In recent years, an increasing number of reports have shown the involvement of osteopontin (OPN), a pleiotropic cytokine and an important component of the extracellular matrix (ECM), in the pathogenesis of liver injury and the development of fibrosis.1,2 OPN is also frequently overexpressed in hepatocellular carcinoma (HCC), where it modulates HCC growth, invasion and metastasis,2 and in cholangiocarcinoma, where its expression bears prognostic significance. Previous studies3 have shown that in injured livers OPN is expressed by hepatic stellate cells (HSC) and upregulates collagen I production. Interestingly, in HSC, OPN expression appears to be downstream of SOX9, a Hedgehog- and Notch-controlled transcription factor that is expressed also in biliary cells and hepatic progenitor cells (HPC)/hepatocytes committed to the biliary fate.4–6 In this issue of Gut, two papers investigate the role of OPN in HPC-driven ductular reaction (DR) in relation to the progression of liver fibrosis.7,8
DR, a histological lesion present in most chronic liver diseases, is a dynamic, multicellular complex characterised by the presence of ‘reactive’ ductular epithelial cells, arranged in irregular strings along the margins of the portal tract in close contact with mesenchymal, inflammatory and endothelial cells. Reactive ductular cells acquire novel functions including the secretion of cytokines, chemokines, growth factors and angiogenic factors, enabling them to establish intense paracrine communications with a variety of cells and to orchestrate the repair of the epithelial wound. DR cells acquire also limited mesenchymal properties that endow them with increased motility and the ability to detach from the epithelial layer. Ductular reaction is driven by the activation of the HPC compartment, a compensatory mechanism of liver repair triggered when proliferative ability of mature hepatocytes or cholangiocytes is compromised9 such as in most human liver diseases.
Several studies indicate that the extent of DR closely correlates with the amount of collagen deposition, ultimately leading to tissue scarring and lobular architectural distortion.10 This process depends upon a complex interplay between paracrine signals released by HPC/DR able to activate a fibrogenic programme and promote changes in the ECM.10 In this context, OPN is a molecule of particular interest, being able to act as an ECM protein directing cell motility and adhesion as well as a cytokine regulating T-lymphocyte and macrophage responses in inflammation.1 The two studies published in this issue of Gut further expand the role of OPN in HPC activation and DR as a critical mechanism responsible for the progression of liver fibrosis across different experimental models of chronic liver injury.7,8
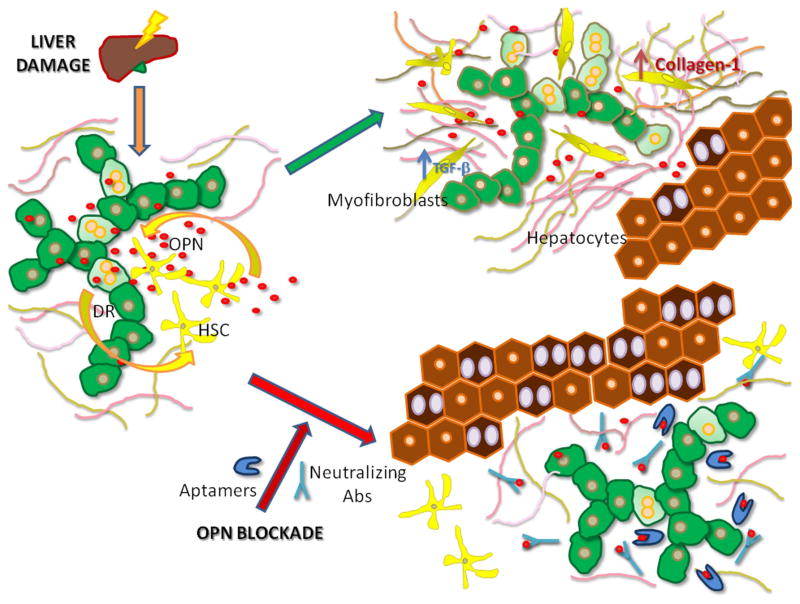
In particular, the two papers show that OPN produced by HPC contributes to DR by stimulating HPC proliferation and migration, while concomitantly it reduces hepatocyte proliferative capability.7,8 Furthermore, they demonstrate that OPN plays an important role in regulating the interaction between HPC and HSC, and in modulating myofibroblast activation and collagen production synergistically with transforming growth factor-β (TGF-β).7,8 In turn, TGF-β influences OPN signalling in cultured HPC cell lines.7 In line with these observations, OPN deficiency or OPN inactivation with OPN-specific aptamers or neutralising antibodies reduces DR, HPC response and fibrogenesis in different experimental models of chronic liver disease (figure 1). Altogether, the two studies nicely complement each other in addressing the importance of OPN in driving HPC expansion and DR.
Figure 1.
