinal SREBP1c, hepatic PPARα, and FAS mRNA |
Comparable TG reductions to ezetimibe supplementation Reductions in blood and hepatic cholesterol levels also observed |
| Ntanios et al. (1998)[40] | Male and female Syrian Golden | Semi-synthetic diet supplemented with phytosterols (0.5 or 1%) from tall oil or soybean oil ~13 wks feeding |
↓ blood TG in males fed 1% soybean oil-phytosterols (no % given) | No effect in female hamsters |
| Ntanios et al. (2003)[41] | Male F1B Syrian Golden | Semi-synthetic diet enriched with graded doses of phytosterol ester (0.24–2.84%). | ↓ blood TG in animals fed >0.96% phytosterol ester (0.96, 1.92, 2.84%) | |
| Vanstone et al. (2001)[34] | Male Syrian Golden | Semi-synthetic diet; tall oil or soybean oil derived PS supplemented in diet or subcutaneously injected (matched to 5 mg/kg BW/day) ~9 wks feeding |
↓ blood TG (−42%) in animals injected with soybean oil derived PS | |
| Jain et al. (2008)[42] | Male Syrian Golden | Semi-synthetic, cholesterol-enriched diet with sitostanol (0.5%) | ↓ blood TG (−22%) in sitostanol group compared with control | |
| Liang et al. (2011)[24] | Male Syrian Golden | Semi-synthetic, cholesterol-enriched diet supplemented with β-sitosterol or stigmasterol (0.1%) | ↓ blood TG (−28%) in both groups | ↓ in intestinal mRNA expression of microsomal triglyceride transfer protein in both groups |
| Ebine et al. (2006)[43] | Male Syrian Golden | Semi-synthetic, cholesterol-enriched diet supplemented with 0.7 and 1.4% disodium ascorbyl phytostanyl phosphate* | ↓ blood TG (−45%) in 1.4% supplemented group | |
| Mouse model | ||||
| Rideout et al. (2010)[25] | Male C57BL/6J | Semi-synthetic ‘Western’ diet2% PS supplementation (Reducol) 6 wks feeding |
↓ blood TG (−28%) ↓ hepatic TG (30%) ↑ fecal 16:0 and 18:0 excretion ↑ hepatic SREBP1c and FAS mRNA; ↓ intestinal PPARα mRNA ↑ de novo lipogenesis (23%) |
Blood total cholesterol not altered No change in a host of intestinal FA absorption & metabolism gene expression |
| Volger et al. (2001)[44] | Female apolipoprotein E*3-Leiden transgenic | Semi-synthetic containing 0.25% cholesterol and 0.0%, 0.25%, 0.5%, 0.75%, or 1.0% plant stanols 8 wk feeding |
↓ hepatic TG (−38%) in the 1% dietary stanol group | No effect on serum TG No effect on hepatic VLDL-TG secretion |
| Plosch et al. (2006)[45] | Male C57BL/6J | Semi-synthetic cholesterol supplemented diet with 0.5% plant sterols or stanols 4 wks feeding |
↑ hepatic TG in both sterol and stanol groups | No change in plasma TG concentrations |
| Brufau et al. (2011)[46] | Male C57BL/6J | Semi-synthetic cholesterol supplemented diet with 1, 2, 4, or 8% plant sterols 2 wks feeding |
↓ blood TG (−26%) in 4% supplemented group ↑ hepatic TG (59%) in the 2% supplemented group |
|
| Looije et al. (2005)[47] | Male C57BL/6J | Low fat semi-synthetic diet supplemented with 2% FM-VP4* | ↓ blood TG (% reduction not specified) | |
| Lukic et al. (2003)[48] | Male apoE knockout | Cholesterol-supplemented chow diet with 0.1, 0.5, 1.0, and 2.0% FM-VP4* 12 wks feeding |
↓ blood TG in 2% supplemented group at 4 and 8 weeks | |
| Rat model | ||||
| Matasuoka et al. (2008)[49] | Male Sprague-Dawley rats | Semi-synthetic diet supplemented with 0.5% free PS (FPS) or free PS egg yolk lipoprotein complex (PSY) 3 wks feeding |
↓ hepatic TG in both groups (FPS, −33%; PSY, −22%) | No change in serum TG concentrations |
| Awaisheh et al. (2012)[50] | Male Sprague-Dawley rats | Semi-purified high fat/cholesterol chow; daily gavage with non-fermented milk with and without PS (5 mg/mL) 8 wks feeding |
↓ blood TG (−16%) ↓ hepatic TG (−92%) |
|
| Ikeda et al. (2006)[51] | Male Sprague-Dawley rats | Semi-synthetic diet supplemented with campestenone (0.5%) | ↓ blood TG (−76%) ↓ hepatic TG (−69%) ↑ expression of β-oxidation genes ↓ hepatic SREBP1c expression |
|
| Tomoyori et al. (2004)[23] | Male Sprague-Dawley rats | Semi-synthetic supplemented with 0.25% PS | ↓ lymphatic transport of TG | |
| Pig model | ||||
| Brufau et al. (2006)[30] | Female Dunkin Hartley guinea pigs | Cholesterol enriched (0.33%), isocaloric diets, chow vs semi-synthetic not specified; Supplemented with 3 doses of PS (0, 1.27, 2.45%); Diets with combination pectin and PS were also examined 4 wks feeding |
↑ apparent absorption of saturated FA in PS-supplemented animals including lauric (12:0) and myristic (14:0) acids ↑ hepatic incorporation of lauric (12:0) and myristic (14:0) acids in PS-supplemented animals vs. animals fed saturated fat diet |
|
| Brufau et al. (2007)[52] | Female Dunkin Hartley guinea pigs | ↓ fecal excretion of lauric (12:0) and myristic (14:0) acids compared with high saturated fat diet ↑ fecal excretion of arachidic (20:0) and behenic (22:0) acids compared with high saturated fat diet |
||
| Brufau et al. (2008)[53] | Female Dunkin Hartley guinea pigs | No change in plasma TG | ||
A semi-synthetic esterified phytostanols-ascorbic acid derivative
This review will provide a thorough assessment of the effects of PS on TG metabolism with discussion of the TG-lowering effects reported in previous clinical interventions and what is known regarding the potential molecular mechanisms that may underlie these responses. We review the extent of our knowledge regarding the metabolic and genetic factors that are thought to influence these responses and discuss future research priorities that must be addressed to more fully evaluate PS as a potential TG-lowering therapy.
