Abstract
Background
We describe nationally representative patterns of utilization and short-term outcomes from robotic versus open thyroidectomy for thyroid cancer.
Methods
Descriptive statistics and multivariable analysis were used to analyze patterns of use of robotic thyroidectomy from the National Cancer Database (2010–2011). Short-term outcomes were compared between patients undergoing robotic versus open thyroidectomy, while adjusting for confounders.
Results
A total of 68,393 patients with thyroid cancer underwent thyroidectomy; 225 had robotic surgery and 57,729 underwent open surgery. Robotic thyroid surgery use increased by 30 % from 2010 to 2011 (p = 0.08). Robotic cases were reported from 93 centers, with 89 centers performing <10 robotic cases. Compared with the open group, the robotic group was younger (51 vs. 47 years; p < 0.01) and included more Asian patients (4 vs. 8 %; p = 0.006) and privately-insured patients (68 vs. 77 %; p = 0.01). Tumor size was similar between patients undergoing robotic versus open surgery. Total thyroidectomy was performed less frequently in the robotic group (67 vs. 84 % open; p < 0.0001). Patients were relatively more likely to undergo robotic surgery if they were female (odds ratio [OR] 1.6; p = 0.04), younger (OR 0.8/10 years; p < 0.0001), or underwent lobectomy (OR 2.4; p < 0.0001). In adjusted multivariable analysis, there were no differences in the number of lymph nodes removed or length of stay between groups; however, there was a non-significant increase in the incidence of positive margins with robotic thyroidectomy.
Conclusions
Use of robotic thyroidectomy for thyroid cancer is limited to a few institutions, with short-term outcomes that are comparable to open surgery. Multi-institutional studies should be undertaken to compare thyroidectomy-specific complications and long-term outcomes.
Thyroid cancer is the most common malignancy of the endocrine system. There has been a near threefold increase in its incidence between 1975 and 2009,1 making it the cancer with the fastest increasing incidence in the US.2 Prognosis is excellent when appropriate therapy is undertaken.3
The mainstay of treatment for differentiated thyroid cancer is surgical resection through an anterior neck incision.4 This technique was initially developed by Emil Kocher in the late 1800s and since then has become the standard approach. Open thyroidectomy is generally a very safe operation in the hands of experienced (high volume) surgeons.5,6
Alternative approaches were developed in order to avoid a neck incision. Robotic thyroidectomy can be performed through an axillary, breast, axillo-breast, posterior auricular, or trans-oral incision.7–10 Robotic thyroidectomy was pioneered by Korean surgeons, and is now the preferred approach for thyroidectomy for both benign and malignant disease in that country.11 It is believed that the popularity of robotic thyroidectomy in Asia is related to a cultural aversion to a scar on the neck.12 Data from Korea have demonstrated that robotic thyroidectomy for thyroid cancer is safe and can provide oncologic outcomes that are equivalent to open thyroidectomy, with improved cosmesis, patient satisfaction, and quality of life.11,13–15 There has been some skepticism in the US about whether these favorable outcomes can be replicated here, given that patients in this country have, on average, a higher body mass index, making the operation more challenging technically and potentially less effective from an oncologic perspective.16–18
To date, there are no data describing the utilization of robotic thyroidectomy for cancer across the US. The aims of our study were to describe patterns of utilization and compare short-term outcomes of robotic thyroidectomy with open thyroidectomy for thyroid cancer at a population level.
METHODS
The National Cancer Database (NCDB) is a joint platform maintained by the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons (ACS). The NCDB is a nationwide, facility-based, comprehensive clinical surveillance system. It contains more than 29 million cancer cases from more than 1,500 CoC-accredited cancer programs representing more than 85 % of all incident thyroid cancer cases in the US.19
Data were coded according to the CoC Registry Operations and Data Standards Manual, the American Joint Committee for Cancer (AJCC) Manual for Staging of Cancer, and the International Classification of Diseases for Oncology, Third Edition (ICD-O-3). To reduce data errors and maintain integrity of the database, all data were extracted from medical records by trained and certified tumor registrars. Our Institutional Review Board granted this study an exemption status due to the de-identified nature of the database.