Osteopontin (OPN) is an important regulator of the interaction between ductular reaction (DR) cells and hepatic stellate cells (HSC): it may represent a molecular target for therapeutic interference. Following chronic liver injury, OPN produced by DR cells, including hepatic progenitor cell (HPC), stimulates HSC recruitment and activation into myofibroblasts, where it induces transforming growth factor-β signalling and increased collagen deposition; on the parenchymal side, OPN simulates HPC proliferation and migration, while concomitantly downregulating hepatocyte proliferation (green arrow). OPN blockade by specific aptamers or neutralising antibodies halts the crosstalk between DR cells and HSC, thereby hampering myofibroblast activation and reducing matrix production, while turning up hepatocyte proliferation (red arrow).
Although these two reports make an important contribution in elucidating the complex role of OPN in chronic liver diseases, a number of issues remain unsolved. Two forms of OPN are known: an intracellular form (iOPN) involved in regulating cell migration and inflammatory signalling in lymphocytes and dendritic cells, and a secreted form (sOPN) with cytokine and ECM protein properties.1 The data of Coombes et al8 using OPN-specific aptamers or neutralising antibodies suggest that sOPN has an important role in fibrogenesis. However, further studies are required to fully understand the role of iOPN. The second question that remains lingering is the cellular origin of OPN in liver diseases. Several cells, including hepatocytes, macrophages, cholangiocytes, T-lymphocytes, natural killer T-cells and HSC, contribute to hepatic OPN production,2 making it difficult to dissect the specific contribution of each cell type to the overall effects. Using immunohistochemistry and morphometry, Wang and coworkers7 studied the relative contribution of different hepatic cell populations to OPN production in control and thioacetamide (TAA)-treated mice. They show that, while in control mice hepatocytes and cholangiocytes are the main sources of OPN in injured livers, HPC and HSC appreciably contribute to OPN production.7 However, even in these conditions more than 90% of OPN staining involves hepatocytes.7 Further studies using animals with selective inactivation of the OPN gene in specific hepatic cell populations are required to better elucidate the origin of OPN that drives DR. Finally, a third question involves the difficulty in discriminating among the pleiotropic actions of OPN. It remains unclear to what extent the capacity of OPN to stimulate HPC response and fibrogenesis depends on its activity as a pro-inflammatory cytokine or by its ability to stimulate HSC activation and collagen production.2,3 Dissecting the pro-inflammatory activity of OPN is important in characterising its role in DR as this response implies a significant contribution of inflammatory cells.9
Altogether, the results presented by Wang et al7 and Coombes et al8 along with previous data suggest OPN as a possible therapeutic target for liver fibrosis. Furthermore, the results of the inhibition studies in these manuscripts represent a further strong piece of evidence that the ‘Holy Grail’ of chronic liver disease progression lies buried in the crosstalk between epithelial and mesenchymal/inflammatory cells,10,11 and whoever follows just one lead will never find it.
Acknowledgments
Funding NIH Grant DK079005 and DK034989 Silvio O. Conte Digestive Diseases Research Core Centers to MS, projects CARIPLO 2011-0470 and PRIN 2009ARYX4T_005 to MS and EA. Telethon (grant #GGP09189) and Progetto di Ricerca Ateneo 2011 (grant #CPD113799/11) to LF.
Footnotes
Contributors All authors were responsible for drafting and editing of the commentary.
Competing interests None.
Provenance and peer review Commissioned; internally peer reviewed.
References
- 1.Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol Int. 2011;61:265–80. doi: 10.1111/j.1440-1827.2011.02649.x. [DOI] [PubMed] [Google Scholar]
- 2.Nagoshi S. Osteopontin: Versatile modulator of liver diseases. Hepatol Res. 2014;44:22–30. doi: 10.1111/hepr.12166. [DOI] [PubMed] [Google Scholar]
- 3.Urtasun R, Lopategi A, George J, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology. 2012;55:594–608. doi: 10.1002/hep.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritchett J, Harvey E, Athwal V, et al. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology. 2012;56:1108–16. doi: 10.1002/hep.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorotto R, Raizner A, Morell CM, et al. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J Hepatol. 2013;59:124–30. doi: 10.1016/j.jhep.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2014 doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Lopategi A, Ge X, et al. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut. 2014;63:1805–18. doi: 10.1136/gutjnl-2013-306373. [DOI] [PubMed] [Google Scholar]
- 8.Coombes JD, Swiderska-Syn M, Dollé L, et al. Osteopontin neutralisation abrogates the liver progenitor cell response and fibrogenesis in mice. Gut. doi: 10.1136/gutjnl-2013-306484. Published Online First: 4 Feb 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guow AS, Clouson AD, Thiese ND. Ductular reaction in human liver; diversity at the interface. Hepatology. 2011;54:1853–63. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 10.Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Semin Liver Dis. 2011;31:11–32. doi: 10.1055/s-0031-1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–56. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]