Clinical Assessment of TG Lowering in Response to PS
A review of the clinical trial database demonstrates a TG-lowering effect of PS ranging from 0.8 to 28% compared to baseline values [7–19]. The TG-lowering response of selected studies are presented in Table 1.
A purview of this data demonstrates that, for the most part, the TG-lowering efficacy of PS increases as baseline TG levels become more prominent. For example, in studies where subjects were hypertriglyceridaemic (>1.7 mmol/L), 1.6–4 g/d PS lowered circulating TG levels 11%–28% [9–14,18]. Conversely, in studies where baseline TG levels were < 1.7 mmol/L, 1.6–4.1 g/d PS lowered TG levels by 0.8–7% [7,11,12,15,16,19]. Pooled analysis of five clinical studies showed that subjects with baseline TG concentrations of 1.0 mmol/L experienced a 1.0%, 3.8% and 4.7% reduction in circulating TG levels with 2.0 g/d, 3.0 g/d or 4.0 g/d plant stanols, respectively [20]. However, across the same dosages of PS, when baseline TG were 2.0 and 3.0 mmol/L, TG levels were decreased by ~1.5% and ~2.0%, ~5.8% and ~7.8%, and, ~7.0% and ~9.7%, respectively [20]. Using data from 12 clinical trials, Demonty et al. [21] showed PS facilitated a mild decrease in circulating TG of 6.0%. However, with the exception of two subjects with mildly elevated baseline TG levels (1.73 mmol/L and 1.93 mmol/L), baseline TG’s were relatively normal. Therefore, the 6.0% reduction in circulating TG aligns with data demonstrated in Table 1. In the same study, when the data were stratified by baseline TG levels, PS lowered TG concentrations by 0.18 mmol/L in subjects with TG levels within the 75th percentile (1.9 mmol/L) [21]. This reduction is substantially greater than the 0.0006 mmol/L and 0.08 mmol/L decrease in circulating TG concentrations among subjects with baseline TGs within the 25th (0.99 mmol/L) and 50th percentile (1.36 mmol/L), respectively [21]. To date, only one clinical trial by Theuwissen et al.[10] has sought to delineate PS TG-lowering efficacy. Using subjects with baseline TG levels of at least 1.73 mmol/L, PS had no effect on circulating TG concentrations. However, among subjects with moderate-to-high baseline TG levels (> 2.3 mmol/L), 2.5 g/d PS lowered TG concentrations by 11% compared with baseline. It is noted that, among normotriglyceridemic subjects (1.2 mmol/L), Davidson et al. [17] observed a 13.3% decrease in TG levels with 3.0 g/d. However, no effect was demonstrated with 6.0 g/d and 9.0 g/d PS [17]. While the TG-lowering results outlined in Table 1 are promising, a more focused approach for delineating PS effects on circulating TG is required. Overall, data suggest that 2.0–4.0 g/PS/d facilitate significant reductions in circulating TG levels in humans. However, the degree of PS-induced TG-lowering could be dependent on the presence and magnitude of hypertriglyceridemia.
In addition to applying hypertriglyceridemia as inclusion criteria in future clinical trials, future studies should also consider the consistency as to how the TG-lowering response is reported. Table 1 shows that the TG-lowering efficacy of PS are reported as comparisons to baseline, the control group or both. This is problematic when deciphering the true of effect PS on circulating TG levels. For example, Jones et al. [18], reported that, compared to baseline, hypertriglyceridemic subjects receiving 1.84 g/d PS decreased circulating TG by 18.9% and 17.4%, respectively. However, compared to the control group, TG levels were modestly decreased by 1.0% [18]. Shaghaghia et al. [19] demonstrated the opposite effect, where 2.0 g/d PS decreased TG relative to baseline and the control group by 0.8% and 13.9%, respectively. Large disparities in relative reductions in TG between baseline and controls values could suggest that background diets, or other lifestyle factors, are imposing a substantial effect on the TG response. Perhaps consistent reporting of absolute TG reductions would address the abovementioned discrepancies in data that support the effects of PS on elevated TG concentrations.