The NCDB Participant User File was used to identify all thyroid cancer patients ≥18 years of age who underwent robotic or open thyroid surgery in 2010 and 2011. Patient-related variables included age at diagnosis, race, sex, level of education, annual income, insurance status, type of insurance, year of diagnosis, distance traveled to treating institution, and comorbidity. Comorbidity was quantified by the modified Charlson–Deyo scoring system.20 Education and annual income were determined by linking a patient's ZIP code to the year 2000 US Census data. Facility-related variables included classification according to CoC accreditation, all thyroidectomy and robotic case volume, and geographic region.
Tumor characteristics included histology, size, and absence or presence of extrathyroidal extension, multifocality, lymph node and distant metastases. Histologic diagnosis was identified based on corresponding ICD-O-3 codes. Data on surgical approach utilized were obtained directly from the database. Patients who underwent either robotic or open thyroidectomy were included. The extent of surgery was categorized as lobectomy (lobectomy and partial lobectomy) or total thyroidectomy (total, near-total, and subtotal thyroidectomy). Patients were excluded if the extent of surgery was not specified.
Short-term outcomes included the number of lymph nodes removed, rate of positive surgical margins, and length of hospital stay (LOS), as measured from the date of surgery to discharge. Number of lymph nodes removed was analyzed only in patients who underwent a lymph node dissection. LOS was dichotomized into ≤1 or >1 day, as thyroidectomy is considered an outpatient procedure with a typical LOS of 23 h.
Statistical Analyses
Intention-to-treat analysis was used; robotic cases converted to open surgery were analyzed as robotic. Comparisons of patient and facility characteristics for patients undergoing robotic versus open thyroidectomy were performed using Chi square tests for categorical variables and non-parametric Kruskal–Wallis tests for continuous variables.
Separate multivariable logistic regression models were employed to analyze factors associated with the use of robotic versus open thyroidectomy and short-term outcomes while adjusting for patient age, sex, race, annual income, insurance type, geographic region, hospital type, presence of nodal disease, multifocality, extrathyroidal extension, extent of thyroid surgery, and open thyroidectomy and robotic case volume of the treating facility. The number of lymph nodes removed was analyzed using negative binomial regression, a form of generalized linear regression model suited to analyze over-dispersed nonnegative continuous variables. Generalized estimating equations were used to account for clustering of patients within hospitals when examining the associations of robotic versus open thyroidectomy with each of the outcomes. Multivariable models for short-term outcomes were limited to patients with papillary thyroid cancer (PTC), given the small number of patients with other forms of thyroid cancer. A backward variable elimination method was used to produce the most parsimonious model by removal of non-significant variables based on a cutoff p value of ≤0.2. The level of statistical significance was set a priori at a two-sided p-value of <0.05. Statistical analyses were performed using SAS Enterprise Guide 5.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 68,393 patients underwent surgery for thyroid cancer in 2010 and 2011; over 90 % of these patients had surgery for PTC. A total of 225 patients underwent robotic thyroidectomy, and 57,729 had open thyroidectomy. The rate of conversion from robotic to open surgery was 4 %. Use of robotic thyroidectomy increased by 30 % from 2010 to 2011 (Table 1; p = 0.08); the increase in the proportion of robotic cases to all thyroidectomy cases was from 0.34 % in 2010 to 0.43 % in 2011. Robotic cases were reported from 93 hospitals across the 2-year study period. Of these 93 hospitals, 57 hospitals performed robotic cases in 2010, but only 14 (25 %) hospitals continued performing robotic thyroidectomy in 2011. There were 50 hospitals that performed robotic cases in 2011, including 36 (72 %) hospitals that started performing robotic cases. The number of robotic cases performed by each hospital varied from 1 to 29 cases (Fig. 1); 96 % performed <10 total cases. Four hospitals performed 32 % (n = 72) of all robotic cases (Fig. 2).
TABLE 1.
