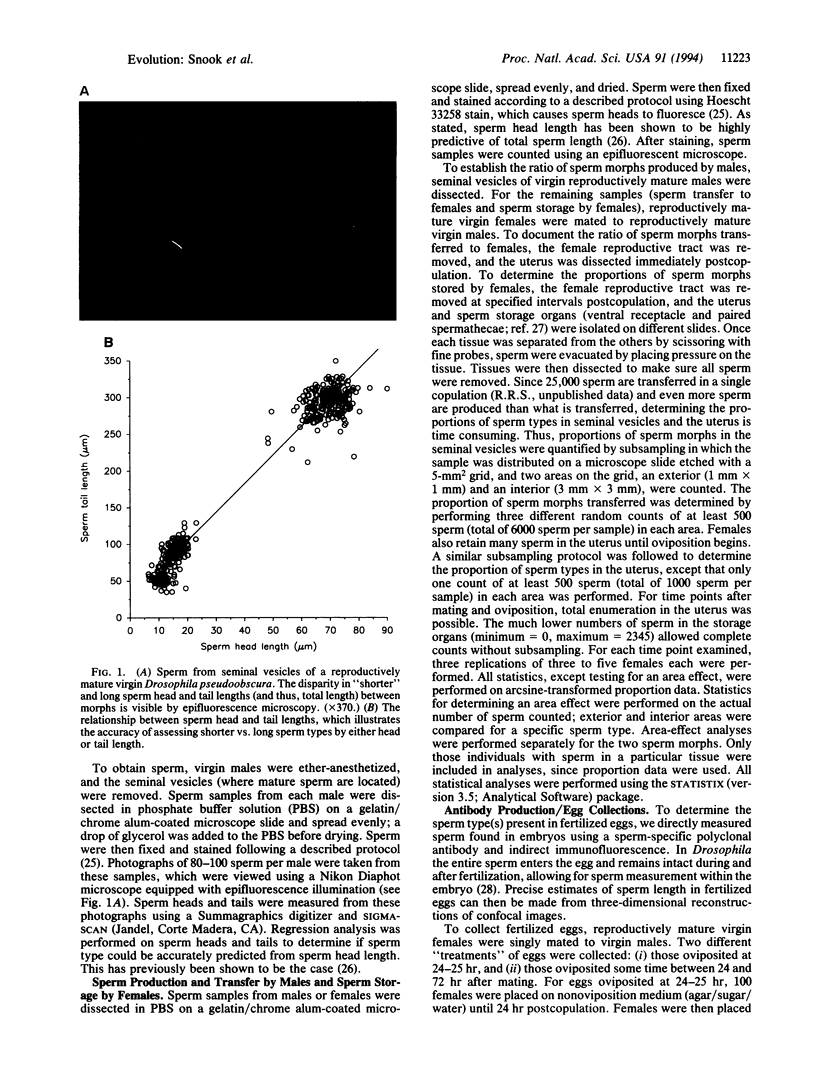
Abstract
We report on a form of sperm polymorphism, termed polymegaly, that occurs in species of the Drosophila obscura group. Individual males of species in this group characteristically produce more than one discrete length of nucleated, motile sperm. Hypotheses suggested to explain the evolutionary significance of sperm polymorphism have been either nonadaptive or adaptive, with the latter focusing on sperm competition or nutrient provisioning. These hypotheses assume all sperm types fertilize eggs; however, no data have been gathered to test this assumption. We found that two size classes of sperm are produced and transferred to females in approximately equal numbers by the male; only long sperm persist in significant numbers in female sperm storage organs. Furthermore, we used a DNA-specific dye (bisbenzimide) and sperm-specific antibodies to ask if both sperm types fertilize eggs in Drosophila pseudoobscura. Confocal microscopy and immunofluorescent analyses of fertilized eggs using anti-sperm polyclonal antisera demonstrated that only long sperm participate in fertilization. These data falsify those hypotheses in which all sperm types are assumed to be functionally equivalent (fertilize eggs). Any remaining or new hypotheses for the evolutionary significance of polymegaly must incorporate these findings. Several new areas of research are suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatty R. A., Burgoyne P. S. Size classes of the head and flagellum of Drosophila spermatozoa. Cytogenetics. 1971;10(3):177–189. [PubMed] [Google Scholar]
- Beatty R. A., Burgoyne P. S. Size classes of the head and flagellum of Drosophila spermatozoa. Cytogenetics. 1971;10(3):177–189. [PubMed] [Google Scholar]
- Bressac C., Joly D., Devaux J., Lachaise D. Can we predict the mating pattern of Drosophila females from the sperm length distribution in males? Experientia. 1991 Jan 15;47(1):111–114. doi: 10.1007/BF02041270. [DOI] [PubMed] [Google Scholar]
- Bressac C., Joly D., Devaux J., Serres C., Feneux D., Lachaise D. Comparative kinetics of short and long sperm in sperm dimorphic Drosophila species. Cell Motil Cytoskeleton. 1991;19(4):269–274. doi: 10.1002/cm.970190405. [DOI] [PubMed] [Google Scholar]
- Dajoz I., Till-Bottraud I., Gouyon P. H. Evolution of pollen morphology. Science. 1991 Jul 5;253(5015):66–68. doi: 10.1126/science.253.5015.66. [DOI] [PubMed] [Google Scholar]
- Karr T. L. Intracellular sperm/egg interactions in Drosophila: a three-dimensional structural analysis of a paternal product in the developing egg. Mech Dev. 1991 Jun;34(2-3):101–111. doi: 10.1016/0925-4773(91)90047-a. [DOI] [PubMed] [Google Scholar]
- O'Kane C. J., Gehring W. J. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]