Pre-Clinical Assessment of TG Lowering in Response to PS
Several animal species, namely hamsters, mouse and rat models, demonstrate fairly consistent reductions in both blood and hepatic TG concentrations following dietary PS incorporation (Table 2). It is of interest to note that these TG reductions are evident despite drastic differences in study design including diverse animal models with distinct lipid metabolism and different background diets (chow vs. semi-synthetic) with variable types of fat, and wide-ranging sources and supplementation levels of PS. With a few exceptions, most of these studies were designed to specifically examine cholesterol-lowering responses and associated mechanisms, however, results of these studies do shed light on potential pathways by which PS may directly or indirectly modulate TG metabolism.
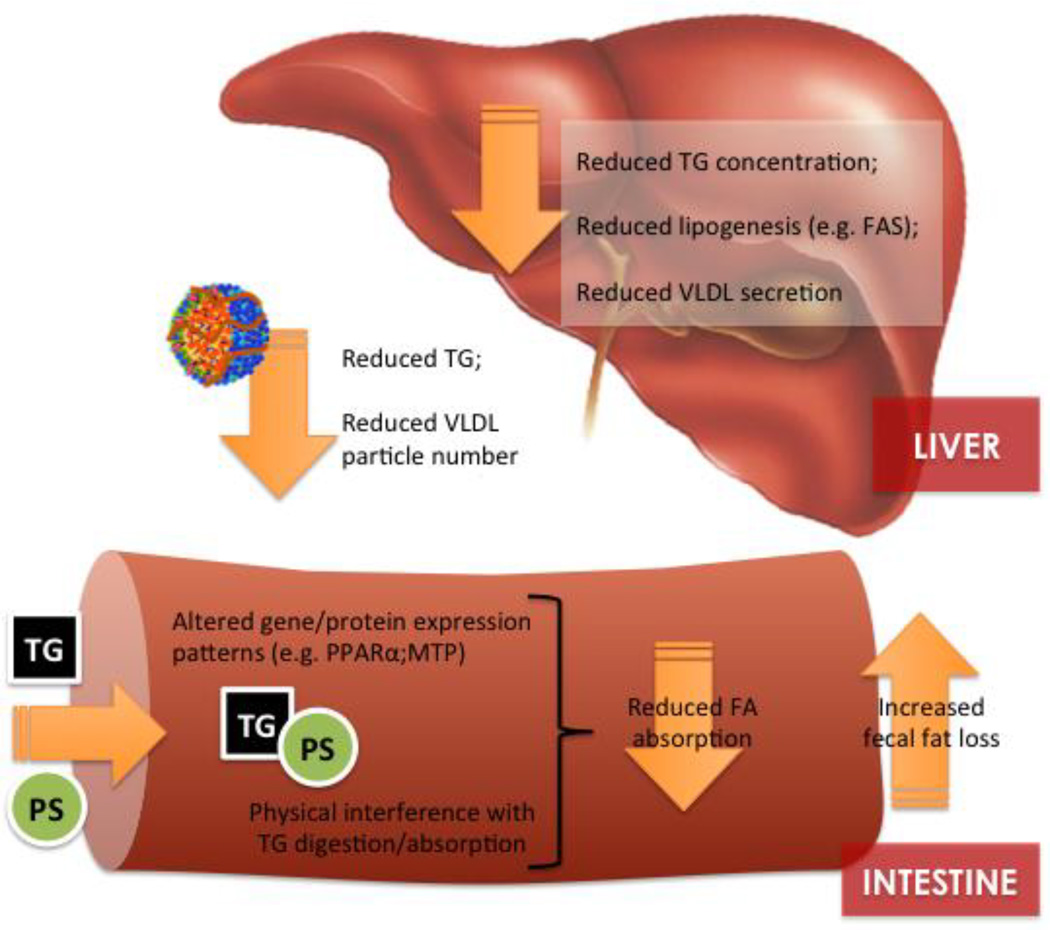
There are multiple lines of evidence to suggest that at least part of the TG-lowering response to PS is related to alterations in TG and/or FA metabolism within the intestine. In what we believe to be one of the first studies explicitly designed to examine the TG-lowering mechanisms of PS, we fed male C57BL/6J mice a semi-synthetic ‘Western’ diet supplemented with 2% PS (Reducol) for 6 weeks. Wild type C57BL/6J mice are a unique model as they exhibit reductions in blood and hepatic TG following PS supplementation but are considered non-responders to the cholesterol-lowering action of PS due their high cholesterol synthetic capacity [22]. Compared with unsupplemented animals, PS-fed mice exhibited reductions in plasma (28%) and hepatic (30%) TG concentrations. At least part of this TG lowering response was associated with changes in intestinal fat metabolism including increased fecal fatty acid excretion, specifically fecal palmitate and stearate. Supporting a potential role of PS in reducing intestinal TG absorption, Tomoyori et al. (2004) reported that PS reduced the postprandial lymphatic transport of TG (5–7 hours following a meal) in thoracic duct–cannulated Sprague-Dawley rats [23]. In our mouse study, we detected no difference in the expression of a host of intestinal genes related to FA absorption and chylomicron assembly, however, PPARα mRNA expression was reduced compared with control animals (discussed below). These results may suggest a physical interference of PS on intestinal FA absorption rather than a direct effect on the expression of genes and proteins that regulate FA uptake, similar to the interference of PS with the incorporation of cholesterol into bile salt micelles. Alternatively, Liang et al. (2011) reported that male Syrian golden hamsters exhibited a 28% reduction in blood TG which was associated with reduced mRNA expression of intestinal microsomal triglyceride transfer protein (MTP) following dietary supplementation with β-sitosterol or stigmasterol (0.1%) [24]. As MTP plays a pivotal role in the assembly and secretion of apolipoprotein B (apoB)-containing chylomicron particles, PS-mediated reductions in the expression of this gene could conceivably be linked to TG lowering and should be confirmed in future mechanistic studies. Although many studies (both animal and human) have directly examined intestinal cholesterol absorption in response to PS consumption, we are not aware of any studies that have employed stable-isotope methodology to directly measure FA absorption following PS intervention. This is a major knowledge gap in our understanding of the potential mechanisms involved in PS-mediated TG reductions and should be addressed in future animal and human studies.