Demographic, clinical, and pathologic characteristics of patients undergoing robotic versus open thyroidectomy for cancer (2010–2011)
| Robotic (N = 225) | Open (N = 57729) | p value | |
|---|---|---|---|
| Patient age (years; mean ± SD) | 47 ± 15 | 51 ± 15 | 0.0002 |
| Female | 186 (82.7) | 44,017 (76.5) | 0.02 |
| Race | 0.01 | ||
| White | 181 (80.4) | 48,597 (84.2) | |
| Black | 14 (6.2) | 4,597 (8) | |
| Asian | 18 (8.0) | 2,488 (4.3) | |
| Hispanic | 11 (4.9) | 4,459 (7.7) | NS |
| Annual incomea | NS | ||
| <$35,000 | 56 (24.9) | 14,050 (24.3) | |
| ≥$35,000 | 156 (69.3) | 39,765 (68.9) | |
| Insurance type | 0.01 | ||
| Private | 174 (77.3) | 39,220 (67.9) | |
| Medicare | 27 (12.0) | 11,354 (19.7) | |
| Medicaid | 12 (5.3) | 3,537 (6.1) | |
| Educationb | NS | ||
| Less educated | 75 (33.3) | 18,706 (32.4) | |
| More educated | 137 (60.9) | 35,102 (60.8) | |
| Urban/rural | NS | ||
| Metro | 186 (82.7) | 45,598 (79) | |
| Urban/rural | 27 (12.0) | 7,716 (13.4 %) | |
| Year of diagnosis | 0.08 | ||
| 2010 | 98 (43.6) | 28,593 (49.5) | |
| 2011 | 127 (56.4) | 29,136 (50.5) | |
| Hospital type | <0.0001 | ||
| Academic | 135 (60.0) | 24,609 (42.6) | |
| Comprehensivec | 72 (32.0) | 28,693 (49.7) | |
| Community | 18 (8.0) | 4,218 (7.3) | |
| Robotic case volume (mean ± SD) | 8 ± 10 | 4 ± 7 | <0.0001 |
| Hospital location | 0.06 | ||
| South | 87 (38.7) | 19,629 (34.0) | |
| Northeast | 64 (28.4) | 15,162 (26.3) | |
| West | 40 (17.8) | 9,868 (17.1) | |
| Midwest | 34 (15.1) | 13,070 (22.6) | |
| Distance traveled (mean miles ± SD)d | 70 ± 237 | 34 ± 259 | NS |
| Comorbiditye | NS | ||
| 0 | 194 (86.2) | 47,505 (82.3) | |
| ≥1 | 31 (13.8) | 10,224 (17.7) | |
| Histology | NS | ||
| Papillary | 212 (94.2) | 51,957 (90.0) | |
| Follicular | 11 (4.9) | 4,162 (7.2) | |
| Insular | 0 | 83 (0.1) | |
| Anaplastic | 0 | 183 (0.3) | |
| Tumor size (cm) | NS | ||
| <1.0 | 88 (39.1) | 21,404 (37.1) | |
| 1.0–2.0 | 73 (32.4) | 18,375 (31.8) | |
| 2.1–4.0 | 43 (19.1) | 11,687 (20.2) | |
| >4.0 | 18 (8.0) | 4,991 (8.7) | |
| Extrathyroidal extension | 28 (12.4) | 9,495 (16.5) | NS |
| Multifocality | 72 (32) | 22,012 (38.1) | NS |
| Nodal disease | 33 (14.7) | 11,735 (20.3) | 0.04 |
| Metastatic diseasef | 0 | 622 (1.1) | NS |
| Extent of surgery | <0.0001 | ||
| Total thyroidectomy | 151 (67.1) | 48,454 (83.9) | |
| Lobectomy | 70 (31.1) | 8,458 (14.7) | |
| Lymph node dissection | 91 (40.4) | 30,382 (52.6) | <0.001 |
Data are expressed as n (%) unless otherwise specified
Percentages were rounded and may not add up to 100. Missing data were not included in the analyses
NS non-significant, SD standard deviation
Median household income for each patient's area of residence is estimated by matching the ZIP code of the patient. Household income is categorized as <$35,000 or ≥$35,000
This variable provides a measure of the number of adults in the patient's ZIP code who did not graduate from high school, and is categorized as less educated: ≥20 % of citizens within ZIP code area with no high-school diploma; educated: <20 % of citizens within ZIP code with no high-school diploma
Comprehensive community hospital
Distance traveled to treating hospital in miles
Comorbidity as defined by Charlson-Deyo score
Metastatic disease at the time of diagnosis
FIG. 1.