As mentioned above, in a previous study designed to examine the TG lowering responses of PS, we have observed reductions in intestinal peroxisome proliferator-activated receptor alpha (PPARα) mRNA expression [25]. PPARα is a nuclear receptor highly expressed within intestinal enterocytes that mediates the effects of nutrients, specifically fatty acids, on gene expression. PPARα activation has been shown to regulate a whole-host of intestinal functions including nutrient transport, fatty acid oxidation, oxidative stress, and inflammation and its expression patterns mimics that of other critical genes involved in fatty acid absorption [26–28]. The broad scope of its regulation and constant exposure to its fatty acid ligands makes PPARα an intriguing therapeutic target for obesity and dyslipidemia. At this point, it is difficult to say whether the observed reduction in PPARα mRNA expression is a direct effect of PS or an indirect consequence, possibly from a direct interference of PS with intestinal TG absorption.
There is also evidence to suggest that PS directly or indirectly influence hepatic FA and TG metabolism. The liver is central to whole-body FA and TG metabolism as a primary site for the de novo lipogenesis of FA, the synthesis and secretion of nascent VLDL particles, and the clearance of fatty acids (FA) from TG-rich remnant lipoprotein particles and HDL species. PS supplementation is regularly associated with a reduction in hepatic TG concentrations in hamster, mouse, and rat models (Table 2). In addition to tissue TG concentrations, there is also evidence to suggest that PS may modulate the hepatic FA profile. We observed a shift in hepatic fatty acid composition toward increased saturated 16:0 (~50%) and reduced monounsaturated FA (16:1, ~40%; and 18:1, ~24%) in PS-supplemented hamsters compared with un-supplemented animals [29]. Similarly, Brufau et al. (2007) also observed an increase in the hepatic incorporation of lauric (12:0) and myristic (14:0) acids in PS-supplemented female Dunkin Hartley guinea pigs vs. animals fed a saturated fat diet [30]. The implications of this apparent shift in hepatic FA toward a more saturated profile is not yet known, although it may be a secondary effect due to a reduction in intestinal TG absorption or modulation of hepatic lipogenesis and/or lipoprotein synthesis. In support of a TG-lowering mechanism of hepatic origin, Plat et al. (2009) reported a reduction in large and medium plasma VLDL particles in dyslipidemic metabolic syndrome subjects consuming 2 g of PS provided in a yogurt drink matrix [11].
We have investigated the modulation of hepatic de novo lipogenesis as a potential mechanism that may underlie TG reductions following PS-supplementation in both mouse and hamster models [25,29]. However, the results of these studies highlight differential model-specific responses that preclude any clear consensus regarding the impact of PS on hepatic lipogenesis. We observed an increase in hepatic de novo lipogenesis in PS-fed C57BL6 that we interpreted to be a compensatory response to interference with intestinal FA absorption as evidenced by increased fecal FA excretion. We have recently identified a similar response in a PS-fed Zucker rat model that demonstrated an increase in the ratio of hepatic 16:0/18:2n-6, an indirect measure of hepatic de novo lipogenesis (Rideout et al; unpublished). However, in a separate study, PS fed Syrian golden hamsters exhibited a reduction in de novo lipogenesis that was supported by a decrease in the protein abundance of fatty acid synthase (FAS), a rate-limiting enzyme in the lipogenic pathway. Given the similarity in the design factors between the two studies, including background diet, PS supplementation level, and stable isotope analysis, this discrepancy highlights the underlying differences in lipid metabolism between mouse and hamster species and clearly demonstrates the need for estimates of de novo lipogenesis as part of a mechanistic human intervention.
It is of interest to note that lipid reductions (both cholesterol and TGs) have been reported following intraperitoneal and subcutaneous PS injections in various animal models [31–33]. Vanstone et al. [34] observed TG reductions in Syrian golden hamsters subcutaneously injected with PS at a dose of 5mg/kg/BW. These results suggest that PS may mediate blood lipid concentrations independent of their direct effects within the intestine, possibly by modulating the expression of genes that regulate hepatic TG balance.
Factors Affecting Responsiveness
Recent work has highlighted a variety of subject specific metabolic and genetic factors that predict the magnitude and direction of the cholesterol response to PS [22,35–37]. Although less work has specifically examined the heterogeneity of responses in blood TG, its range has been estimated to be between 6–20%[3]. As discussed previously, the TG-lowering efficacy of PS seems to depend on the presence and magnitude of hypertriglyceridaemia. This stands to reason as TG reductions in response to ezetimibe, the well-characterized intestinal cholesterol absorptive inhibitor, have also been shown to be dependent on baseline TG concentrations [38].
Although a genetic basis for the variable TG-lowering effects of PS is probable, we are only aware of two studies that have examined a potential genetic link, both with polymorphisms in the apolipoprotein E (apoE) gene. ApoE is an apolipoprotein component of lipoproteins including chlyomicrons, VLDL, LDL, and HDL and is therefore heavily involved in directing lipoprotein metabolism and remodeling with the plasma compartment. Apolipoprotein E is polymorphic with three common alleles in the population, namely ε4, ε3 and ε2, that are thought to underlie the lipid-lowering responses to drug and diet-based therapies. Sanchez-Muniz et al. (2009) observed TG reductions in apoE2 but not in apoE3 or E4 subjects following PS intervention in hypercholesterolemic adults [8]. Although not significant, Geelen et al. (2002) reported that E4 subjects tended (p=0.13) to have a greater TG-lowering response compared with E3/3 subjects consuming 3.2 g of daily PS intake in margarine (difference of 0.08 mmol/L)[39]. There is a need to conduct further studies to understand the genetic basis of responsiveness with a focus on genes that are known to modulate plasma TG concentrations and lipoprotein metabolism. Furthermore, there has yet to be any studies to examine more readily identifiable patient characteristics such as ethnicity, age, gender, and BMI that may underlie the TG-lowering response to PS.
Summary
Although PS are well-substantiated for their LDL-C lowering effects in hypercholesterolemic patients, the efficacy of PS as a TG-lowering therapy has only recently gained momentum within the nutrition community through a limited number of animal and human studies specifically designed to examine this response and the potential mechanisms involved. Nonetheless, clinical trial data has shown that, among subjects with hypertriglyceridemia (TG > 1.73 mmol/L), 2–4 g/PS/d can facilitate a decrease in TG concentrations of ≥ 11%. Results from a number of different animal studies suggested that the TG-lowering mechanisms of PS may be multifactorial including interference with FA absorption within the intestinal lumen, modulation of hepatic de novo lipogenesis, and a reduction in circulating medium and large VLDL particles. The effects of PS on the expression of a variety of gene and protein targets, including FAS and PPARα, suggest that there may be a molecular component to the TG lowering response. However, to fully substantiate the utility of PS as a TG-lowering therapy, human clinical trials that are specifically powered to detect an effect of PS on TG concentrations in hypertriglyceridemic subjects are required. Furthermore, human interventions should explore a mechanistic basis for the TG-lowering response with a direct examination of FA absorption and whole-body lipogenesis in response to PS supplementation. Finally, responsiveness studies that identify both metabolic and genetic factor that determine the magnitude of PS-induced TG reductions will be critical in defining the clinical utility of PS as a TG-lowering therapy.
Figure 1.
Potential mechanisms involved in the triglyceride-lowering response to physterols/phytostanols.
References
- 1.Anitschkow N, Chalatow S. Classics in arteriosclerosis research: On experimental cholesterin steatosis and its significance in the origin of some pathological processes, translated by Mary Z. Pelias, 1913. Arteriosclerosis. 1983;3:178–182. [PubMed] [Google Scholar]
- 2.Coronary heart disease in seven countries. Summary. Circulation. 1970;41:I186–I195. [PubMed] [Google Scholar]
- 3.Gylling H, Plat J, Turley S, Ginsberg HN, Ellegard L, Jessup W, Jones PJ, Lutjohann D, Maerz W, Masana L, Silbernagel G, Staels B, Boren J, Catapano AL, De Backer G, Deanfield J, Descamps OS, Kovanen PT, Riccardi G, Tokgozoglu L, Chapman MJ European Atherosclerosis Society Consensus Panel on P. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis. 2014;232:346–360. doi: 10.1016/j.atherosclerosis.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 4.De Smet E, Mensink RP, Plat J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Molecular Nutrition & Food Research. 2012;56:1058–1072. doi: 10.1002/mnfr.201100722. [DOI] [PubMed] [Google Scholar]