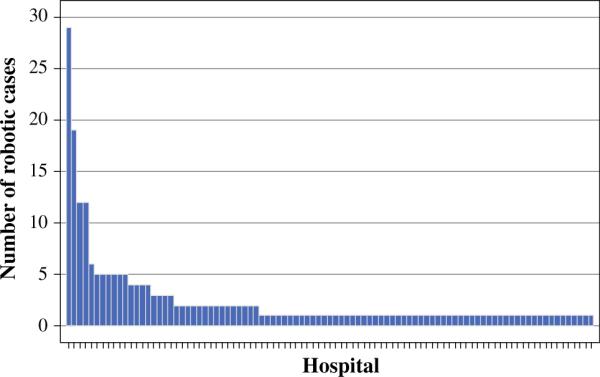
Variation in robotic thyroidectomy case volume among hospitals performing robotic cases for thyroid cancer (2010–2011)
FIG. 2.
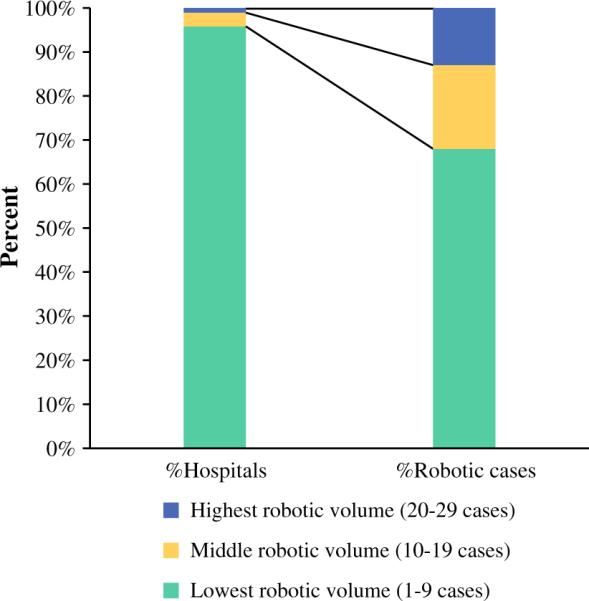
Summary of the distribution of hospitals performing robotic thyroidectomy and corresponding robotic cases in the three robotic volume categories; hospitals in the top two volume categories (four hospitals) performed 30 % of all robotic cases for cancer (2010–2011)
Compared with open thyroidectomy patients, robotic thyroidectomy patients were younger, more often female, Asian, privately insured, and cared for at academic hospitals (Table 1). The robotic group included a higher percentage of patients who underwent lobectomy (15 % open vs. 30 % robotic; p < 0.0001), and fewer patients who underwent a lymph node dissection (53 % open vs. 40 % robotic; p < 0.0001).
Patients undergoing robotic thyroidectomy had fewer nodal metastases than those who had open surgery (15 vs. 20 %; p = 0.04). In bivariate analyses, there were no differences between the robotic and open thyroidectomy groups with regard to the number of lymph nodes removed in patients who had any lymph nodes resected (median three vs. four nodes, respectively; p = 0.63); there were no differences in the rate of positive surgical margins (11 % for both groups; p = 0.83), LOS of >1 day (16 vs. 19 %; p = 0.41), unplanned readmissions (1.3 vs. 1.9 %; p = 0.81), and deaths in the first 30 days following thyroid surgery (0.4 vs. 0.2 %; p = 0.33).
After adjustment, among patients who had a lymph node dissection, there was no difference between robotic versus open thyroidectomy in the number of lymph nodes removed (relative risk [RR] 0.98; 95 % confidence interval [CI] 0.79–1.22; p = 0.89). The likelihood of an LOS > 1 day was similar between robotic and open thyroidectomy (odds ratio [OR] 1.08; 95 % CI 0.69–1.69; p = 0.74), but there was a non-significant trend suggestive of a higher likelihood of positive surgical margins in the robotic group (OR 1.55; 95 % CI 0.92–2.63; p = 0.1) compared with open thyroidectomy.
In the backward selection model, factors associated with the use of robotic thyroidectomy included female sex (p = 0.04), having surgery in the South [vs. Midwest] (p = 0.02), and undergoing lobectomy (p < 0.0001). Patients were less likely to undergo robotic thyroidectomy if they were older (p < 0.0001) or had lymph node metastases (p = 0.06) (Table 2).
TABLE 2.