- 5.Amir Shaghaghi M, Abumweis SS, Jones PJ. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: results of a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113:1494–1503. doi: 10.1016/j.jand.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Ras RT, Geleijnse JM, Trautwein EA. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. Br J Nutr. 2014;112:214–219. doi: 10.1017/S0007114514000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki KC, Lawless AL, Reeves MS, Kelley KM, Dicklin MR, Jenks BH, Shneyvas E, Brooks JR. Lipid effects of a dietary supplement softgel capsule containing plant sterols/stanols in primary hypercholesterolemia. Nutrition. 2013;29:96–100. doi: 10.1016/j.nut.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Muniz FJ, Maki KC, Schaefer EJ, Ordovas JM. Serum lipid and antioxidant responses in hypercholesterolemic men and women receiving plant sterol esters vary by apolipoprotein E genotype. J Nutr. 2009;139:13–19. doi: 10.3945/jn.108.090969. [DOI] [PubMed] [Google Scholar]
- 9.Sialvera TE, Pounis GD, Koutelidakis AE, Richter DJ, Yfanti G, Kapsokefalou M, Goumas G, Chiotinis N, Diamantopoulos E, Zampelas A. Phytosterols supplementation decreases plasma small and dense LDL levels in metabolic syndrome patients on a westernized type diet. Nutr Metab Cardiovasc Dis. 2012;22:843–848. doi: 10.1016/j.numecd.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Theuwissen E, Plat J, van der Kallen CJ, van Greevenbroek MM, Mensink RP. Plant stanol supplementation decreases serum triacylglycerols in subjects with overt hypertriglyceridemia. Lipids. 2009;44:1131–1140. doi: 10.1007/s11745-009-3367-6. [DOI] [PubMed] [Google Scholar]
- 11.Plat J, Mensink RP. Plant stanol esters lower serum triacylglycerol concentrations via a reduced hepatic VLDL-1 production. Lipids. 2009;44:1149–1153. doi: 10.1007/s11745-009-3361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rideout TC, Chan YM, Harding SV, Jones PJ. Low and moderate-fat plant sterol fortified soymilk in modulation of plasma lipids and cholesterol kinetics in subjects with normal to high cholesterol concentrations: report on two randomized crossover studies. Lipids Health Dis. 2009;8:45. doi: 10.1186/1476-511X-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plat J, Brufau G, Dallinga-Thie GM, Dasselaar M, Mensink RP. A plant stanol yogurt drink alone or combined with a low-dose statin lowers serum triacylglycerol and non-HDL cholesterol in metabolic syndrome patients. J Nutr. 2009;139:1143–1149. doi: 10.3945/jn.108.103481. [DOI] [PubMed] [Google Scholar]
- 14.Clifton PM, Mano M, Duchateau GS, van der Knaap HC, Trautwein EA. Dose-response effects of different plant sterol sources in fat spreads on serum lipids and C-reactive protein and on the kinetic behavior of serum plant sterols. Eur J Clin Nutr. 2008;62:968–977. doi: 10.1038/sj.ejcn.1602814. [DOI] [PubMed] [Google Scholar]
- 15.Plana N, Nicolle C, Ferre R, Camps J, Cos R, Villoria J, Masana L. Plant sterol-enriched fermented milk enhances the attainment of LDL-cholesterol goal in hypercholesterolemic subjects. Eur J Nutr. 2008;47:32–39. doi: 10.1007/s00394-007-0693-4. [DOI] [PubMed] [Google Scholar]
- 16.Judd JT, Baer DJ, Chen SC, Clevidence BA, Muesing RA, Kramer M, Meijer GW. Plant sterol esters lower plasma lipids and most carotenoids in mildly hypercholesterolemic adults. Lipids. 2002;37:33–42. doi: 10.1007/s11745-002-0861-y. [DOI] [PubMed] [Google Scholar]
- 17.Davidson MH, Maki KC, Umporowicz DM, Ingram KA, Dicklin MR, Schaefer E, Lane RW, McNamara JR, Ribaya-Mercado JD, Perrone G, Robins SJ, Franke WC. Safety and tolerability of esterified phytosterols administered in reduced-fat spread and salad dressing to healthy adult men and women. J Am Coll Nutr. 2001;20:307–319. doi: 10.1080/07315724.2001.10719051. [DOI] [PubMed] [Google Scholar]
- 18.Jones PJ, Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Feng JY, Parsons WE. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res. 2000;41:697–705. [PubMed] [Google Scholar]
- 19.Shaghaghia MA, Harding SV, Jones PJH. Water dispersible plant sterol formulation shows improved effect on lipid profile compared to plant sterol esters. Journal of Functional Foods. 2014;6:280–289. [Google Scholar]
- 20.Naumann E, Plat J, Kester AD, Mensink RP. The baseline serum lipoprotein profile is related to plant stanol induced changes in serum lipoprotein cholesterol and triacylglycerol concentrations. J Am Coll Nutr. 2008;27:117–126. doi: 10.1080/07315724.2008.10719683. [DOI] [PubMed] [Google Scholar]