Factors independently associated with the use of robotic versus open thyroidectomy for thyroid cancer (2010–2011)
| Adjusted odds ratio | 95 % CI | p value | |
|---|---|---|---|
| Age (in decades) | 0.81 | 0.73–0.90 | <0.001 |
| Sex (ref = male) | |||
| Female | 1.58 | 1.03–2.42 | 0.04 |
| Race (ref = White) | |||
| Asian | 1.64 | 0.94–2.86 | 0.08 |
| Geographic location (ref = Midwest) | |||
| South | 1.75 | 1.11–2.76 | 0.02 |
| Hospital type (ref = community) | |||
| Academic | 1.83 | 0.98–3.44 | 0.06 |
| Nodal metastases (ref = absent) | |||
| Present | 0.67 | 0.43–1.02 | 0.06 |
| Extent of surgery (ref = total thyroidectomy) | |||
| Lobectomy | 2.38 | 1.68–3.36 | <0.001 |
DISCUSSION
This is the first study to examine utilization of robotic thyroid surgery for thyroid cancer in the US. We show that utilization remains limited to a few institutions; however, the great majority of these surgeries are being performed in low-volume centers. Robotic and conventional thyroidectomy appear to have comparable length of hospital stay. With regard to adequacy of the oncologic resection, the two approaches are associated with a similar number of lymph nodes removed, but there was a non-significant trend toward a higher likelihood of positive surgical margins with robotic thyroidectomy.
In our study, <1 % of all thyroid cancer surgeries were performed robotically. This is consistent with the few published studies from single institutions in the US. The largest of these series included just 24 patients.18 Other studies included 3–11 patients.21–23 The limited use of robotic thyroidectomy in the US may reflect the controversy among experts regarding its role in the management of thyroid cancer.16–18 Compared with conventional thyroidectomy, robotic thyroidectomy is argued to have higher rates of hemorrhage, chest paresthesia, and injury to the brachial plexus, trachea, and large blood vessels.8,24–27 Most of the literature reporting on the safety of robotic thyroidectomy is from Korea, where patients are generally leaner, with smaller body frames than many Americans. As a result, the distance from the axilla to the thyroid is shorter, which could at least in part account for fewer technical difficulties.12
In our analysis, the great majority of hospitals where robotic thyroidectomy was performed were low-volume institutions (96 %), with <10 robotic cases over the 2 years; they accounted for approximately 70 % of all robotic cases. This is concerning since robotic thyroidectomy is technically challenging, with a steep learning curve.24,28 The New Technology Task Force, a multidisciplinary body for implementation of robotic thyroidectomy in the US, stressed the importance of adequate surgeon experience.29 Studies from the US and Korea have shown that at least 40 cases are required to be performed by a surgeon to reach proficiency in robotic thyroid lobectomy, and at least 50 robotic cases for total thyroidectomy.24,28 In our study, there was no hospital that performed >40 cases; however, this study did not include robotic cases that were performed for benign thyroid disease. While it is possible that some high-volume centers have not published their experience with robotic thyroidectomy, it is still notable that there is only one center in the US that has performed more than 40 robotic cases and reported its outcomes.18
In the current study, patients who were female and younger were more likely to undergo robotic thyroidectomy. This finding has been demonstrated in a meta-analysis of 726 patients undergoing robotic thyroidectomy for benign and malignant etiologies collated from 11 US and Korean studies.30 In our study, a higher proportion of patients undergoing robotic surgery had lobectomies than those in the open group. This has been shown in previous studies and highlights the possible selection bias that could account for the different demographic profiles for patients who undergo robotic versus open thyroid surgery.18,21
In multivariable analysis, we have shown that robotic thyroidectomy was associated with comparable length of hospital stay. In addition, the number of lymph nodes removed at the time of thyroidectomy was similar. These results are in agreement with published studies from Korea and small, single-institutional studies from high-volume centers in the US.11,18,21 In a single-institution report of 59 thyroid cancer patients from the US, Noureldine et al. retrospectively analyzed data from 24 thyroid cancer patients who underwent robotic thyroidectomy and 35 patients who had open thyroidectomy.18 All surgical margins were negative, and the two groups had similar rates of perioperative complications; their stimulated thyroglobulin levels were also similar 2 months after surgery. The robotic surgery group had shorter length of hospital stay; however, these patients were carefully selected and, on average, were younger and had a lower body mass index, and no concurrent thyroiditis or Graves’ disease.18 Therefore, these findings should be interpreted with caution because of the selection bias favoring robotic surgery.18,21,31
There are several possible limitations of this study. Due to the small sample size, we were not able to definitively discern the impact of robotic thyroidectomy on the higher incidence of positive surgical margins. Studies using databases are subject to the possibility of coding errors; however, the NCDB uses a standardized coding system and is heavily audited. Certain variables of interest, including patient body mass index, operative time, costs, and thyroidectomy-specific complications, such as hypoparathyroidism and recurrent laryngeal nerve injury, are not available in the dataset and therefore could not be analyzed. Also, selection bias is always a concern in observational studies when comparing outcomes between interventions. Our description of patients undergoing both procedures in this large series provides important information regarding the demographic of patients selected for robotic thyroid surgery.
CONCLUSIONS
The use of robotic surgery for thyroid cancer in the US is limited to a few institutions, with the majority of cases performed at low-volume institutions. While the overall number of robotic cases is small, robotic thyroidectomy in carefully selected patients appears to have some short-term outcomes similar to open thyroidectomy. Ideally, a prospective, multi-institutional clinical trial could better evaluate short- and long-term clinical and economic outcomes of robotic versus open thyroidectomy for thyroid cancer.
Footnotes
A portion of the results of this study was presented at the 67th Society of Surgical Oncology Annual Cancer Symposium, Phoenix, Arizona, USA, 12–15 March 2014.
DISCLOSURE The authors report no conflicts of interest. The data used in this study are derived from a de-identified NCDB file. The ACS and the CoC have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
REFERENCES
- 1.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–22. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society [1 Mar 2014];Cancer facts & figures. 2013 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf.
- 3.Shaha AR. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope. 2004;114(3):393–402. doi: 10.1097/00005537-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hannan SA. The magnificent seven: a history of modern thyroid surgery. Int J Surg. 2006;4(3):187–91. doi: 10.1016/j.ijsu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Giddings AE. The history of thyroidectomy. J R Soc Med. 1998;91(Suppl 33):3–6. doi: 10.1177/014107689809133s02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228(3):320–30. doi: 10.1097/00000658-199809000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer MC, Heffernan A, Terris DJ. Defining anatomical landmarks for robotic facelift thyroidectomy. World J Surg. 2014;38(1):92–5. doi: 10.1007/s00268-013-2246-8. [DOI] [PubMed] [Google Scholar]
- 8.Song CM, Ji YB, Bang HS, Park CW, Kim H, Tae K. Long-term sensory disturbance and discomfort after robotic thyroidectomy. World J Surg. doi: 10.1007/s00268-014-2456-8. Epub 8 Feb 2014. [DOI] [PubMed] [Google Scholar]
- 9.Nam KH, Owen R, Inabnet WB., 3rd Prevention of complications in transaxillary single-incision robotic thyroidectomy. Thyroid. 2012;22(12):1266–74. doi: 10.1089/thy.2012.0068. [DOI] [PubMed] [Google Scholar]
- 10.Richmon JD, Holsinger FC, Kandil E, Moore MW, Garcia JA, Tufano RP. Transoral robotic-assisted thyroidectomy with central neck dissection: preclinical cadaver feasibility study and proposed surgical technique. J Robot Surg. 2011;5(4):279–82. doi: 10.1007/s11701-011-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Kwon IS, Bae EH, Chung WY. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J Clin Endocrinol Metab. 2013;98(7):2701–8. doi: 10.1210/jc.2013-1583. [DOI] [PubMed] [Google Scholar]
- 12.Duh QY. Robot-assisted endoscopic thyroidectomy: has the time come to abandon neck incisions? Ann Surg. 2011;253(6):1067–8. doi: 10.1097/SLA.0b013e31821c4e48. [DOI] [PubMed] [Google Scholar]
- 13.Tae K, Ji YB, Cho SH, Lee SH, Kim DS, Kim TW. Early surgical outcomes of robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach for papillary thyroid carcinoma: 2 years’ experience. Head Neck. 2012;34(5):617–25. doi: 10.1002/hed.21782. [DOI] [PubMed] [Google Scholar]
- 14.Lee YM, Yi O, Sung TY, Chung KW, Yoon JH, Hong SJ. Surgical outcomes of robotic thyroid surgery using a double incision gasless transaxillary approach: analysis of 400 cases treated by the same surgeon. Head Neck. doi: 10.1002/hed.23472. Epub 22 Aug 2013. [DOI] [PubMed] [Google Scholar]