- 21.Demonty I, Ras RT, van der Knaap HC, Meijer L, Zock PL, Geleijnse JM, Trautwein EA. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomised controlled trials. Eur J Nutr. 2013;52:153–160. doi: 10.1007/s00394-011-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rideout TC, Harding SV, Mackay D, Abumweis SS, Jones PJ. High basal fractional cholesterol synthesis is associated with nonresponse of plasma LDL cholesterol to plant sterol therapy. Am J Clin Nutr. 2010;92:41–46. doi: 10.3945/ajcn.2009.29073. [DOI] [PubMed] [Google Scholar]
- 23.Tomoyori H, Kawata Y, Higuchi T, Ichi I, Sato H, Sato M, Ikeda I, Imaizumi K. Phytosterol oxidation products are absorbed in the intestinal lymphatics in rats but do not accelerate atherosclerosis in apolipoprotein E-deficient mice. J Nutr. 2004;134:1690–1696. doi: 10.1093/jn/134.7.1690. [DOI] [PubMed] [Google Scholar]
- 24.Liang YT, Wong WT, Guan L, Tian XY, Ma KY, Huang Y, Chen ZY. Effect of phytosterols and their oxidation products on lipoprotein profiles and vascular function in hamster fed a high cholesterol diet. Atherosclerosis. 2011;219:124–133. doi: 10.1016/j.atherosclerosis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Rideout TC, Harding SV, Jones PJ. Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J mice. Mol Nutr Food Res. 2010;54(Suppl 1):S7–S13. doi: 10.1002/mnfr.201000027. [DOI] [PubMed] [Google Scholar]
- 26.Uchida A, Slipchenko MN, Cheng JX, Buhman KK. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, alters triglyceride metabolism in enterocytes of mice. Biochim Biophys Acta. 2011;1811:170–176. doi: 10.1016/j.bbalip.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunger M, van den Bosch HM, van der Meijde J, Kersten S, Hooiveld GJ, Muller M. Genome-wide analysis of PPARalpha activation in murine small intestine. Physiol Genomics. 2007;30:192–204. doi: 10.1152/physiolgenomics.00198.2006. [DOI] [PubMed] [Google Scholar]
- 28.de Vogel-van den Bosch HM, Bunger M, de Groot PJ, Bosch-Vermeulen H, Hooiveld GJ, Muller M. PPARalpha-mediated effects of dietary lipids on intestinal barrier gene expression. BMC Genomics. 2008;9:231. doi: 10.1186/1471-2164-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rideout TC, Ramprasath V, Griffin JD, Browne RW, Harding SV, Jones PJ. Phytosterols protect against diet-induced hypertriglyceridemia in Syrian golden hamsters. Lipids Health Dis. 2014;13:5. doi: 10.1186/1476-511X-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brufau G, Canela MA, Rafecas M. A high-saturated fat diet enriched with phytosterol and pectin affects the fatty acid profile in guinea pigs. Lipids. 2006;41:159–168. doi: 10.1007/s11745-006-5084-8. [DOI] [PubMed] [Google Scholar]
- 31.Gerson T, Shorland FB, Dunckley GG. The effect of beta-sitosterol on the metabolism of cholesterol and lipids in rats on a diet containing coconut oil. Biochem J. 1965;96:399–403. doi: 10.1042/bj0960399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konlande JE, Fisher H. Evidence for a nonabsorptive antihypercholesterolemic action of phytosterols in the chicken. J Nutr. 1969;98:435–442. doi: 10.1093/jn/98.4.435. [DOI] [PubMed] [Google Scholar]
- 33.Malini T, Vanithakumari G. Rat toxicity studies with beta-sitosterol. J Ethnopharmacol. 1990;28:221–234. doi: 10.1016/0378-8741(90)90032-o. [DOI] [PubMed] [Google Scholar]
- 34.Vanstone CA, Raeini-Sarjaz M, Jones PJ. Injected phytosterols/stanols suppress plasma cholesterol levels in hamsters. J Nutr Biochem. 2001;12:565–574. doi: 10.1016/s0955-2863(01)00175-9. [DOI] [PubMed] [Google Scholar]
- 35.Plat J, Mensink RP. Relationship of genetic variation in genes encoding apolipoprotein A-IV, scavenger receptor BI, HMG-CoA reductase, CETP and apolipoprotein E with cholesterol metabolism and the response to plant stanol ester consumption. Eur J Clin Invest. 2002;32:242–250. doi: 10.1046/j.1365-2362.2002.00982.x. [DOI] [PubMed] [Google Scholar]
- 36.Rudkowska I, AbuMweis SS, Nicolle C, Jones PJ. Association between non-responsiveness to plant sterol intervention and polymorphisms in cholesterol metabolism genes: a case-control study. Appl Physiol Nutr Metab. 2008;33:728–734. doi: 10.1139/H08-041. [DOI] [PubMed] [Google Scholar]
- 37.Houweling AH, Vanstone CA, Trautwein EA, Duchateau GS, Jones PJ. Baseline plasma plant sterol concentrations do not predict changes in serum lipids, C-reactive protein (CRP) and plasma plant sterols following intake of a plant sterol-enriched food. Eur J Clin Nutr. 2009;63:543–551. doi: 10.1038/sj.ejcn.1602969. [DOI] [PubMed] [Google Scholar]