- 15.Kang SW, Park JH, Jeong JS, et al. Prospects of robotic thyroidectomy using a gasless, transaxillary approach for the management of thyroid carcinoma. Surg Laparosc Endosc Percutan Tech. 2011;21(4):223–9. doi: 10.1097/SLE.0b013e3182266f31. [DOI] [PubMed] [Google Scholar]
- 16.Perrier ND. Why I have abandoned robot-assisted transaxillary thyroid surgery. Surgery. 2012;152(6):1025–6. doi: 10.1016/j.surg.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 17.Inabnet WB., 3rd Robotic thyroidectomy: must we drive a luxury sedan to arrive at our destination safely? Thyroid. 2012;22(10):988–90. doi: 10.1089/thy.2012.2210.com2. [DOI] [PubMed] [Google Scholar]
- 18.Noureldine SI, Jackson NR, Tufano RP, Kandil E. A comparative North American experience of robotic thyroidectomy in a thyroid cancer population. Langenbecks Arch Surg. 2013;398(8):1069–74. doi: 10.1007/s00423-013-1123-0. [DOI] [PubMed] [Google Scholar]
- 19.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99(8):488–90. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Aliyev S, Taskin HE, Agcaoglu O, et al. Robotic transaxillary total thyroidectomy through a single axillary incision. Surgery. 2013;153(5):705–10. doi: 10.1016/j.surg.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Landry CS, Grubbs EG, Warneke CL, et al. Robot-assisted transaxillary thyroid surgery in the United States: is it comparable to open thyroid lobectomy? Ann Surg Oncol. 2012;19(4):1269–74. doi: 10.1245/s10434-011-2075-7. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson CE, Gardner DF, Grover AC. Patient factors affecting operative times for single-incision trans-axillary robotic-assisted (STAR) thyroid lobectomy: does size matter? Ann Surg Oncol. 2012;19(5):1460–5. doi: 10.1245/s10434-011-1972-0. [DOI] [PubMed] [Google Scholar]
- 24.Kandil EH, Noureldine SI, Yao L, Slakey DP. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg. 2012;214(4):558–64. doi: 10.1016/j.jamcollsurg.2012.01.002. discussion 564–556. [DOI] [PubMed] [Google Scholar]
- 25.Kuppersmith RB, Holsinger FC. Robotic thyroid surgery: an initial experience with North American patients. Laryngoscope. 2011;121(3):521–6. doi: 10.1002/lary.21347. [DOI] [PubMed] [Google Scholar]
- 26.Landry CS, Grubbs EG, Morris GS, et al. Robot assisted transaxillary surgery (RATS) for the removal of thyroid and parathyroid glands. Surgery. 2011;149(4):549–55. doi: 10.1016/j.surg.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Yun JH, Nam KH, Choi UJ, Chung WY, Soh EY. Peri-operative clinical outcomes after robotic thyroidectomy for thyroid carcinoma: a multicenter study. Surg Endosc. 2011;25(3):906–12. doi: 10.1007/s00464-010-1296-3. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Yun JH, Nam KH, Soh EY, Chung WY. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol. 2011;18(1):226–32. doi: 10.1245/s10434-010-1220-z. [DOI] [PubMed] [Google Scholar]
- 29.Perrier ND, Randolph GW, Inabnet WB, Marple BF, VanHeerden J, Kuppersmith RB. Robotic thyroidectomy: a framework for new technology assessment and safe implementation. Thyroid. 2010;20(12):1327–32. doi: 10.1089/thy.2010.1666. [DOI] [PubMed] [Google Scholar]
- 30.Sun GH, Peress L, Pynnonen MA. Systematic review and meta-analysis of robotic vs conventional thyroidectomy approaches for thyroid disease. Otolaryngol Head Neck Surg. 2014;150(4):520–32. doi: 10.1177/0194599814521779. [DOI] [PubMed] [Google Scholar]
- 31.Foley CS, Agcaoglu O, Siperstein AE, Berber E. Robotic transaxillary endocrine surgery: a comparison with conventional open technique. Surg Endosc. 2012;26(8):2259–66. doi: 10.1007/s00464-012-2169-8. [DOI] [PubMed] [Google Scholar]