- 38.Gazi IF, Daskalopoulou SS, Nair DR, Mikhailidis DP. Effect of ezetimibe in patients who cannot tolerate statins or cannot get to the low density lipoprotein cholesterol target despite taking a statin. Curr Med Res Opin. 2007;23:2183–2192. doi: 10.1185/030079907X226267. [DOI] [PubMed] [Google Scholar]
- 39.Geelen A, Zock PL, de Vries JH, Katan MB. Apolipoprotein E polymorphism and serum lipid response to plant sterols in humans. Eur J Clin Invest. 2002;32:738–742. doi: 10.1046/j.1365-2362.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- 40.Ntanios FY, Jones PJ. Effects of variable dietary sitostanol concentrations on plasma lipid profile and phytosterol metabolism in hamsters. Biochim Biophys Acta. 1998;1390:237–244. doi: 10.1016/s0005-2760(97)00196-3. [DOI] [PubMed] [Google Scholar]
- 41.Ntanios FY, van de Kooij AJ, de Deckere EA, Duchateau GS, Trautwein EA. Effects of various amounts of dietary plant sterol esters on plasma and hepatic sterol concentration and aortic foam cell formation of cholesterol-fed hamsters. Atherosclerosis. 2003;169:41–50. doi: 10.1016/s0021-9150(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 42.Jain D, Ebine N, Jia X, Kassis A, Marinangeli C, Fortin M, Beech R, Hicks KB, Moreau RA, Kubow S, Jones PJ. Corn fiber oil and sitostanol decrease cholesterol absorption independently of intestinal sterol transporters in hamsters. J Nutr Biochem. 2008;19:229–236. doi: 10.1016/j.jnutbio.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Ebine N, Demonty I, Jia X, Jones PJ. Plant stanol ascorbate esters reduce body weight gain through decreased energy absorption in hamsters. Int J Obes (Lond) 2006;30:751–757. doi: 10.1038/sj.ijo.0803191. [DOI] [PubMed] [Google Scholar]
- 44.Volger OL, van der Boom H, de Wit EC, van Duyvenvoorde W, Hornstra G, Plat J, Havekes LM, Mensink RP, Princen HM. Dietary plant stanol esters reduce VLDL cholesterol secretion and bile saturation in apolipoprotein E*3-Leiden transgenic mice. Arterioscler Thromb Vasc Biol. 2001;21:1046–1052. doi: 10.1161/01.atv.21.6.1046. [DOI] [PubMed] [Google Scholar]
- 45.Plosch T, Kruit JK, Bloks VW, Huijkman NC, Havinga R, Duchateau GS, Lin Y, Kuipers F. Reduction of cholesterol absorption by dietary plant sterols and stanols in mice is independent of the Abcg5/8 transporter. J Nutr. 2006;136:2135–2140. doi: 10.1093/jn/136.8.2135. [DOI] [PubMed] [Google Scholar]
- 46.Brufau G, Kuipers F, Lin Y, Trautwein EA, Groen AK. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS One. 2011;6:e21576. doi: 10.1371/journal.pone.0021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Looije NA, Risovic V, Stewart DJ, Debeyer D, Kutney J, Wasan KM. Disodium Ascorbyl Phytostanyl Phosphates (FM-VP4) reduces plasma cholesterol concentration, body weight and abdominal fat gain within a dietary-induced obese mouse model. J Pharm Pharm Sci. 2005;8:400–408. [PubMed] [Google Scholar]
- 48.Lukic T, Wasan KM, Zamfir D, Moghadasian MH, Pritchard PH. Disodium ascorbyl phytostanyl phosphate reduces plasma cholesterol concentrations and atherosclerotic lesion formation in apolipoprotein E-deficient mice. Metabolism. 2003;52:425–431. doi: 10.1053/meta.2003.50084. [DOI] [PubMed] [Google Scholar]
- 49.Matsuoka R, Muto A, Kimura M, Hoshina R, Wakamatsu T, Masuda Y. Cholesterol-lowering activity of plant sterol-egg yolk lipoprotein complex in rats. J Oleo Sci. 2008;57:309–314. doi: 10.5650/jos.57.309. [DOI] [PubMed] [Google Scholar]
- 50.Awaisheh SS, Khalifeh MS, Al-Ruwaili MA, Khalil OM, Al-Ameri OH, Al-Groom R. Effect of supplementation of probiotics and phytosterols alone or in combination on serum and hepatic lipid profiles and thyroid hormones of hypercholesterolemic rats. J Dairy Sci. 2013;96:9–15. doi: 10.3168/jds.2012-5442. [DOI] [PubMed] [Google Scholar]
- 51.Ikeda I, Konno R, Shimizu T, Ide T, Takahashi N, Kawada T, Nagao K, Inoue N, Yanagita T, Hamada T, Morinaga Y, Tomoyori H, Imaizumi K, Suzuki K. Campest-5-en-3-one, an oxidized derivative of campesterol, activates PPARalpha, promotes energy consumption and reduces visceral fat deposition in rats. Biochim Biophys Acta. 2006;1760:800–807. doi: 10.1016/j.bbagen.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Brufau G, Canela MA, Rafecas M. Phytosterols, but not pectin, added to a high-saturated-fat diet modify saturated fatty acid excretion in relation to chain length. J Nutr Biochem. 2007;18:580–586. doi: 10.1016/j.jnutbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Brufau G, Canela MA, Quílez J, Rafecas M. Phytosterols and pectin added to a high-saturated fat diet do not show hypocholesterolemic activity in female guinea pigs. European Journal of Lipid Science and Technology. 2008;110:206–215. [Google Scholar]